Simple Summary
Neogobius melanostomus is a highly invasive fish that has colonized most major European rivers and is dispersing into their tributaries. Its foraging behaviour does not show particular prey preferences, which makes predicting its interactions with endangered members of the macrozoobenthic community in tributaries a challenge. We observed the interaction of N. melanostomus and crayfish juvenile or A. aquaticus in single- and multiple-prey systems to better predict its ecological impact. The results suggest an impact of N. melanostomus on crayfish similar to that on A. aquaticus, potentially making it a threat to crayfish population stability. Destabilization of a keystone species such as crayfish in river tributaries may lead to a trophic cascade in the ecosystem with irreversible consequences.
Abstract
Despite the spread of round goby Neogobius melanostomus into freshwater streams, there is a lack of information with respect to its effect on macroinvertebrate communities, especially crustaceans. We studied foraging efficiency of N. melanostomus on Procambarus virginalis and Asellus aquaticus, using a functional response (FR) approach. Stocking density of the prey species was manipulated to determine its effect on consumer utilization, with prey offered separately or combined at 1:1, 3:1, and 1:3 at each tested density. For both prey species, N. melanostomus exhibited type II FR, occasionally with a high proportion of non-consumptive mortality. Procambarus virginalis suffered a significantly higher attack rate compared to A. aquaticus. Neogobius melanostomus killed significantly more of the most prevalent prey, regardless of species. In trials with prey species of equal proportions, a difference in the number of each species killed was observed only at the highest density, at which P. virginalis was preferred. Neogobius melanostomus may be an important driver of population dynamics of prey species in the wild. The non-selective prey consumption makes N. melanostomus a potential threat to macrozoobenthic communities of river tributaries.
1. Introduction
Crayfish have an impact at multiple trophic levels through predation, shedding, burrowing, and competition [1,2,3] and are considered keystone species influencing stability and functionality of ecosystems, particularly in tributaries to major streams [4,5,6]. Crayfish populations worldwide are threatened by multiple stressors: Climate change, water pollution, habitat modification, invasive species, and disease [5,7]. Nearly one third of crayfish species worldwide are threatened with extinction [7]. Although interventions in the EU [8] and throughout the world [9,10] aim to improve the ecological status of freshwater lotic ecosystems, the threat presented by non-indigenous species is ever-increasing [11]. In addition to interactions with non-indigenous crayfish, native crayfish interact with small benthic fishes, including non-native species [1].
The round goby Neogobius melanostomus (Pallas 1814), among the most invasive of freshwater fish species [12], has expanded substantially beyond its native range the Ponto-Caspian region. It poses a serious threat to freshwater and brackish ecosystems [13] causing critical food web disruptions, shifts in trophic levels, extermination of native species through direct predation and/or competition for resources and habitat, and spread of disease [14,15,16,17]. In major rivers, after establishing a viable population, N. melanostomus spreads both down- and up-stream [18,19]. It is increasingly found in tributaries of major rivers [20,21,22] that are often used as refugia for native species [23] and contain unique highly diverse macrozoobenthic communities including endangered species such as crayfish [24]. These communities may be seriously threated by N. melanostomus invasion and dispersion [25,26].
Macrozoobenthos represent a predominant proportion of the N. melanostomus diet [27,28], reflecting the community structure in a given locality [29,30]. In contrast to major rivers and lakes, which often harbour several non-native macrozoobenthos species, in small streams with highly diverse macrozoobenthic communities, N. melanostomus remains a generalist omnivore [30]. This can lead to a significant transformation of the community structure with severe consequences to endangered species, since even partial depletion of a single prey population can alter the predator food selectivity [31]. Nevertheless, crayfish are rarely reported in N. melanostomus diet in invaded regions [32,33], possibly the result of a unique flip-tail escape strategy, as observed for dragonfly nymph predation on early-stage crayfish [34].
With respect to the coexistence of small benthic fish and crayfish, due to similar body size, the primary focus has been on competition for food and shelter and on behaviour interactions in the presence of a common predator, as opposed to their mutual predation relationship [1]. However, crayfish juveniles that have become independent after leaving the female are threatened by fish predation due to their small size [35,36] and limited antipredator defences, usually restricted to the tail-flip escape movement [35,36,37,38]. The impact of small voracious benthic fish such as N. melanostomus on early crayfish stages may be intensified when sharing a common habitat. The ecological impact of N. melanostomus on crayfish populations has not been quantified.
Understanding and predicting novel predator-prey interaction dynamics and their consequences for invaded freshwater communities is a critical issue in invasion management [39]. Invasive predators, often possessing better foraging efficiency and/or resource utilization, may have higher maximum feeding rates than the analogous native predators and therefore greater ecological impact [40,41] with especially pronounced consequences in aquatic environments [42].
Resource availability represents a crucial determinant of feeding rate as illustrated by a functional response (FR) curve [43,44]. The shape and asymptote of the curve depict important parameters of consumer-resource interactions and population community dynamics [45,46]. Invasive species often display elevated FRs compared to native or low-impact non-native ecologically analogous species [47,48,49] making comparative FR a valuable tool for invasion biologists [48,49,50]. Functional response has been calculated for comparison of N. melanostomus foraging efficiency with native [51] as well as non-native analogous species [52] and can be employed for comparison of predator impact on prey components, since predator response to prey may be prey species–dependent [53,54,55,56,57]. A higher FR asymptote denotes more effective prey exploitation, possibly due to greater prey attractiveness or palatability and/or greater predator adaptation to prey antipredation behaviour. Currently, knowledge of the relationship between N. melanostomus and crayfishes is lacking, especially in tributaries serving as refuges for native aquatic biota and sources of genetic diversity for main stream ecosystems.
The aim of our study was to characterize N. melanostomus foraging efficiency on early juvenile crayfish. While natural ecosystems generally consist of multiple prey species per predator, the majority of research experiments address interaction between a single predator and prey species. We observed the predation behaviour of N. melanostomus in the presence of two prey species differing in escape behaviour at several densities and stocking proportions. We hypothesized that prey defence, as well as the presence of an alternative prey in various proportions, may significantly influence predator foraging efficiency.
2. Materials and Methods
2.1. Predator and Prey Acquisition and Acclimatization
Neogobius melanostomus were collected with a backpack pulsed-DC electrofishing unit (FEG 1500, EFKO, Leutkirch, Germany) in early October 2018 from a recently colonized locality in the Elbe River (50.6524583 N, 14.0441314 E). Specimens (TL = 55.9 ± 2.6 mm; W = 2.1 ± 0.3 g) were transported to the Institute of Aquaculture and Protection of Water and acclimated in a 1600 L recirculating aquaculture system for 7 days. They were fed frozen chironomid larvae to satiation twice daily. Water temperature (20.3 ± 0.3 °C), dissolved oxygen (100.6 ± 2.9%), and pH (7.7 ± 0.2) were measured twice daily with an HQ40d digital multimeter (Hach Lange GmbH, Düsseldorf, Germany).
We used two hard-bodied benthic invertebrate prey species of similar body mass differing in escape strategy: The native water louse Asellus aquaticus (L.) (W = 5.56 ± 1.94 mg) is representative of isopods that form a component of the N. melanostomus diet [58,59]. Isopod locomotion is restricted to slow crawling with no escape strategy [60]. The second species was the juvenile non-native marbled crayfish Procambarus virginalis (Lyko 2017) (W = 5.45 ± 0.66 mg), a common crayfish model species for laboratory research [61], which exhibits a flip-tail escape strategy as the native crayfish species [34]. Both native crayfish species in the Czech Republic (i.e., Astacus astacus and Austropotamobius torretium) are classified as critically endangered species in the Red list of threatened species of the Czech Republic with a continual populations decline [62]. Therefore, their use for experiments performance is strongly forbidden and dispensation from law is impossible.
Asellus aquaticus was collected with hand nets in late September 2018 in the Kyselá voda stream (49.0195475 N, 14.4640344 E). The P. virginalis were obtained from the Laboratory of Ethology of Fish and Crayfish, FFPW USB. Both prey species were housed in 200 L glass aquaria equipped with PVC trickling filter media (Hewitech GmbH, Ochtrup, Germany) that served as shelter and filter. Half the water volume was exchanged daily with dechlorinated tap water.
2.2. Experiment Design
Transparent plastic boxes (295 × 185 × 155 mm; total volume = 6000 mL) filled with 5000 mL dechlorinated tap water and 200 mL fine aquarium sand (particle size < 0.3 mm) were used as experimental arenas. Five prey exposures were tested: A. aquaticus and P. virginalis separately and combined at respective ratios of 1:1, 1:3, and 3:1. Each exposure included prey densities of 4, 8, 20, 36, 60, and 100 individuals/box with six replicates per density. Overall, 180 N. melanostomus specimens were used in the experiment, whereas each predator was used only once. Baseline prey mortality was assessed with control groups of the same combinations, ratios, and densities in six replications without predators. Neogobius melanostomus were starved for 24 h before each trial to standardize hunger level and placed individually into the experimental arenas 1 h after prey insertion. A light regime of 500 lux m2 was maintained in a 12 L:12 D photoperiod. The predator was removed from the arena after 24 h, and the number and species of surviving prey and non-consumptive mortality (NCM) were determined. Non-consumptive mortality was calculated as in [63] including dead prey not ingested by the predator. Each predator was used once to avoid experience bias.
2.3. Data Analysis
The FR of N. melanostomus was fitted separately for each prey organism and ratio and calculated as a total number of killed prey (sum of NCM and eaten prey). Hence, FR quantified the overall impact of N. melanostomus on prey. The FRs of N. melanostomus on prey were compared between species and among stocking ratios. The type of FR was determined by fitting of logistic regression on the basis of the relationship between the killed prey (Ne) and the initial prey density (N0):
where P0, P1, P2, and P3 represent intercept, linear, quadratic, and cubic coefficients, respectively, estimated using the method of maximum likelihood. If P1 reaches a positive value with P2 negative, the proportion of prey killed is positively density-dependent, which is peculiar to type III FR. However, if P1 is a negative value, the proportion of prey killed declines monotonically from initial prey density, indicating type II FR [46]. Based on logistic regression, we used Rogers’s random predator equation [64] for type II FR in all prey types and ratios, which is suitable for non-replacement design:
where T is time of prey exposure to predator (24 h), a is predator attack rate (predator relative consumption rate corresponds to search efficiency in low prey density manifested in an initial slope steepness on FR curve; L day-1), and h is predator handling time (time pursuing, subduing, and eating of prey combined with time spent prey searching and digestive pause; days prey-1) [65]. For bordering of the Rogers’s random-predator equation by Ne on both sides of the equation, we used the Lambert W function for solving Equation [66]:
We estimated parameters a and h using non-linear least-squares regression and Lamber W function included in the EMDBOOK package [66]. Differences in parameters among prey species and ratios were evaluated based on an overlap of 95% confidence intervals. If no overlap was observed, the parameters significantly differed among the treatments [67].
The effects of prey species, ratio, density, and their interaction upon the number of prey eaten, NCM, and killed prey were tested using a generalized linear model (GLM) with Gaussian distribution. Tukey’s HSD post-hoc test was subsequently used for determination of significant differences among exposures. Since the survival rate in all control treatments exceeded 97% (97.2–100.0%), the mortality of predator-exposed prey was attributed exclusively to the presence of N. melanostomus, and datasets were not adjusted for natural mortality. All analyses were conducted in R version 4.0.3 (R Development Core Team 2018).
3. Results
3.1. Functional Response Type
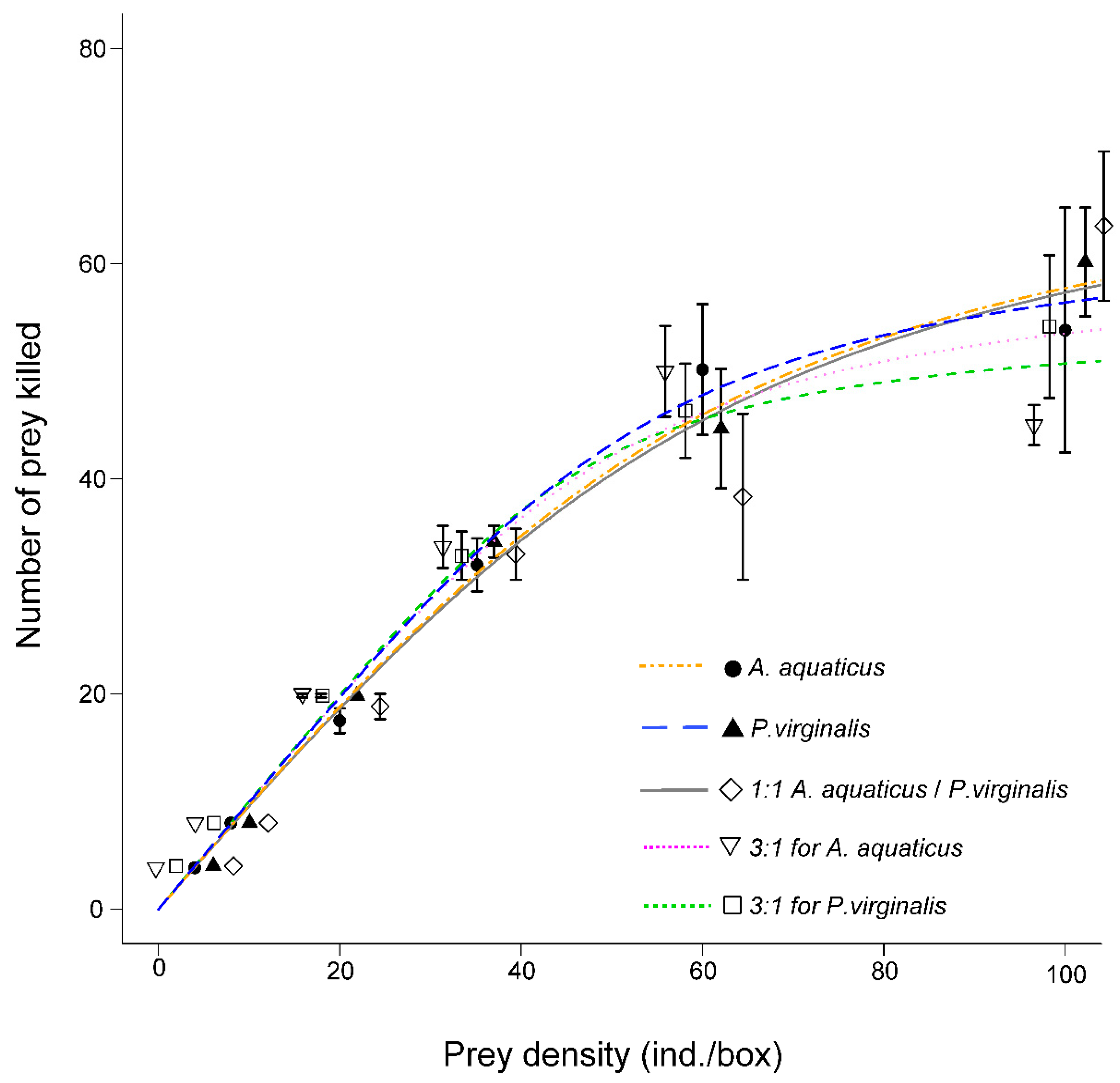
In all exposures, N. melanostomus exhibited the type II functional response (Figure 1): Significant negative linear coefficients in logistic regressions (Table 1).
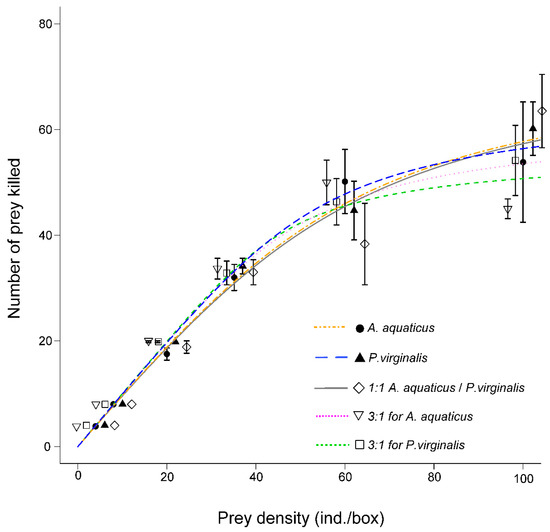
Figure 1.
Functional response (mean ± SE) of Neogobius melanostomus. Asellus aquaticus is represented by the orange dot-dash line and Procambarus virginalis by the blue dashed line. Prey were offered separately and combined 1:1 (grey solid line), 3:1 for A. aquaticus (pink dotted line), and 3:1 for P. virginalis (green dotted line).

Table 1.
Linear coefficient P1 of logistic regression of Neogobius melanostomus relative to prey species and stocking ratio.
3.2. Attack Rate and Handling Time
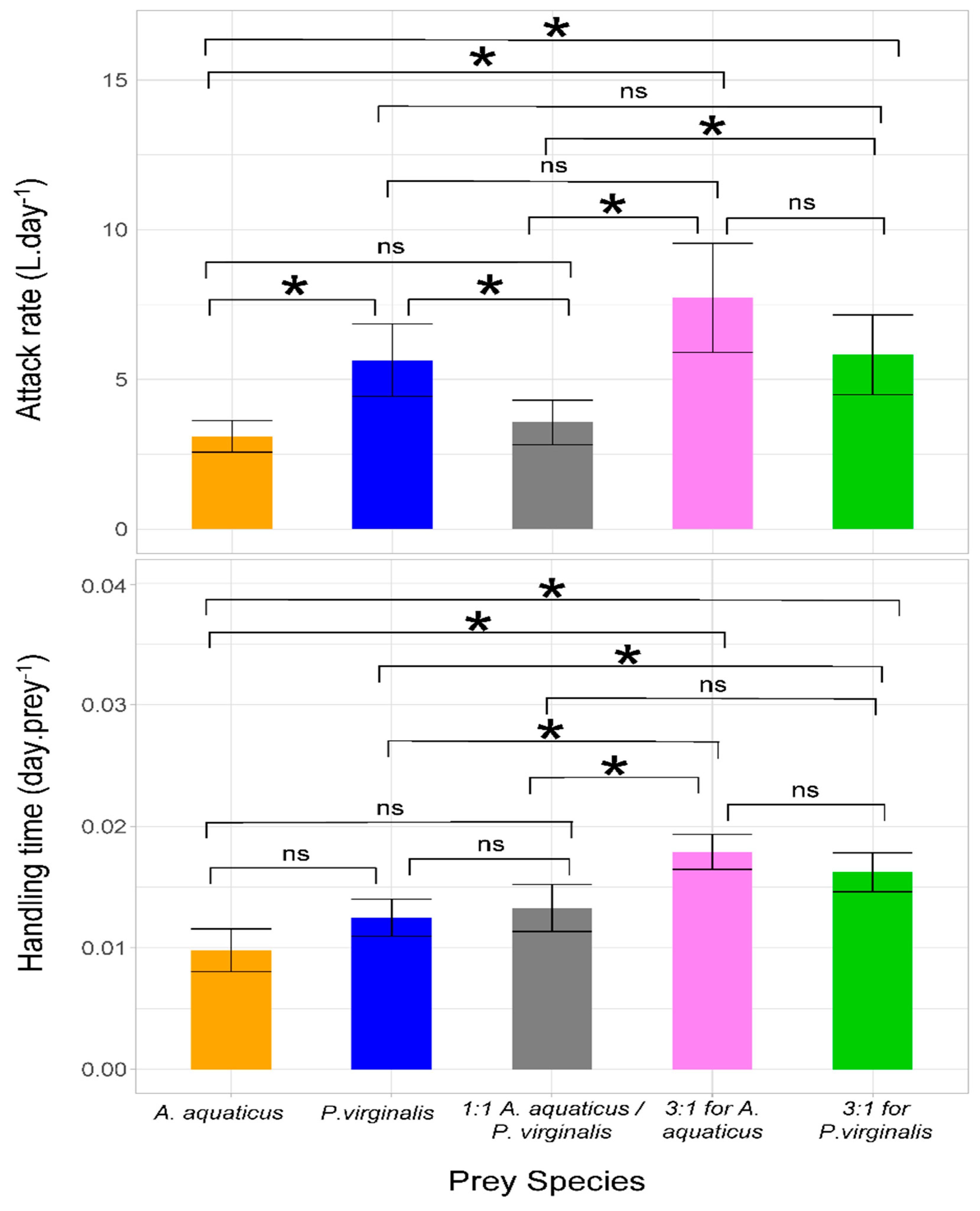
Significantly higher values of attack rate were observed in the trial with P. virginalis offered separately as well as in both 3:1 prey combinations compared with the 1:1 combination and A. aquaticus offered separately. Neogobius melanostomus displayed the highest handling time in the 3:1 trials, with no significant differences among groups in which prey species were offered separately or at 1:1 (Table 2 and Figure 2).

Table 2.
Confidence intervals (95% CI) of handling time and attack rate of Neogobius melanostomus relative to prey species and presentation (separately or mixed). In multiple prey trials, Asellus aquaticus and Procambarus virginalis were offered at ratios of 1:1, 3:1 or 1:3.
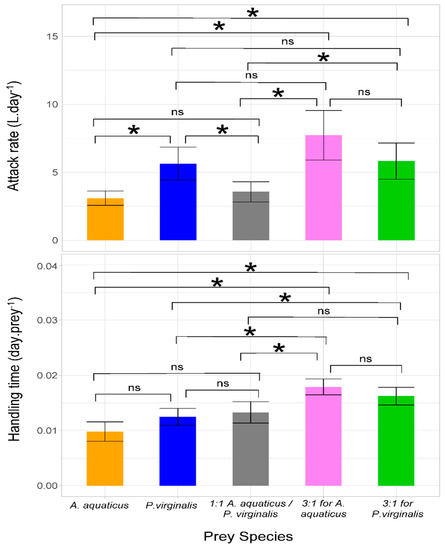
Figure 2.
Attack rate and handling time (error bars denote 95% confidence intervals) of Neogobius melanostomus with respect to prey species separately and combined. In multiple prey trials, Asellus aquaticus and Procambarus virginalis were offered at ratios of 1:1, 3:1, and 1:3. Asterisks denote significant (p < 0.05) differences among trials and NS indicates non-significant difference.
3.3. Number of Killed and Eaten Prey and Non-Consumptive Mortality
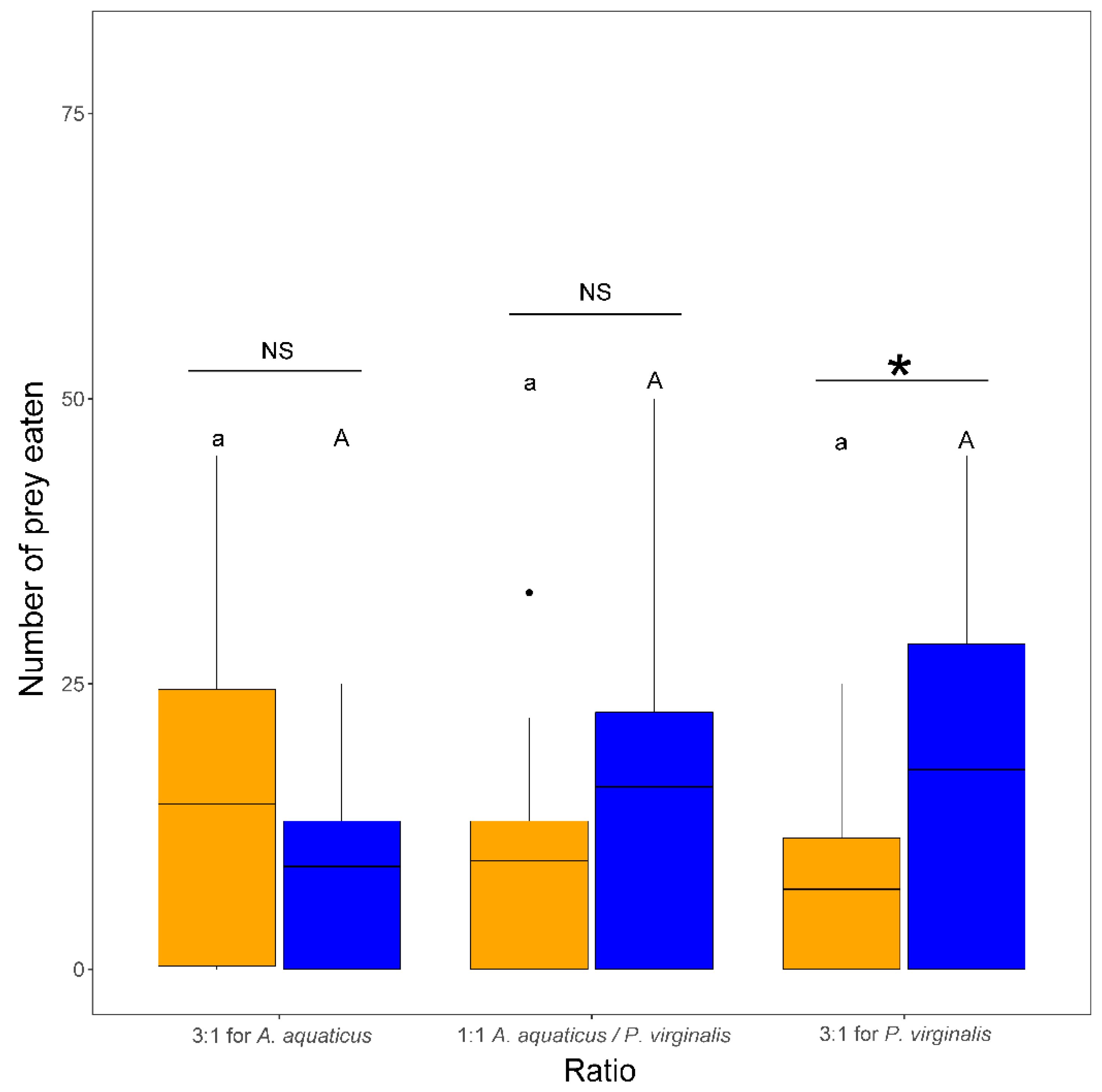
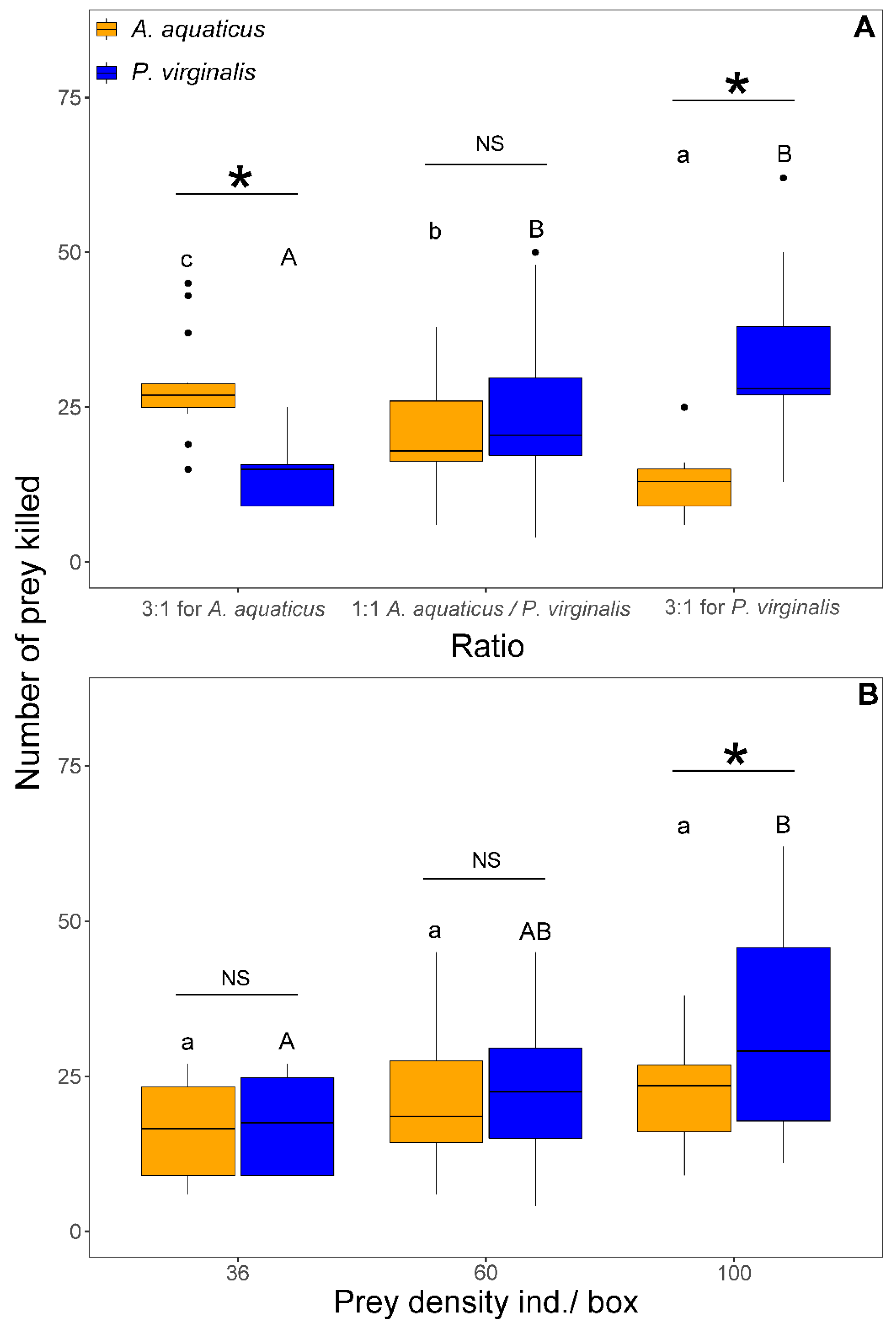
The number of prey eaten by N. melanostomus was significantly affected by the interaction of species and ratio (F2,102 = 4.71, p = 0.011). This was reflected in a significantly higher number of P. virginalis consumed than A. aquaticus in the group with 3:1 for P. virginalis. There were no other significant differences among trials in the number of prey eaten (Figure 3).
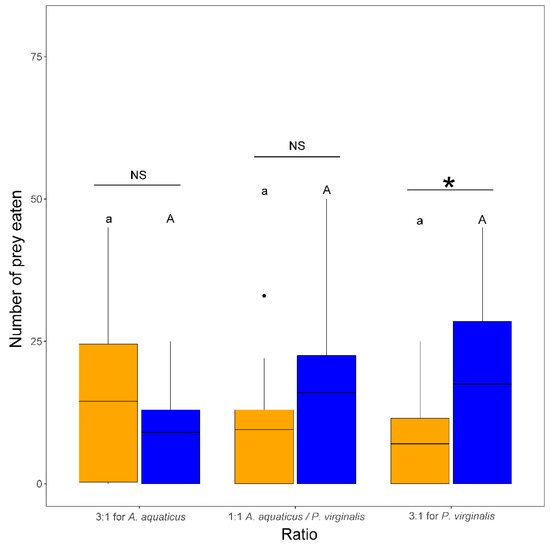
Figure 3.
Number of Asellus aquaticus (orange) vs. Procambarus virginalis (blue) consumed by Neogobius melanostomus is prey species ratio–dependent. Exposures with the same letter do not significantly differ (p > 0.05). Asterisk denotes significant difference (p < 0.05) between species and NS indicates non-significant difference. The points denote outliers.
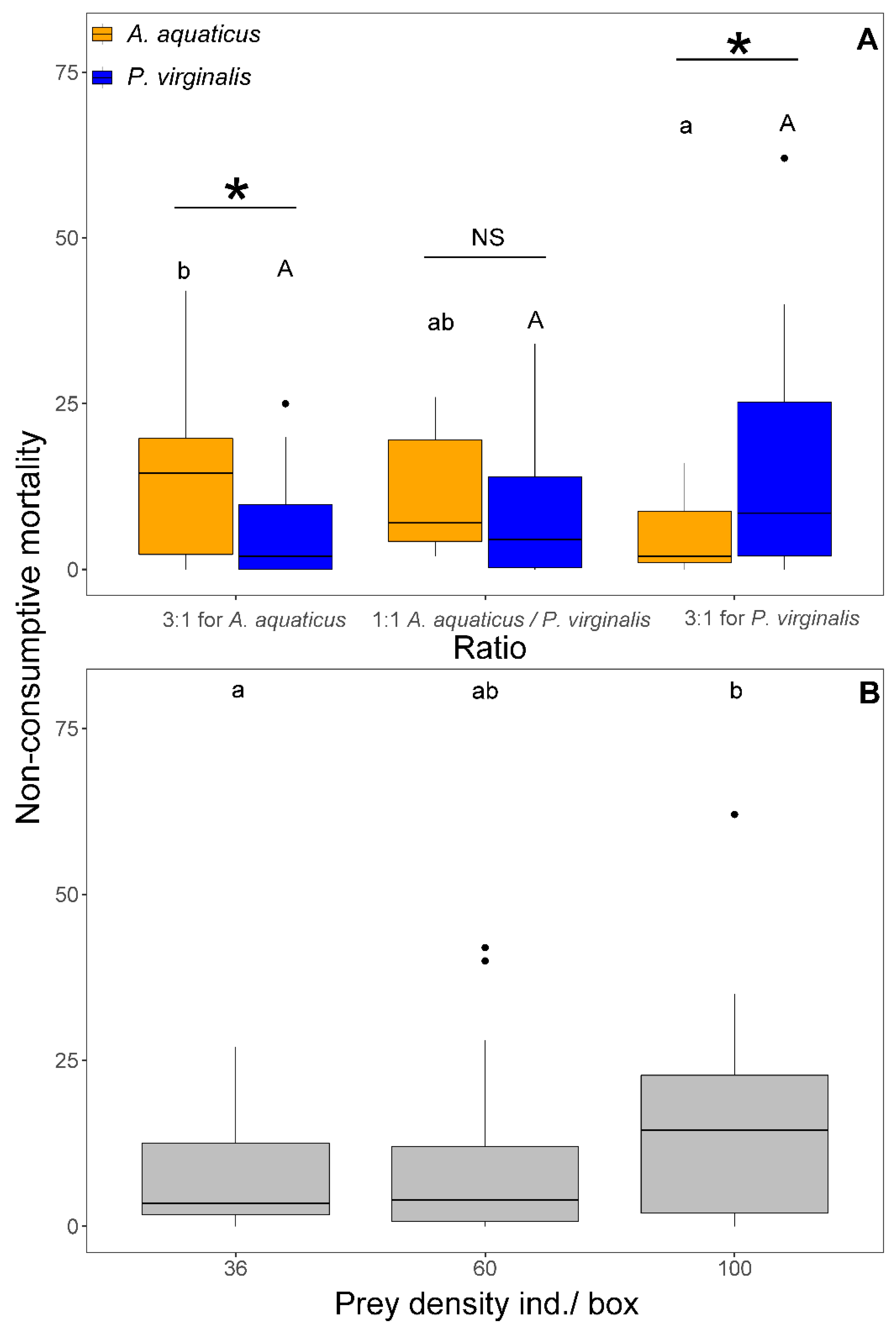
The NCM was affected by prey density (F1,103 = 7.33, p = 0.008) and the interaction between prey species and ratio (F2,101 = 5.87, p = 0.004). The NCM at 1:1 was significantly higher at the highest density (100 ind/box) than at densities < 60 ind/box at the same ratio (Figure 4B). There was no difference in NCM of A. aquaticus among the three ratios. In contrast, the NCM of P. virginalis at 3:1 for P. virginalis was significantly higher than 3:1 for A. aquaticus. The NCM was always significantly higher in the prevalent prey species than in the less abundant (Figure 4A). At 1:1, no significant species differences were observed in NCM (Figure 4B). In all exposures, NCM ranged from 0 to 100% of killed prey.
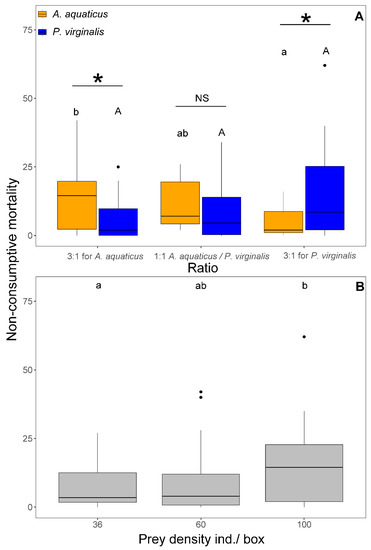
Figure 4.
Number of non-consumptive mortality of Asellus aquaticus (orange) and Procambarus virginalis (blue) by Neogobius melanostomus relative to the prey species ratio (A) and density (B). Effect of density, regardless of prey species, is shown for 1:1 (grey colour) at the highest prey densities (36, 60, and 100 individuals/box). Treatments with the same letter did not differ significantly (p > 0.05). Asterisk indicates significant difference (p < 0.05) and NS indicates non-significant difference. The points denote outliers.
The number of killed prey was significantly affected by prey density (F1,103 = 29.82, p < 0.001), species (F1,106 = 4.21, p = 0.042), and interaction of prey species with prey ratio (F2,101 = 40.07, p < 0.001) and density (F1,100 = 6.13, p = 0.015). In both 3:1 trials, N. melanostomus killed a significantly higher number of the prevalent prey species. The number of killed A. aquaticus differed significantly with the proportion and reflected the number offered. In contrast, the number of killed P. virginalis reached similar values at 1:1 and 3:1 for P. virginalis only at 3:1 for A. aquaticus and was significantly lower than at other ratios (Figure 5A). At 1:1, there was no significant difference between species in the number of killed prey at densities <60 individuals/box. With 100 individuals/box at 1:1, N. melanostomus killed significantly more P. virginalis than A. aquaticus (Figure 5B).
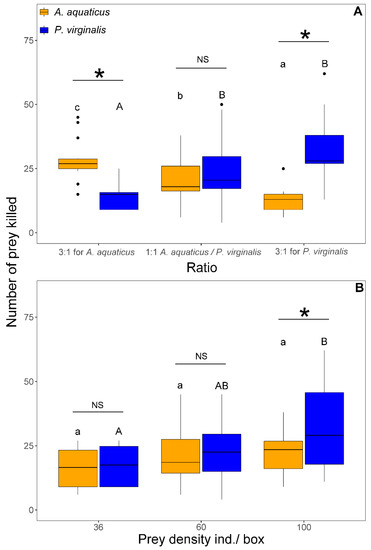
Figure 5.
Number of killed Asellus aquaticus (orange) and Procambarus virginalis (blue) by Neogobius melanostomus relative to prey species ratio (A) and density (B). Effect of density is shown only for 1:1 at 36, 60, and 100 individuals/box. Exposures with the same letter did not significantly differ (p > 0.05). Asterisk indicates significant difference (p < 0.05) between groups and NS indicates non-significant difference. The points denote outliers.
4. Discussion
The ability to utilize different prey sources and to switch among prey species as required is an attribute of successful invasive predators that can negatively affect not only prey species populations but also coenoses stability [31,68]. Neogobius melanostomus significantly changes composition of the macrozoobenthic communities in the invaded freshwater ecosystems [25,69]. Tributaries of major rivers serve as refuges for native aquatic biota and as sources of genetic diversity for the main streams [23] that are currently heavily affected by biological invasions [70].
Neogobius melanostomus exhibited type II FR toward prey organisms differing in escape strategy regardless of presentation. This type of functional response is typical of carnivorous predators [63,71] and is usually associated with destabilization of prey organism populations [72]. Type II FR was previously observed in N. melanostomus towards amphipods [49,51,73], A. aquaticus [49], and common carp Cyprinus carpio L. larvae [52] under experimental conditions. With increasing habitat complexity [74], switching among prey types [45] and consumption of less preferred prey [75] or prey with a well-developed antipredator defence [76] commonly involves a shift from type II FR to type III FR. However, this expected phenomenon was not observed in our two prey–species system, although prey organisms displayed different escape abilities. This is consistent with Gebauer et al. [77] who found no shift in N. melanostomus FR with increased habitat complexity, suggesting that N. melanostomus is a highly effective predator irrespective of habitat conditions [76] and prey behaviour (this study).
Handling time, as the ability to find and process prey, determines the predator maximum feeding rate [50]. This parameter closely correlates with habitat complexity [57,77,78] and, especially, with prey morphology and behaviour [53,79]. The typical crayfish flip-tail escape is generally considered a successful antipredation strategy [34,80] that reduces predator success or at least requires higher predator energy [81,82]. Contrary to expectations, we observed no significant differences in handling time of A. aquaticus and P. virginalis, suggesting that the crayfish escape strategy is ineffective against N. melanostomus predation, at least in early crayfish ontogenetic stages and in sandy substrates. In the trials with a single prey species at low density, N. melanostomus exploited P. virginalis more effectively than A. aquaticus, reflected in its significantly higher attack rate on P. virginalis.
Based on these results, we can conclude that crayfish populations, including native species (e.g., genus Astacus and Austropotamobius for European regions), in freshwater ecosystems may be exposed to predation stress by N. melanostomus similar to that on A. aquaticus. Lawton et al. [83] reported that the predator attack rate decreases and handling time is elevated when alternative prey items are available [83], and Colton [53] demonstrated that, in a multi-prey system, both handling time and attack rate vary with quantity and characteristics of the second most available prey item [53]. However, our experimental design did not allow analysis of those parameters with respect to prey species separately in the multiple-prey exposures. Handling time and attack rate in our multi-prey trials reached values different from those that would be expected in single-prey exposures. Regardless of the proportion of P. virginalis, the prey item considered to be a driver of the N. melanostomus attack rate, on the overall offered prey amount, N. melanostomus showed a higher attack rate in both 3:1 ratios compared to 1:1 or A. aquaticus offered separately. At 1:1, the attack rate was similar to that in the system with only A. aquaticus. In both 3:1 trials at lower densities, the attack rate was positively affected, while, at higher densities, N. melanostomus handling time was prolonged compared to expectations based on results gained in the single-prey systems, implying ongoing predator switch to the alternative prey. The prey alternation could be more challenging when prey species occur in unequal quantities. This is in agreement with Colton [53], who stated that the addition of a prey species to a system leads to additional interactions and behaviour changes, and the food system becomes unpredictable. Lawton et al. [83] reported reduced predator pressure on individual prey in such conditions due to the increased handling time and depressed attack rate. However, our data clearly showed that addition of a second prey item led to an increase in N. melanostomus attack rate as well as elevated impact on the prey community. In addition, our study confirms the value of multi-species experimental design in ecological studies to gain a more realistic assessment of predator impact upon prey communities.
Several studies have documented N. melanostomus feed selectivity [30,84,85] that differs with locality. The optimal foraging theory states that a predator will maximize energy profit to cost with respect to prey acquisition and processing [86]. Prey selectivity in aquatic ecosystems is affected by multiple factors including prey availability [87]; predator experience [56]; prey size, morphology, and colour [87,88]; and water turbidity [89,90]. The latter is demonstrated by N. melanostomus diet shift to easily available prey under experimental conditions of high turbidity [89]. Therefore, it can be assumed that prey exhibiting an effective escape response and/or high mobility will be less preferred by predators [86]. However, studies of N. melanostomus feed selectivity have often shown contradictory results, with respect to preferences for native [91,92] or non-native [93] species. In addition, overexploitation of certain benthic species regardless of abundance has been observed [30,84,85] and confirmed by our findings of no species-differences in the number of prey killed when presented in equal numbers, while at 3:1, N. melanostomus killed significantly more specimens of the prevalent species. These findings support the hypothesis that N. melanostomus often shows indiscriminate foraging, taking the most readily available prey and easily switching to another source [30,94]. The ineffectiveness of crayfish tail-flip escape strategy against N. melanostomus predation was also shown. An exception was 1:1 presentation of prey at density of 100 ind/box, when N. melanostomus killed significantly more P. virginalis than A. aquaticus, possibly showing predator food preference after satiation [45].
Although the focus is generally on the direct consumption of prey, this is not the only means by which predator ecological impact may occur [95]. We observed that non-consumptive mortality (NCM) may have an even higher effect on prey populations than direct predation [96]. This component of predator behaviour, also known as waste or surplus killing, has been observed in invertebrates [63,97,98] and mammals [99,100,101]. Ignoring NCM may cause a significant underestimation of predator ecological impact [102] as well as energy transfer among trophic levels [97]. In our experimental exposures, N. melanostomus exhibited a high rate of NCM, indicating its potential role in the effect of this predator on prey population abundance and ecosystem function. Our observed NCM is in contrast with previous studies of N. melanostomus FR with fish larvae as prey [52,77] in which no NCM was observed. In mammals, NCM is usually connected either with an ineffective anti-predator response due to lack of co-evolution with the predator [101] or to lack of prey escape response as a consequence of isolated short-term events [102]. In invertebrates, it seems that the satiation level determines whether the prey is consumed. However, hunting and killing of prey are probably directed by mechanisms [97] in invertebrates that differ from that of vertebrates [103]. Johnson et al. [97] assumed that an empty midgut may stimulate predatory damselfly nymphs to capture more prey than can be processed due to filled foregut. It seems that an effect of satiation was not confirmed in our experiment, since N. melanostomus killed both prey species without their consumption after 24 h starvation, even at the lowest densities. Although the NCM has usually been reported to increase as prey density rises [63,97,98], we did not find a correlation of NCM rate and prey density in N. melanostomus, and the proportion of NCM in total prey mortality ranged from 0–100% (33.4 ± 39.2%).
Fantinou et al. [63] described NCM elevation at temperatures outside the predator thermal optimum, i.e., in stressful conditions. Similarly, Veselý et al. [98] in a study of Aeschna cyanea nymphs, and Jedrzejewska and Jederzejewski [100] in Mustela nivalis, described higher NCM at lower temperature. However, it is unclear whether the low temperature directly caused change of predator behaviour or influenced prey occurrence and/or behaviour and subsequently predator response. In our study, the temperature ranged within the optimum range reported for N. melanostomus [104]. We can assume that a potential reason for observed high NCM values might be the absence of shelter as a possible trigger of stress, although we have no evidence supporting this assumption or quantifying its importance in the wild in N. melanostomus.
Neogobius melanostomus successfully exploited both hard-bodied prey species differing in escape strategy without showing a distinct preference. The simultaneous effects of high N. melanostomus foraging efficiency on P. virginalis and previously documented successful competition of N. melanostomus for shelter with crayfish [105] may demonstrate a potential to regulate P. virginalis populations in the wild. Bovy et al. [106] pointed out that a destabilization effect of predator presence on prey populations is negatively correlated with prey reproduction and dispersal abilities. Therefore, despite a strong interaction between P. virginalis and N. melanostomus as invasive non-native species, an eradication effect is less likely in established P. virginalis populations due to its high fertility rate and overall reproduction ability [107]. However, for native crustaceans, including indigenous European crayfishes that are threatened for many reasons [7,62] and exhibit lower fecundity [108], N. melanostomus may pose a serious risk. Particularly with regards to increasing records of N. melanostomus in smaller tributaries [30,109,110] inhabited by native crayfish, this can be crucial for continuing crayfish existence. More attention should be focused on identifying and clarifying non-consumptive mortality in the wild as a potential element of N. melanostomus foraging behaviour. The reason for ineffective predation in N. melanostomus is unclear, and this is one of the first laboratory foraging studies to report non-consumptive predation in fish. Both indiscriminate foraging behaviour and non-consumptive mortality are important factors that should be taken into consideration for quantification of N. melanostomus impact on native crustaceans in freshwater ecosystems.
5. Conclusions
Although N. melanostomus shows comparable predation pressure on both preys, it can be a threat to the population stability of already endangered crustaceans such as crayfish. Effective control to limit further spreading of N. melanostomus to tributaries should be a priority. There is a need for more multiple-prey studies, as quantification of N. melanostomus impact on the macrozoobenthic community based on the single prey model may be insufficient. In addition to prey species, their density and relative proportions can significantly influence the N. melanostomus foraging efficiency.
Author Contributions
Conceptualization and formal analysis, P.F., R.G., M.B. and B.D.; methodology, P.F. and R.G.; investigation, P.F., R.G. and N.Z.S.; data curation, L.V. and P.F.; writing—original draft preparation, P.F. and R.G.; writing—review and editing, R.G., M.B. and B.D.; visualization, R.G. and L.V.; supervision, B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic—projects CENAKVA (LM2018099) and the project MoBI-aqua: Cross border monitoring of biological invasions as a tool for freshwater biodiversity conservation (project number 100314623, program of cross-border cooperation with Free State of Saxony; Hallo Nachbar, Ahoj Sousede, Interreg VA/2014–2020). R.G. would like to thank the project Reproductive and Genetic Procedures for Preserving Fish Biodiversity and Aquaculture (CZ.02.1.01/0.0/0.0/16_025/0007370).
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the character of the experiment. The vertebrates were held in captivity and fed natural food under laboratory conditions for a short time with unnecessary handling. Therefore, this study was not subject to authorization under the Czech Republic legislation.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request.
Acknowledgments
Thanks to my colleagues and students: Petr Kovář, Marcellin Rutegwa, Anna Pavlovna Ivanovna, Jan Dofek, Jan Matoušek, and Pavel Šablatura who helped with both collecting experimental animals and preparation of experimental trials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dorn, N.; Mittelbach, G. More than predator and prey: A review of interactions between fish and crayfish. Vie Milieu 1999, 49, 229–237. [Google Scholar]
- Creed, R.P., Jr.; Reed, J.M. Ecosystem engineering by crayfish in a headwater stream community. J. N. Am. Benthol. Soc. 2004, 23, 224–236. [Google Scholar] [CrossRef]
- Dorn, N.J.; Wojdak, J.M. The role of omnivorous crayfish in littoral communities. Oecologia 2004, 140, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Stenroth, P.; Holmqvist, N.; Nyström, P.; Berglund, O.; Larsson, P.; Granéli, W. The influence of productivity and width of littoral zone on the trophic position of a large-bodied omnivore. Oecologia 2008, 156, 681–690. [Google Scholar] [CrossRef]
- Edwards, B.A.; Jackson, D.A.; Somers, K.M. Multispecies crayfish declines in lakes: Implications for species distributions and richness. J. N. Am. Benthol. Soc. 2009, 28, 719–732. [Google Scholar] [CrossRef]
- Reynolds, J.; Souty-Grosset, C.; Richardson, A. Ecological roles of crayfish in freshwater and terrestrial habitats. Freshw. Crayfish 2013, 19, 197–218. [Google Scholar]
- Richman, N.I.; Böhm, M.; Adams, S.B.; Alvarez, F.; Bergey, E.A.; Bunn, J.J.S.; Burnham, Q.; Cordeiro, J.; Coughran, J.; Crandall, K.A.; et al. Multiple drivers of decline in the global status of freshwater crayfish (Decapoda: Astacidea). Philos. Trans. R. Soc. B 2015, 370, 20140060. [Google Scholar] [CrossRef]
- European Parliament and of the Council on Minimum Requirements for Water Reuse. 337 Final 2018/0169 (COD) Brussels. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52018PC0337 (accessed on 28 May 2018).
- Ding, L.; Chen, L.; Ding, C.; Tao, J. Global trends in dam removal and related research: A systematic review based on associated datasets and bibliometric analysis. Chin. Geogr. Sci. 2019, 29, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wohl, E.; Lane, S.N.; Wilcox, A.C. The science and practice of river restoration. Water Resour. Res. 2015, 51, 5974–5997. [Google Scholar] [CrossRef] [Green Version]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Vilà, M.; Basnou, C.; Gollasch, S.; Josefsson, M.; Pergl, J.; Scalera, R. One hundred of the most invasive alien species in Europe. In Handbook of Alien Species in Europe; Hulme, P.E., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 265–268. [Google Scholar]
- Kornis, M.S.; Mercado-Silva, N.; Vander Zanden, M.J. Twenty years of invasion: A review of round goby Neogobius melanostomus biology, spread and ecological implications. J. Fish Biol. 2012, 80, 235–285. [Google Scholar] [CrossRef]
- French, J.R.; Jude, D.J. Diets and diet overlap of nonindigenous gobies and small benthic native fishes co-inhabiting the St. Clair River, Michigan. J. Great Lakes Res. 2001, 27, 300–311. [Google Scholar] [CrossRef]
- Almqvist, G.; Strandmark, A.K.; Appelberg, M. Has the invasive round goby caused new links in Baltic food webs? Environ. Biol. Fishes 2010, 89, 79–93. [Google Scholar] [CrossRef]
- Emde, S.; Rueckert, S.; Kochmann, J.; Knopf, K.; Sures, B.; Klimpel, S. Nematode eel parasite found inside acanthocephalan cysts-a Trojan horse strategy? Parasite Vector 2014, 7, 1–5. [Google Scholar]
- Pagnucco, K.S.; Remmal, Y.; Ricciardi, A. An invasive benthic fish magnifies trophic cascades and alters pelagic communities in an experimental freshwater system. Freshw. Sci. 2016, 35, 654–665. [Google Scholar] [CrossRef] [Green Version]
- Janáč, M.; Šlapanský, L.; Valová, Z.; Jurajda, P. Downstream drift of round goby (Neogobius melanostomus) and tubenose goby (Proterorhinus semilunaris) in their non-native area. Ecol. Freshw. Fish 2013, 22, 430–438. [Google Scholar] [CrossRef]
- Šlapanský, L.; Janáč, M.; Roche, K.; Mikl, L.; Jurajda, P. Expansion of round gobies in a non-navigable river system. Limnologica 2017, 67, 27–36. [Google Scholar] [CrossRef]
- Kornis, M.S.; Vander Zanden, M.J. Forecasting the distribution of the invasive round goby (Neogobius melanostomus) in Wisconsin tributaries to Lake Michigan. Can. J. Fish. Aquat. Sci. 2010, 67, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Poos, M.; Dextrase, A.J.; Schwalb, A.N.; Ackerman, J.D. Secondary invasion of the round goby into high diversity Great Lakes tributaries and species at risk hotspots: Potential new concerns for endangered freshwater species. Biol. Invasions 2010, 12, 1269–1284. [Google Scholar] [CrossRef]
- Verliin, A.; Kesler, M.; Svirgsden, R.; Taal, I.; Saks, L.; Rohtla, M.; Hubel, K.; Eschbaum, R.; Vetemaa, M.; Saat, T. Invasion of round goby to the temperate salmonid streams in the Baltic Sea. Ichthyol. Res. 2017, 64, 155–158. [Google Scholar] [CrossRef]
- Meyer, J.L.; Strayer, D.L.; Wallace, J.B.; Eggert, S.L.; Helfman, G.S.; Leonard, N.E. The contribution of headwater streams to biodiversity in river networks. JAWRA J. Am. Water Resour. 2017, 43, 86–103. [Google Scholar] [CrossRef] [Green Version]
- Bottcher, J.L.; Walsworth, T.E.; Thiede, G.P.; Budy, P.; Speas, D.W. Frequent usage of tributaries by the endangered fishes of the upper Colorado River basin: Observations from the San Rafael River, Utah. N. Am. J. Fish. Manag. 2013, 33, 585–594. [Google Scholar] [CrossRef]
- Kuhns, L.A.; Berg, M.B. Benthic invertebrate community responses to round goby (Neogobius melanostomus) and zebra mussel (Dreissena polymorpha) invasion in southern Lake Michigan. J. Great Lakes Res. 1999, 25, 910–917. [Google Scholar] [CrossRef]
- Lederer, A.M.; Janssen, J.; Reed, T.; Wolf, A. Impacts of the introduced round goby (Apollonia melanostoma) on dreissenids (Dreissena polymorpha and Dreissena bugensis) and on macroinvertebrate community between 2003 and 2006 in the littoral zone of Green Bay, Lake Michigan. J. Great Lakes Res. 2008, 34, 690–697. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Miyasaka, H.; Genkai-Kato, M.; Taniguchi, Y.; Nakano, S. Seasonal change in the gastric evacuation rate of rainbow trout feeding on natural prey. J. Fish Biol. 2007, 71, 1873–1878. [Google Scholar] [CrossRef]
- Perello, M.M.; Simon, T.P.; Thompson, H.M.; Kane, D.D. Feeding ecology of the invasive round goby, Neogobius melanostomus (Pallas, 1814), based on laboratory size preference and field diet in different habitats in the western basin of Lake Erie. Aquat. Invasions 2015, 10, 463–474. [Google Scholar] [CrossRef]
- Polačik, M.; Janáč, M.; Jurajda, P.; Adámek, Z.; Ondračková, M.; Trichkova, T.; Vassilev, M. Invasive gobies in the Danube: Invasion success facilitated by availability and selection of superior food resources. Ecol. Freshw. Fish 2009, 18, 640–649. [Google Scholar] [CrossRef]
- Pennuto, C.; Krakowiak, P.; Janik, C. Seasonal abundance, diet, and energy consumption of round gobies (Neogobius melanostomus) in Lake Erie tributary streams. Ecol. Freshw. Fish 2010, 19, 206–215. [Google Scholar] [CrossRef]
- Townsend, C.R.; Winfield, I.J. The application of optimal foraging theory to feeding behaviour in fish. In Fish Energetics: New Perspectives, 1st ed.; Tytler, P., Calow, P., Eds.; Croom Helm: Sydney, Australia, 1985; pp. 67–98. [Google Scholar]
- Shemonaev, E.; Kirilenko, E. Features of biology of the round goby Neogobius melanostomus (Perciformes, Gobiidae) in waters of Kuibyshev Reservoir. J. Ichthyol. 2009, 49, 454–459. [Google Scholar] [CrossRef]
- Kirilenko, E.; Shemonaev, E. Feeding of the round goby Neogobius melanostomus (Perciformes, Gobiidae) in two Volga reservoirs. J. Ichthyol. 2012, 52, 291–295. [Google Scholar] [CrossRef]
- Herberholz, J.; Sen, M.M.; Edwards, D.H. Escape behavior and escape circuit activation in juvenile crayfish during prey–predator interactions. J. Exp. Biol. 2004, 207, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- Stein, R.A. Selective predation, optimal foraging, and the predator-prey interaction between fish and crayfish. Ecology 1977, 58, 1237–1253. [Google Scholar] [CrossRef]
- Sandeman, R.; Sandeman, D. Development, growth, and plasticity in the crayfish olfactory system. Microsc. Res. Tech. 2003, 60, 266–277. [Google Scholar] [CrossRef]
- Lang, F.; Govind, C.; Costello, W.J.; Greene, S.I. Developmental neuroethology: Changes in escape and defensive behavior during growth of the lobster. Science 1977, 197, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Kellie, S.; Greer, J.; Cooper, R.L. Alterations in habituation of the tail flip response in epigean and troglobitic crayfish. J. Exp. Zool. 2001, 290, 163–176. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L.; Douglas, M.R.; Douglas, M.E.; Fausch, K.D. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004, 19, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dick, J.T.; Platvoet, D.; Kelly, D.W. Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can. J. Fish. Aquat. Sci. 2002, 59, 1078–1084. [Google Scholar] [CrossRef] [Green Version]
- Dick, J.T.; Gallagher, K.; Avlijas, S.; Clarke, H.C.; Lewis, S.E.; Leung, S.; Minchin, D.; Caffrey, J.; Alexander, M.E.; Maguire, C. Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol. Invasions 2013, 15, 837–846. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; Macdonald, D.W. Are invasives worse in freshwater than terrestrial ecosystems? WIREs Water 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Solomon, M. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Holling, C.S. Some Characteristics of Simple Types of Predation and Parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Oaten, A. Predation and population stability. Adv. Ecol. Res. 1975, 9, 1–131. [Google Scholar]
- Juliano, S.A. Nonlinear curve fitting: Predation and functional response curve. In Design and Analysis of Ecological Experiments, 2nd ed.; Scheiner, S.M., Gurevitch, J., Eds.; Chapman and Hall: New York, NY, USA, 2001; pp. 178–196. [Google Scholar]
- Bollache, L.; Dick, J.T.; Farnsworth, K.D.; Montgomery, W.I. Comparison of the functional responses of invasive and native amphipods. Biol. Lett. 2008, 4, 166–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, M.E.; Dick, J.T.; Weyl, O.L.; Robinson, T.B.; Richardson, D.M. Existing and emerging high impact invasive species are characterized by higher functional responses than natives. Biol. Lett. 2014, 10, 20130946. [Google Scholar] [CrossRef] [PubMed]
- Laverty, C.; Green, K.D.; Dick, J.T.; Barrios-O’Neill, D.; Mensink, P.J.; Médoc, V.; Spataro, T.; Caffrey, J.M.; Lucy, F.E.; Boets, P. Assessing the ecological impacts of invasive species based on their functional responses and abundances. Biol. Invasions 2017, 19, 1653–1665. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Mu, X.; Dick, J.T.; Fang, M.; Gu, D.; Luo, D.; Zhang, J.; Luo, J.; Hu, Y. Comparative functional responses predict the invasiveness and ecological impacts of alien herbivorous snails. PLoS ONE 2016, 11, e0147017. [Google Scholar] [CrossRef] [Green Version]
- Dubs, D.O.; Corkum, L.D. Behavioral interactions between round gobies (Neogobius melanostomus) and mottled sculpins (Cottus bairdi). J. Great Lakes Res. 1996, 22, 838–844. [Google Scholar] [CrossRef]
- Gebauer, R.; Veselý, L.; Kouba, A.; Buřič, M.; Drozd, B. Forecasting impact of existing and emerging invasive gobiids under temperature change using comparative functional responses. Aquat. Invasions 2018, 13, 289–297. [Google Scholar] [CrossRef]
- Colton, T.F. Extending functional response models to include a second prey type: An experimental test. Ecology 1987, 68, 900–912. [Google Scholar] [CrossRef]
- Dodd, J.A.; Dick, J.T.A.; Alexander, M.E.; Macneil, C.; Dunn, A.M.; Aldridge, D.C. Predicting the ecological impacts of a new freshwater invader: Functional responses and prey selectivity of the ‘killer shrimp’, Dikerogammarus villosus, compared to the native Gammarus pulex. Freshw. Biol. 2014, 59, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, R.N.; Dickey, J.W.; McMorrow, C.; Laverty, C.; Dick, J.T. Resistance is futile: Lack of predator switching and a preference for native prey predict the success of an invasive prey species. R. Soc. Open Sci. 2018, 5, 180339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudová, P.; Boukal, D.S.; Klecka, J. Prey selectivity and the effect of diet on growth and development of a dragonfly, Sympetrum sanguineum. PeerJ. 2019, 7, e7881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- South, J.; McCard, M.; Khosa, D.; Mofu, M.; Madzivanzira, T.C.; Dick, J.T.; Weyl, O.L. The effect of prey identity and substrate type on the functional response of a globally invasive crayfish. NeoBiota 2019, 52, 9. [Google Scholar] [CrossRef] [Green Version]
- Vašek, M.; Všetičková, L.; Roche, K.; Jurajda, P. Diet of two invading gobiid species (Proterorhinus semilunaris and Neogobius melanostomus) during the breeding and hatching season: No field evidence of extensive predation on fish eggs and fry. Limnologica 2014, 46, 31–36. [Google Scholar] [CrossRef]
- Hempel, M.; Magath, V.; Neukamm, R.; Thiel, R. Feeding ecology, growth and reproductive biology of round goby Neogobius melanostomus (Pallas, 1814) in the brackish Kiel Canal. Mar. Biodivers. 2018, 49, 795–807. [Google Scholar] [CrossRef]
- Hay, A.M. Foraging Behaviour of the Ruffe (Gymnocephalus cernuus) and Predator Avoidance by the Freshwater Isopod Asellus aquaticus: Implication for Predator-Prey Interaction. Ph.D. Thesis, University of Glasgow, Glasgow, UK, September 1999. [Google Scholar]
- Hossain, M.S.; Patoka, J.; Kouba, A.; Buřič, M. Clonal crayfish as biological model: A review on marbled crayfish. Biologia 2018, 73, 841–855. [Google Scholar] [CrossRef]
- Kouba, A.; Petrusek, A.; Kozák, P. Continental-wide distribution of crayfish species in Europe: Update and maps. Knowl. Manag. Aquat. Ecosyst. 2017, 413, 05. [Google Scholar] [CrossRef]
- Fantinou, A.; Perdikis, D.C.; Maselou, D.; Lambropoulos, P. Prey killing without consumption: Does Macrolophus pygmaeus show adaptive foraging behaviour? Biol. Control 2008, 47, 187–193. [Google Scholar] [CrossRef]
- Rogers, D. Random search and insect population models. J. Anim. Ecol. 1972, 369–383. [Google Scholar] [CrossRef]
- Holling, C.S. The Functional Response of Predators to Prey Density and its Role in Mimicry and Population Regulation. Mem. Entomol. Soc. Can. 1965, 97, 5–60. [Google Scholar] [CrossRef] [Green Version]
- Bolker, B. Ecological Models and Data in R; Princeton University Press: Princeton, NJ, USA, 2008; pp. 127–135. [Google Scholar]
- Sentis, A.; Hemptinne, J.; Brodeur, J. How functional response and productivity modulate intraguild predation. Ecosphere 2013, 4, 1–14. [Google Scholar] [CrossRef]
- David, P.; Thebault, E.; Anneville, O.; Duyck, P.F.; Chapuis, E.; Loeuille, N. Impacts of invasive species on food webs: A review of empirical data. Adv. Ecol. Res. 2017, 56, 1–60. [Google Scholar]
- Mikl, L.; Adámek, Z.; Všetičková, L.; Janáč, M.; Roche, K.; Šlapanský, L.; Jurajda, P. Response of benthic macroinvertebrate assemblages to round (Neogobius melanostomus, Pallas 1814) and tubenose (Proterorhinus semilunaris, Heckel 1837) goby predation pressure. Hydrobiologia 2017, 785, 219–232. [Google Scholar] [CrossRef]
- Gebauer, R.; Divíšek, J.; Buřič, M.; Večeřa, M.; Kouba, A.; Drozd, B. Distribution of alien animal species richness in the Czech Republic. Ecol. Evol. 2018, 8, 4455–4464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeuwen, E.V.; Jansen, V.; Bright, P. How population dynamics shape the functional response in a one-predator–two-prey system. Ecology 2007, 88, 1571–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dick, J.T.; Alexander, M.E.; Jeschke, J.M.; Ricciardi, A.; MacIsaac, H.J.; Robinson, T.B.; Kumschick, S.; Weyl, O.L.; Dunn, A.M.; Hatcher, M.J. Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol. Invasions 2014, 16, 735–753. [Google Scholar] [CrossRef] [Green Version]
- Paton, R.A.; Gobin, J.; Rooke, A.C.; Fox, M.G. Population density contributes to the higher functional response of an invasive fish. Biol. Invasions 2019, 21, 1737–1749. [Google Scholar] [CrossRef]
- Alexander, M.E.; Dick, J.T.; O’Connor, N.E.; Haddaway, N.R.; Farnsworth, K.D. Functional responses of the intertidal amphipod Echinogammarus marinus: Effects of prey supply, model selection and habitat complexity. Mar. Ecol. Prog. Ser. 2012, 468, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Akre, B.G.; Johnson, D.M. Switching and sigmoid functional response curves by damselfly naiads with alternative prey available. J. Anim. Ecol. 1979, 703–720. [Google Scholar] [CrossRef]
- Hammill, E.; Petchey, O.L.; Anholt, B.R. Predator functional response changed by induced defenses in prey. Am. Nat. 2010, 176, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, R.; Veselý, L.; Vanina, T.; Buřič, M.; Kouba, A.; Drozd, B. Prediction of ecological impact of two alien gobiids in habitat structures of differing complexity. Can. J. Fish. Aquat. Sci. 2019, 76, 1954–1961. [Google Scholar] [CrossRef]
- Alexander, M.; Kaiser, H.; Weyl, O.; Dick, J. Habitat simplification increases the impact of a freshwater invasive fish. Environ. Biol. Fishes 2015, 98, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, J.M.; Tollrian, R. Density-dependent effects of prey defences. Oecologia 2000, 123, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.; Hart, P. The vulnerability of juvenile signal crayfish to perch and eel predation. Freshw. Biol. 1995, 33, 233–244. [Google Scholar] [CrossRef]
- Webb, P. Effect of body form and response threshold on the vulnerability of four species of teleost prey attacked by largemouth bass (Micropterus salmoides). Can. J. Fish. Aquat. Sci. 1986, 43, 763–771. [Google Scholar] [CrossRef]
- Sih, A.; Christensen, B. Optimal diet theory: When does it work, and when and why does it fail? Anim. Behav. 2001, 61, 379–390. [Google Scholar] [CrossRef]
- Lawton, J.; Beddington, J.; Bonser, R. Switching in invertebrate predators. In Ecological Stability; Usher, M.B., Williamson, M.H., Eds.; Chapman and Hall: London, UK, 1974; pp. 141–158. [Google Scholar]
- Kipp, R.; Hébert, I.; Lacharité, M.; Ricciardi, A. Impacts of predation by the Eurasian round goby (Neogobius melanostomus) on molluscs in the upper St. Lawrence River. J. Great Lakes Res. 2012, 38, 78–89. [Google Scholar] [CrossRef]
- Bhagat, Y.; Ruetz, C.R., III; Akins, A.L. Differential habitat use by the round goby (Neogobius melanostomus) and Dreissena spp. in coastal habitats of eastern Lake Michigan. J. Great Lakes Res. 2015, 41, 1087–1093. [Google Scholar] [CrossRef]
- Tytler, P.; Calow, P. Fish Energetics: New Perspectives; Croom Helm: Sydney, Australia, 1985; p. 348. [Google Scholar]
- Hart, P.; Ison, S. The influence of prey size and abundance, and individual phenotype on prey choice by the three-spined stickleback, Gasterosteus aculeatus L. J. Fish Biol. 1991, 38, 359–372. [Google Scholar] [CrossRef]
- Kislalioglu, M.; Gibson, R. Prey ‘handling time’and its importance in food selection by the 15-spined stickleback, Spinachia spinachia (L.). J. Exp. Mar. Biol. Ecol. 1976, 25, 151–158. [Google Scholar] [CrossRef]
- Diggins, T.P.; Kaur, J.; Chakraborti, R.K.; DePinto, J.V. Diet choice by the exotic round goby (Neogobius melanostomus) as influenced by prey motility and environmental complexity. J. Great Lakes Res. 2002, 28, 411–420. [Google Scholar] [CrossRef]
- Sohel, S.; Mattila, J.; Lindström, K. Effects of turbidity on prey choice of three-spined stickleback Gasterosteus aculeatus. Mar. Ecol. Prog. Ser. 2017, 566, 159–167. [Google Scholar] [CrossRef]
- Błońska, D.; Grabowska, J.; Kobak, J.; Jermacz, Ł.; Bącela-Spychalska, K. Feeding preferences of an invasive Ponto-Caspian goby for native and non-native gammarid prey. Freshw. Biol. 2015, 60, 2187–2195. [Google Scholar] [CrossRef]
- Beggel, S.; Brandner, J.; Cerwenka, A.; Geist, J. Synergistic impacts by an invasive amphipod and an invasive fish explain native gammarid extinction. BMC Ecol. 2016, 16, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandner, J.; Auerswald, K.; Cerwenka, A.F.; Schliewen, U.K.; Geist, J. Comparative feeding ecology of invasive Ponto-Caspian gobies. Hydrobiologia 2013, 703, 113–131. [Google Scholar] [CrossRef]
- Carman, S.M.; Janssen, J.; Jude, D.J.; Berg, M.B. Diel interactions between prey behaviour and feeding in an invasive fish, the round goby, in a North American river. Freshw. Biol. 2006, 51, 742–755. [Google Scholar] [CrossRef]
- Siepielski, A.M.; Wang, J.; Prince, G. Nonconsumptive predator-driven mortality causes natural selection on prey. Evolution 2014, 68, 696–704. [Google Scholar] [CrossRef]
- Preisser, E.L.; Bolnick, D.I.; Benard, M.F. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 2005, 86, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.M.; Akre, B.G.; Crowley, P.H. Modeling arthropod predation: Wasteful killing by damselfly naiads. Ecology 1975, 56, 1081–1093. [Google Scholar] [CrossRef]
- Veselý, L.; Boukal, D.S.; Buřič, M.; Kozák, P.; Kouba, A.; Sentis, A. Effects of prey density, temperature and predator diversity on nonconsumptive predator-driven mortality in a freshwater food web. Sci. Rep. 2017, 7, 18075. [Google Scholar] [CrossRef]
- Kruuk, H. Surplus killing by carnivores. J. Zool. 1972, 166, 233–244. [Google Scholar] [CrossRef]
- Jedrzejewska, B.; Jedrzejewski, W. Seasonal surplus killing as hunting strategy of the weasel Mustela nivalis-test of a hypothesis. Acta Theriol. 1989, 34, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Short, J.; Kinnear, J.; Robley, A. Surplus killing by introduced predators in Australia—Evidence for ineffective anti-predator adaptations in native prey species? Biol. Conserv. 2002, 103, 283–301. [Google Scholar] [CrossRef]
- McKee, M.; Wrona, F.; Scrimgeour, G.; Culp, J. Importance of consumptive and non-consumptive prey mortality in a coupled predator–prey system. Freshw. Biol. 1997, 38, 193–201. [Google Scholar] [CrossRef]
- Holling, C.S. The functional response of invertebrate predators to prey density. Mem. Entomol. Soc. Can. 1966, 98, 5–86. [Google Scholar] [CrossRef]
- Lee, V.A.; Johnson, T.B. Development of a bioenergetics model for the round goby (Neogobius melanostomus). J. Great Lakes Res. 2005, 31, 125–134. [Google Scholar] [CrossRef]
- Church, K.; Iacarella, J.C.; Ricciardi, A. Aggressive interactions between two invasive species: The round goby (Neogobius melanostomus) and the spinycheek crayfish (Orconectes limosus). Biol. Invasions 2017, 19, 425–441. [Google Scholar] [CrossRef]
- Bovy, H.C.; Barrios-O’Neill, D.; Emmerson, M.C.; Aldridge, D.C.; Dick, J.T. Predicting the predatory impacts of the “demon shrimp” Dikerogammarus haemobaphes, on native and previously introduced species. Biol. Invasions 2015, 17, 597–607. [Google Scholar] [CrossRef]
- Martin, P. Parthenogenesis: Mechanisms, evolution, and its relevance to the role of marbled crayfish as model organism and potential invader. In Freshwater Crayfish: A Global Overview; Kawai, T., Faulkes, Z., Scholtz, G., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 63–82. [Google Scholar]
- Holdich, D.; Reynolds, J.; Souty-Grosset, C.; Sibley, P. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Managt. Aquat. Ecosyst. 2009, 11, 394–395. [Google Scholar] [CrossRef] [Green Version]
- Pennuto, C.; Rupprecht, S. Upstream range expansion by invasive round gobies: Is functional morphology important? Aquat. Ecol. 2016, 50, 45–57. [Google Scholar] [CrossRef]
- Kornis, M.S.; Weidel, B.C.; Vander Zanden, M.J. Divergent life histories of invasive round gobies (Neogobius melanostomus) in Lake Michigan and its tributaries. Ecol. Freshw. Fish 2017, 26, 563–574. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).