Establishment and Characterization of Feline Mammary Tumor Patient-Derived Xenograft Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Tumor Samples
2.2. Tissue Implantation of PDXs
2.3. Isolation of Primary FMT Cells from PDX Tumor
2.4. Histopathological and Immunohistochemistry Examination
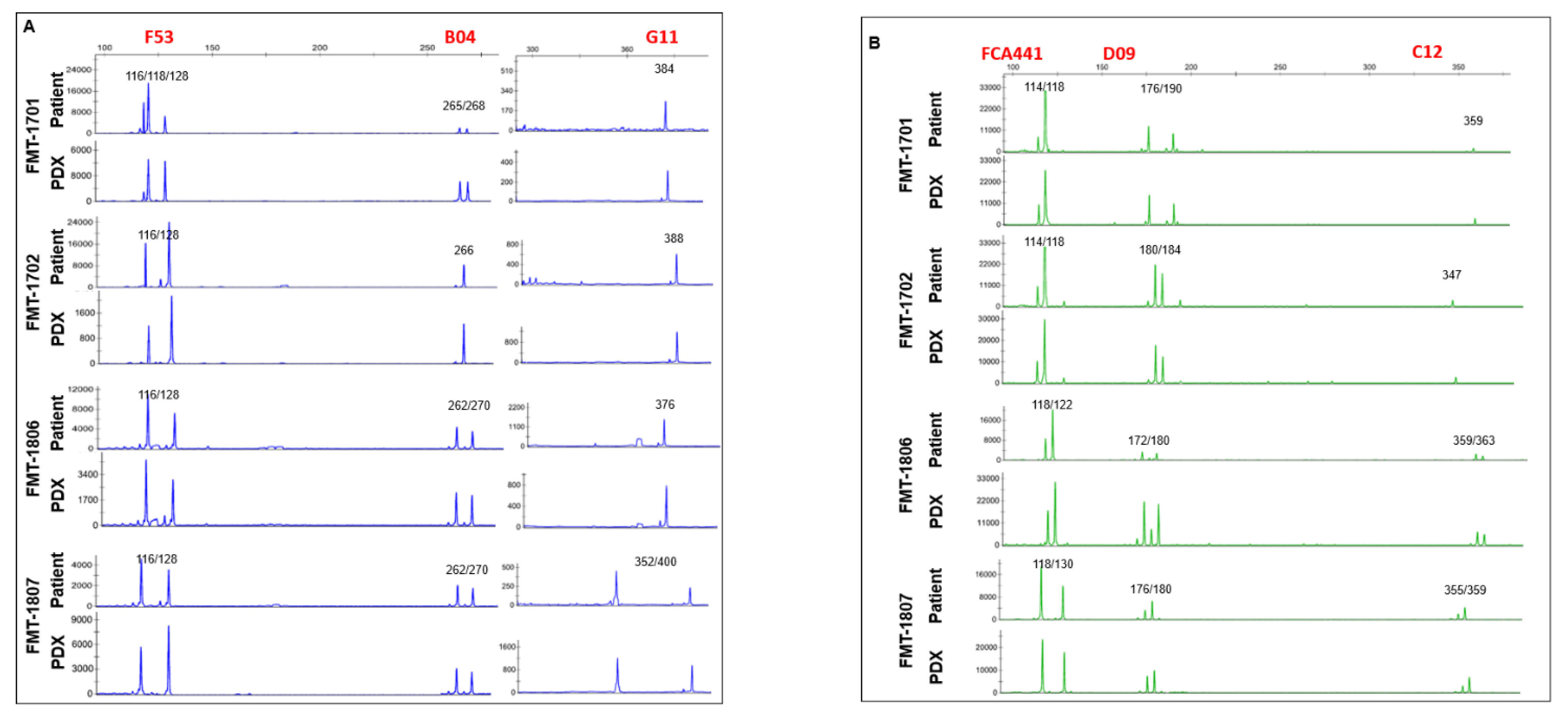
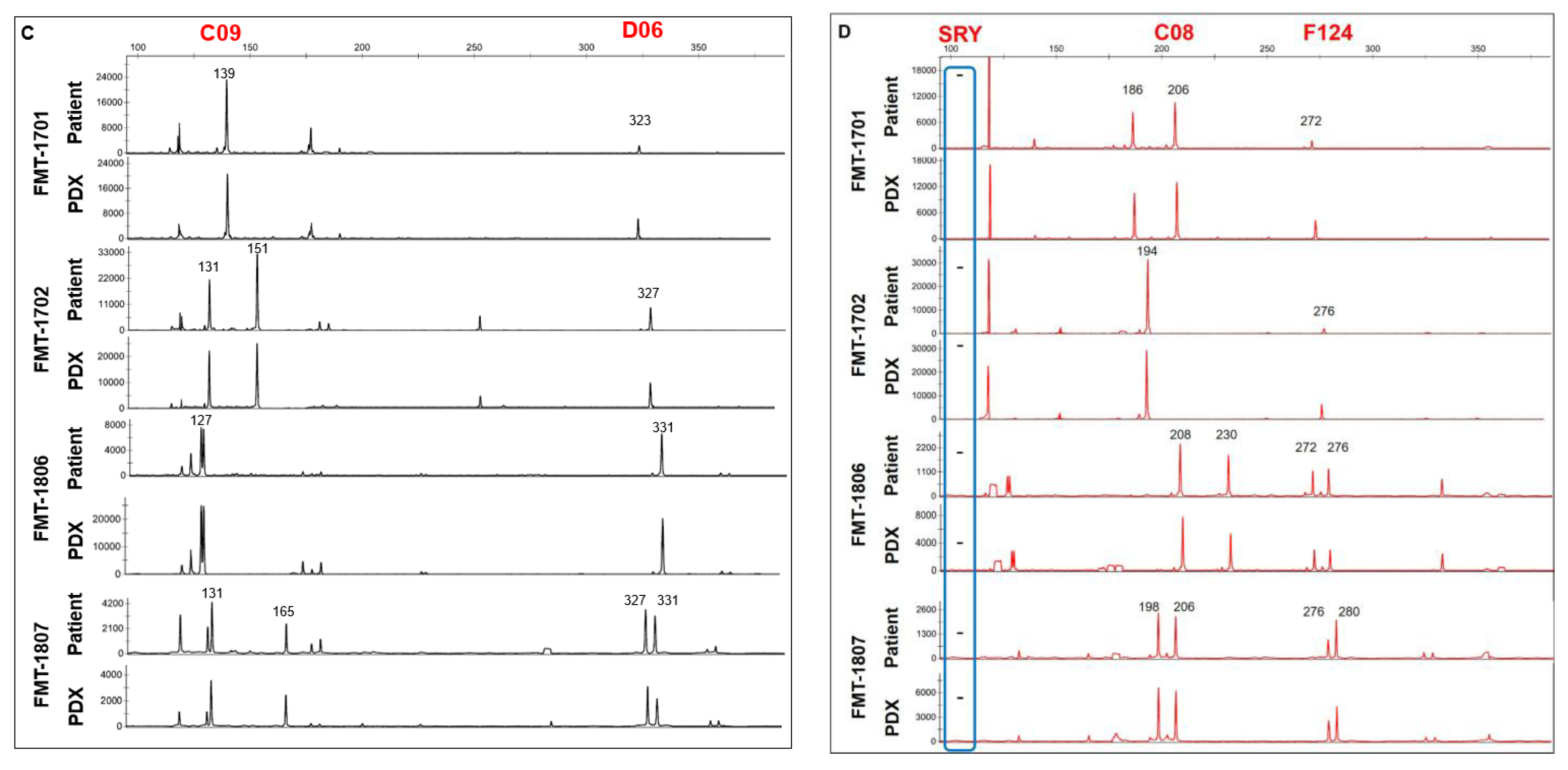
2.5. Short Tandem Repeat (STR) Analysis
2.6. Statistical Analysis
3. Results
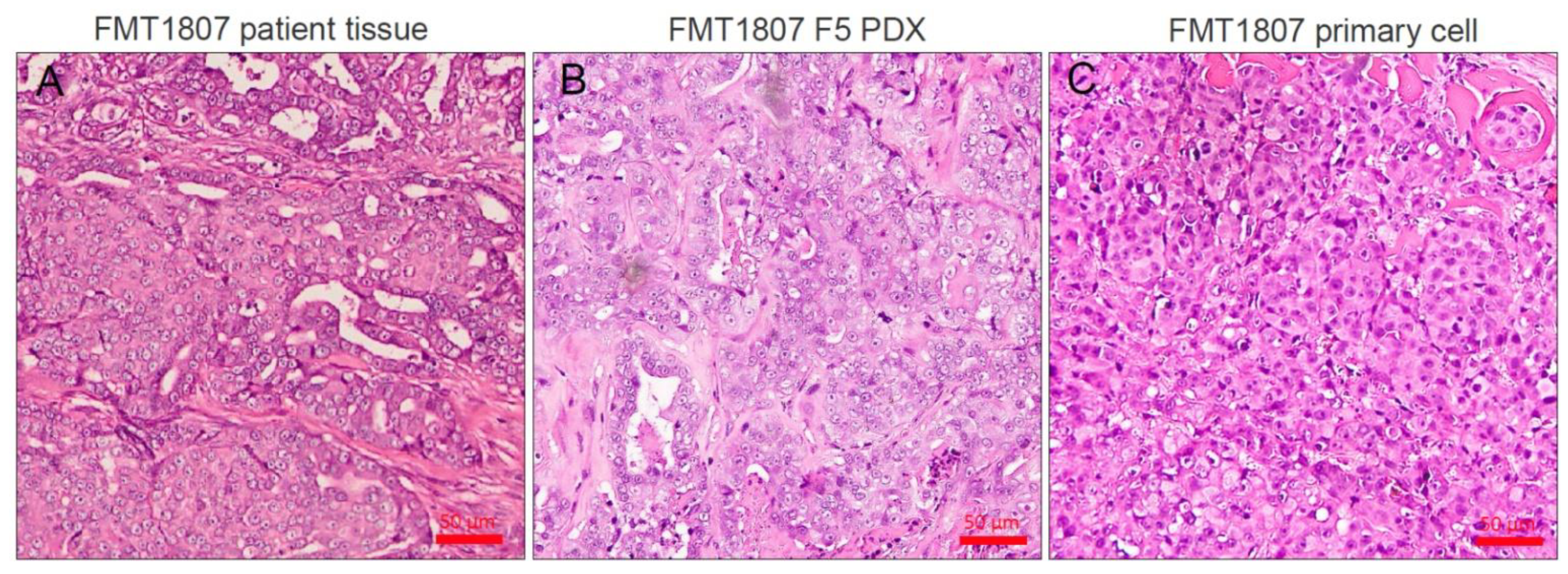
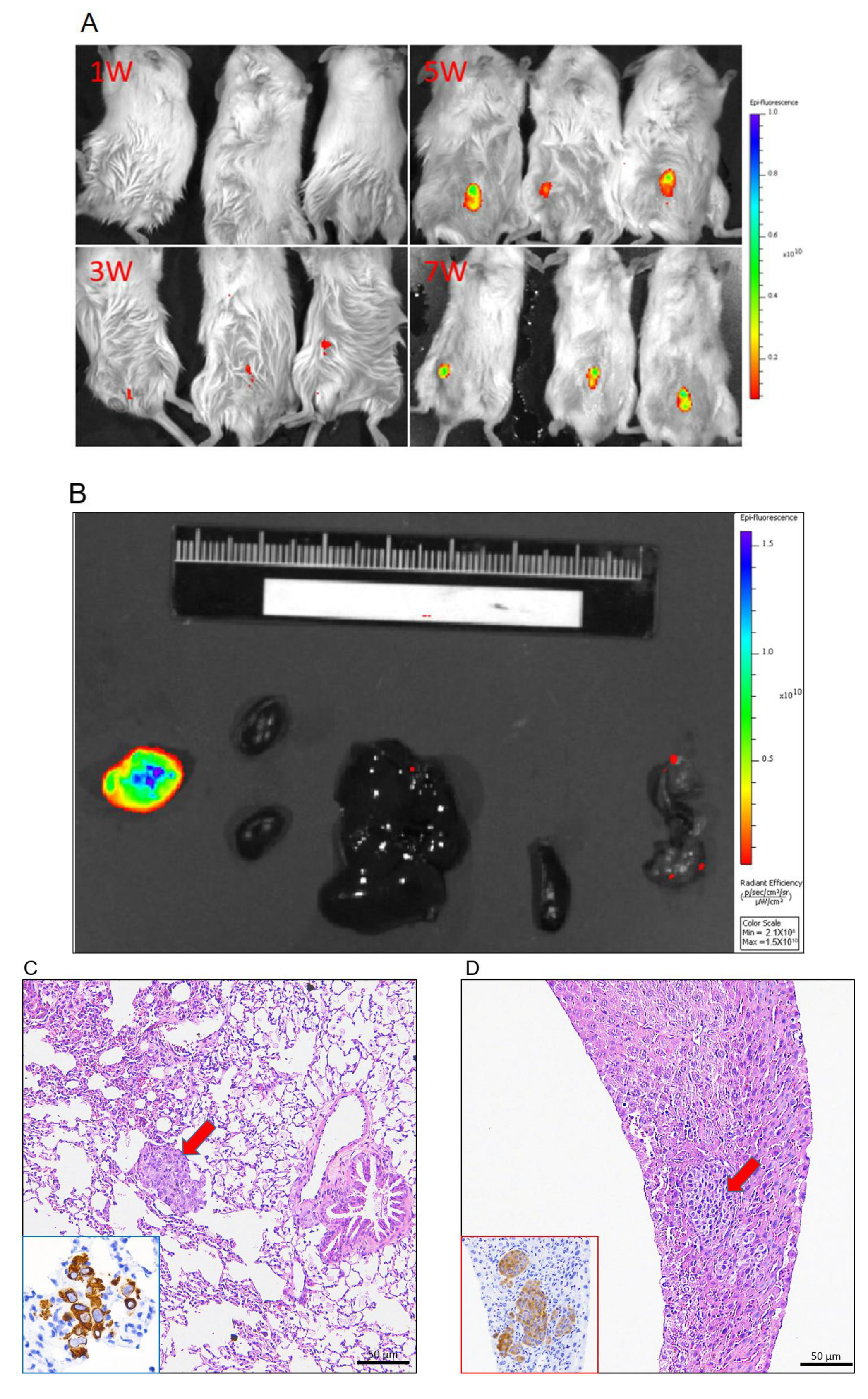
3.1. Generation of Tumor Grafts for Feline Mammary Carcinoma
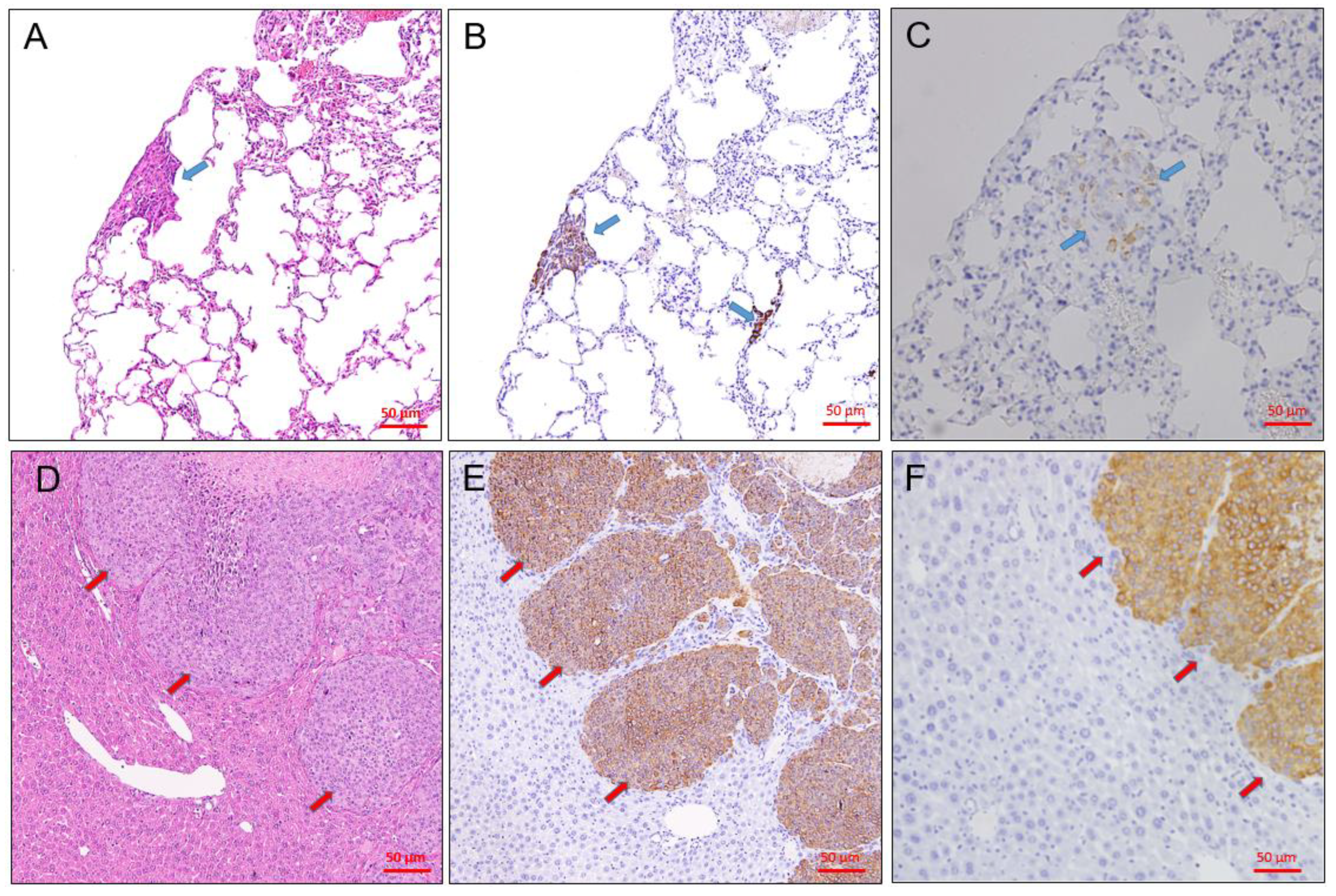
3.2. Tumor Grafts Emulate Metastasis Seen in PDX Mice
3.3. Immunohistochemical Characteristics of PDX Tumors
3.4. Establishment of Primary FMT Cells from PDX
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Di Masi, J.A.; Reichert, J.M.; Feldman, L.; Malins, A. Clinical approval success rates for investigational cancer drugs. Clin. Pharmacol. Ther. 2013, 94, 329–335. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kang, W.; Han, J.Y.; Min, S.; Kang, J.; Lee, A.; Kwon, J.Y.; Lee, C.; Park, H.S. An integrative approach to precision cancer medicine using patientderived xenografts. Mol. Cells 2016, 39, 77–86. [Google Scholar] [PubMed]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Tentler, J.J.; Tan, A.C.; Weekes, C.D.; Jimeno, A.; Leong, S.; Pitts, T.M.; Arcaroli, J.J.; Messersmith, W.A.; Eckhardt, S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012, 9, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Chuang, H.L.; Yin, J.H.; Liao, J.W.; Chen, T.H.; Wang, Y.C. Significance of sphingosine kinase 1 expression in feline mammary tumors. BMC Vet. Res. 2019, 15, 155. [Google Scholar] [CrossRef]
- Burrai, G.P.; Mohammed, S.I.; Miller, M.A.; Marras, V.; Pirino, S.; Addis, M.F.; Uzzau, S.; Antuofermo, E. Spontaneous feline mammary intraepithelial lesions as a model for human estrogen receptor- and progesterone receptor-negative breast lesions. BMC Cancer 2010, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Hayes, H.M., Jr.; Milne, K.L.; Mandell, C.P. Epidemiological features of feline mammary carcinoma. Vet. Rec. 1981, 108, 476–479. [Google Scholar] [CrossRef]
- Borges, A.; Adega, F.; Chaves, R. Establishment and characterization of a new feline mammary cancer cell line, FkMTp. Cytotechnology 2016, 68, 1529–1543. [Google Scholar] [CrossRef]
- Granados-Soler, J.L.; Junginger, J.; Hewicker-Trautwein, M.; Bornemann-Kolatzki, K.; Beck, J.; Brenig, B.; Betz, D.; Schille, J.T.; Murua Escobar, H.; Nolte, I. TiHo-0906: A new feline mammary cancer cell line with molecular, morphological, and immunocytological characteristics of epithelial to mesenchymal transition. Sci. Rep. 2018, 8, 13231. [Google Scholar] [CrossRef]
- Frazier, J.P.; Beirne, E.; Ditzler, S.H.; Tretyak, I.; Casalini, J.R.; Thirstrup, D.J.; Knoblaugh, S.; Ward, J.G.; Tripp, C.D.; Klinghoffer, R.A. Establishment and characterization of a canine soft tissue sarcoma patient-derived xenograft model. Vet. Comp. Oncol 2017, 15, 754–763. [Google Scholar] [CrossRef]
- Michishita, M.; Ohtsuka, A.; Nakahira, R.; Tajima, T.; Nakagawa, T.; Sasaki, N.; Arai, T.; Takahashi, K. Anti-tumor effect of bevacizumab on a xenograft model of feline mammary carcinoma. J. Vet. Med. Sci. 2016, 78, 685–689. [Google Scholar] [CrossRef]
- Maruo, K.; Sugimoto, T.; Suzuki, K.; Shirota, K.; Ejima, H.; Nomura, T. Xenotransplantation and high tumorigenicity of feline tumors in SCID mice. J. Vet. Med. Sci. 1995, 57, 967–969. [Google Scholar] [CrossRef][Green Version]
- Soares, M.; Correia, J.; Peleteiro, M.C.; Ferreira, F. St Gallen molecular subtypes in feline mammary carcinoma and paired metastases-disease progression and clinical implications from a 3-year follow-up study. Tumor Biol. 2016, 37, 4053–4064. [Google Scholar] [CrossRef] [PubMed]
- Eirew, P.; Steif, A.; Khattra, J.; Ha, G.; Yap, D.; Farahani, H.; Gelmon, K.; Chia, S.; Mar, C.; Wan, A.; et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature 2015, 518, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Correia, J.; Rodrigues, P.; Simões, M.; de Matos, A.; Ferreira, F. Feline HER2 protein expression levels and gene status in feline mammary carcinoma: Optimization of immunohistochemistry (IHC) and in situ hybridization (ISH) techniques. Microsc. Microanal. 2013, 19, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Kuperwasser, C.; Chavarria, T.; Wu, M.; Magrane, G.; Gray, J.W.; Carey, L.; Richardson, A.; Weinberg, R.A. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 4966–4971. [Google Scholar] [CrossRef]
- Mattar, M.; McCarthy, C.R.; Kulick, A.R.; Qeriqi, B.; Guzman, S.; de Stanchina, E. Establishing and maintaining an extensive library of patient-derived xenograft models. Front. Oncol. 2018, 8, 19. [Google Scholar] [CrossRef]
- Dobrolecki, L.E.; Airhart, S.D.; Alferez, D.G.; Aparicio, S.; Behbod, F.; Bentires-Alj, M.; Bentires-Alj, M.; Brisken, C.; Bult, C.J.; Cai, S.; et al. Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev. 2016, 35, 547–573. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.L.; Wallace, K.; Mays, S.; Ryan, P.; Coats, K.S. An immunohistochemical assay to detect trophoblasts in frozen feline placenta. J. Vet. Diagn. Investig. 2011, 23, 275–281. [Google Scholar] [CrossRef]
- Darbès, J.; Majzoub, M.; Hermanns, W. Evaluation of the cross-reactivity between human and feline or canine leucocyte antigens using commercially available antibodies. J. Vet. Diagn. Investig. 1997, 9, 94–97. [Google Scholar] [CrossRef]
- Ladiges, W.C.; Van Hoosier, G.L., Jr. Heterotransplantation of feline malignant tumors in nude thymusless mice. Am. J. Vet. Res. 1980, 41, 840–842. [Google Scholar] [PubMed]
- Sugimoto, T.; Maruo, K.; Imaeda, Y.; Suzuki, K.; Shirota, K.; Ejima, H.; Endo, S.; Nomura, T. Xenotransplantation of canine tumors into severe combined immunodeficient (SCID) mice. J. Vet. Med. Sci. 1994, 56, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Umeki, S.; Ema, Y.; Suzuki, R.; Kubo, M.; Hayashi, T.; Okamura, Y.; Yamazaki, J.; Tsujimoto, H.; Tani, K.; Hiraoka, H.; et al. Establishment of five canine lymphoma cell lines and tumor formation in a xenotransplantation model. J. Vet. Med. Sci. 2013, 75, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Behbod, F.; Kittrell, F.S.; LaMarca, H.; Edwards, D.; Kerbawy, S.; Heestand, J.C.; Young, E.; Mukhopadhyay, P.; Yeh, H.W.; Allred, D.C.; et al. An intraductal human-in-mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res. 2009, 11, R66. [Google Scholar] [CrossRef] [PubMed]




| Target | Clone | Dilution | Manufacturer | Reference |
|---|---|---|---|---|
| ER | 6 F11 | 1:100 | Thermo Scientific | Burrai et al., 2010 [14] |
| PR | 10A9 | 1:50 | Meridian Life Science | Burrai et al., 2010 [14] |
| HER2 | CB11 | 1:200 | Invitrogen | Soares et al., 2016 [13,15] |
| Pan-cytokertin | AE1/AE3 | 1:100 | Dakocytomation | Scott et al., 2011 [16] |
| β-catenin | 14 | 1:100 | BD Biosciences | Zappulli et al., 2012 [17] |
| Vimentin | V9 | 1:100 | Dakocytomation | Peñafiel-Verdu et al., 2012 [18] |
| CD3 | SP7 | 1:50 | Abcam | Furukawa et al., 2017 [19] |
| CD20 | B-Ly1 | 1:100 | Santa Cruz Biotechnology | Darbès et al., 1997 [20] |
| Cytokeratin 5/6 | D5/16B4 | 1:100 | Sigma-Aldrich | Soares et al., 2016 [13,15] |
| Ki-67 | polyclonal | 1:500 | Thermo Scientific | Soares et al., 2016 [13,15] |
| STR Marker | Concentration (uM) | Dye | Primer Sequence |
|---|---|---|---|
| F53 | 0.9 | 6FAM | Forward: CCTATGTTGGGAGTAGAGATCACCT |
| 0.9 | Reverse: GTGTCTTGAGTGGCTGTGGCATTTCC | ||
| C08 | 0.9 | Forward: GATCCATCAATAGGTAAATGGATAAAGAAGATG | |
| 0.9 | ROX | Reverse: TGGCTGAGTAATATTCCACTGTCTCTC | |
| B04 | 0.9 | 6FAM | Forward: TGAAGGCTAAGGCACGATAGATAGTC |
| 0.9 | Reverse: GTGTCTTCCACCCAGGTGTCCTGCTTC | ||
| G11 | 1.44 | 6FAM | Forward: ATCCATCTGTCCATCCATCTATT |
| 1.44 | Reverse: GGTCAGCATCTCCACTTGAGG | ||
| SRY | 0.04 | ROX | Forward: TGCGAACTTTGCACGGAGAG |
| 0.04 | Reverse: GCGTTCATGGGTCGTTTGACG | ||
| FCA441 | 0.6 | Forward: GTGTCTTGATCGGTAGGTAGGTAGATATAG | |
| 0.6 | VIC | Reverse: ATATGGCATAAGCCTTGAAGCAAA | |
| D09 | 0.16 | VIC | Forward: CCGAGCTCTGTTCTGGGTATGAA |
| 0.16 | Reverse: GTGTCTTTCTAGTTGGTCGGTCTGTCTATCTG | ||
| F124 | 0.6 | ROX | Forward: TGTGCTGGGTATGAAGCCTACTG |
| 0.6 | Reverse: GTGTCTTCCATGCCCATAAAGGCTCTGA | ||
| C12 | 0.6 | VIC | Forward: GAGGAGCTTACTTAAGAGCATGCGTTC |
| 0.6 | Reverse: GTGTCTTAAACCTATATTCGGATTGTGCCTGCT | ||
| C09 | 1.2 | NED | Forward: AAATTTCAATGTCTTGACAACGCATAAG |
| 1.2 | Reverse: GTGTCTTCCAGGAACACCATGTTGGGCTA | ||
| F85 | 1.44 | NED | Forward: TAAATCTGGTCCTCACGTTTTC |
| 1.44 | Reverse: GCCTGAAAATGTATCCATCACTTCAGAT | ||
| D06 | 1.2 | NED | Forward: CCAAGGAGCTCTGTGATGCAAA |
| 1.2 | Reverse: GTTCCCACAGGTAAACATCAACCAA |
| Subject Information | Xenograft Information | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Breed | Tumor Grade | Histological Type | Histological Grade a | ER | PR | HER2 | Molecular Subgroup | Prognosis | ER | PR | HER2 | Duration in First Passage (weeks) b | Metastasis |
| FMT-1701 | Mixed | T1N0M0 | Tubulopapillary | II | - | - | - | Triple negative basal-like | Not available | - | - | - | 12–14 | Not detected |
| FMT-1702 | Mixed | T1N0M0 | Tubulopapillary | I | + | - | - | Luminal A | Not available | + | - | - | >6 month | LN |
| FMT-1806 | American shorthair | T3N0M0 | Solid | III | - | - | 2+ | HER2-positive | Rrcurrence, lung metastasis | - | - | 2+ | 6–7 | Lung |
| FMT-1807 | Mixed | T2N0M0 | Tubulopapillary | III | - | - | - | Triple negative basal-like | Rrcurrence, lung metastasis | - | - | - | 10–11 | Lung, liver |
| PDX | % of Tumor Growth | Time of 500 mm3 Volume (days) | % of Animals with Metastasis |
|---|---|---|---|
| FMT-1806PDX P3 (n = 6) | 100 | 34.2 ± 2.4 | 83.3% Lung (5/6) |
| FMT-1807PDX P2 (n = 6) | 100 | 56.5 ± 3.2 | 100% Lung (6/6), liver (2/6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, H.-L.; Chang, Y.-C.; Huang, Y.-T.; Liao, J.-W.; Kao, P.-L.; Chen, Y.-F.; Lin, B.-Y.; Lin, Y.-L.; Chen, T.-H.; Wang, Y.-C. Establishment and Characterization of Feline Mammary Tumor Patient-Derived Xenograft Model. Animals 2021, 11, 2380. https://doi.org/10.3390/ani11082380
Chuang H-L, Chang Y-C, Huang Y-T, Liao J-W, Kao P-L, Chen Y-F, Lin B-Y, Lin Y-L, Chen T-H, Wang Y-C. Establishment and Characterization of Feline Mammary Tumor Patient-Derived Xenograft Model. Animals. 2021; 11(8):2380. https://doi.org/10.3390/ani11082380
Chicago/Turabian StyleChuang, Hsiao-Li, Yi-Chih Chang, Yi-Ting Huang, Jiunn-Wang Liao, Pei-Ling Kao, Yi-Fei Chen, Bin-Yin Lin, Yi-Lo Lin, Ter-Hsin Chen, and Yu-Chih Wang. 2021. "Establishment and Characterization of Feline Mammary Tumor Patient-Derived Xenograft Model" Animals 11, no. 8: 2380. https://doi.org/10.3390/ani11082380
APA StyleChuang, H.-L., Chang, Y.-C., Huang, Y.-T., Liao, J.-W., Kao, P.-L., Chen, Y.-F., Lin, B.-Y., Lin, Y.-L., Chen, T.-H., & Wang, Y.-C. (2021). Establishment and Characterization of Feline Mammary Tumor Patient-Derived Xenograft Model. Animals, 11(8), 2380. https://doi.org/10.3390/ani11082380






