Simple Summary
The conservation status of a native fish species is often a key indicator of the state of habitat alteration, which supports strong anthropogenic disturbance. Ecuador contains the Guayas basin, the largest basin in the Pacific Ocean, which is a biodiversity reserve. However, there is little information regarding the morphometric characterization of Brycon dentex and its variations within this basin, although its plasticity has been proposed as an indicator of the maintenance of biodiversity. The goal of this study was to analyze the effects of anthropogenic activity and habitat modification on the morphological variation of Brycon dentex and to determine the usefulness of discriminant analysis in the morphometric differentiation of three populations of Brycon dentex in Ecuador. The Brycon dentex morphometric model could be used as a framework in conservation and, thus, an indicator of habitat status by quickly detecting changes in fish shape.
Abstract
The Guayas, located in Ecuador, is the largest basin in the Pacific Ocean and has an inventory of 123 native freshwater species. Most of these are endemic species that are threatened or at-risk due to anthropogenic activity and the modification, fragmentation, and destruction of habitats. The aim of this study was to determine the morphometric variation in three wild populations of Brycon dentex in the Guayas basin rivers and their connections to fishing management and environmental conditions. A total of 200 mature fish were captured, and 26 morphometric parameters were measured. The fishing policies (Hypothesis 1) and environmental conditions (Hypothesis 2) were considered fixed factors and were validated by t-tests. The morphological variation among the three populations (Hypothesis 3) was validated through a discriminant analysis. Fishing policies and resource management were found to generate morphological differences associated with body development. In addition, the environmental conditions were found to influence the size and structure of Brycon dentex populations. The analyzed populations were discriminated by the generated morphometric models, which differentiated Cluster 1 (Quevedo and Mocache rivers) with high fishing pressure from Cluster 2 (Pintado river) with medium–low fishing pressure. Morphometric differentiation by discriminant analysis is a direct and economic methodology that can be applied as an indicator of diversity maintenance.
1. Introduction
The ecological theory of diversification [1] and studies of wild populations explain how changes in environmental factors could induce changes in behavior, morphology, and physiology [2,3]. Selection pressure in new environments favors the divergence of populations, and there is a strong link between environmental variations and the morphological diversification of a population [2,4,5]. Furthermore, habitat modification may result in changes in the composition, geographical spread, and population structure of a species [6,7,8], while diversity is considered to be an indicator of ecosystem restoration [9]. In this sense, fishing policies in each country seek food sovereignty and the maintenance of genetic resources and biodiversity through economic incentives and regulatory measures [10]. The construction of habitat conservation indicators in Latin America is complex due to a lack of data and the absence of characterization and productive behavior studies [11]. The characterization of animal genetic resources covers all activities associated with the identification and quantitative and qualitative descriptions of populations, as well as the natural habitat and production systems to which they are adapted [12]. In this sense, morphological analysis has been widely used for breed and population characterization [13,14]. It has recently been used in comparative morphometric studies of native freshwater species from Ecuador. It could also be useful for the implementation of a livestock development program and as a restoration indicator. The evaluation of morphological variations in a native freshwater species living under different conditions within the same habitat could help to identify the factors responsible for these differences [15]. According to Dauda et al. [16], this knowledge is key for the management of fisheries, as having stocks with different life-history traits is essential to enhance stock management programs [17]. A study by Ndiwas et al. [2] noted that the geographical spread of a species across a broad range of environmental conditions is accompanied by equally diverse morphological variations that are strongly correlated to the environmental conditions. Many factors can cause morphological variations between populations, and they are hard to identify [18]. Previous studies using geometric morphometrics to investigate variations in fish have found differences based on sex [15], diet composition [19,20], the geographic location of a population [21], and the habitat and water characteristics [3,22]. Morphometry is a cost-effective technique that is frequently used for the differentiation of populations [13,23,24] and is employed to describe fish body shapes, delineate stock status, discriminate between fish populations, and link ontogeny with functional morphology [18]. It is also necessary to collect actual biological information on fish such as data on their ecology, evolution, behavior, and stock assessment [25,26] associated with diverse species, breeds, and populations [27]. Thus, the characterization of native fish and the level of diversity are essential for the development of conservation programs and knowledge of morphometric characteristics. This constitutes the first step in the classification of animal genetic resources [16,28].
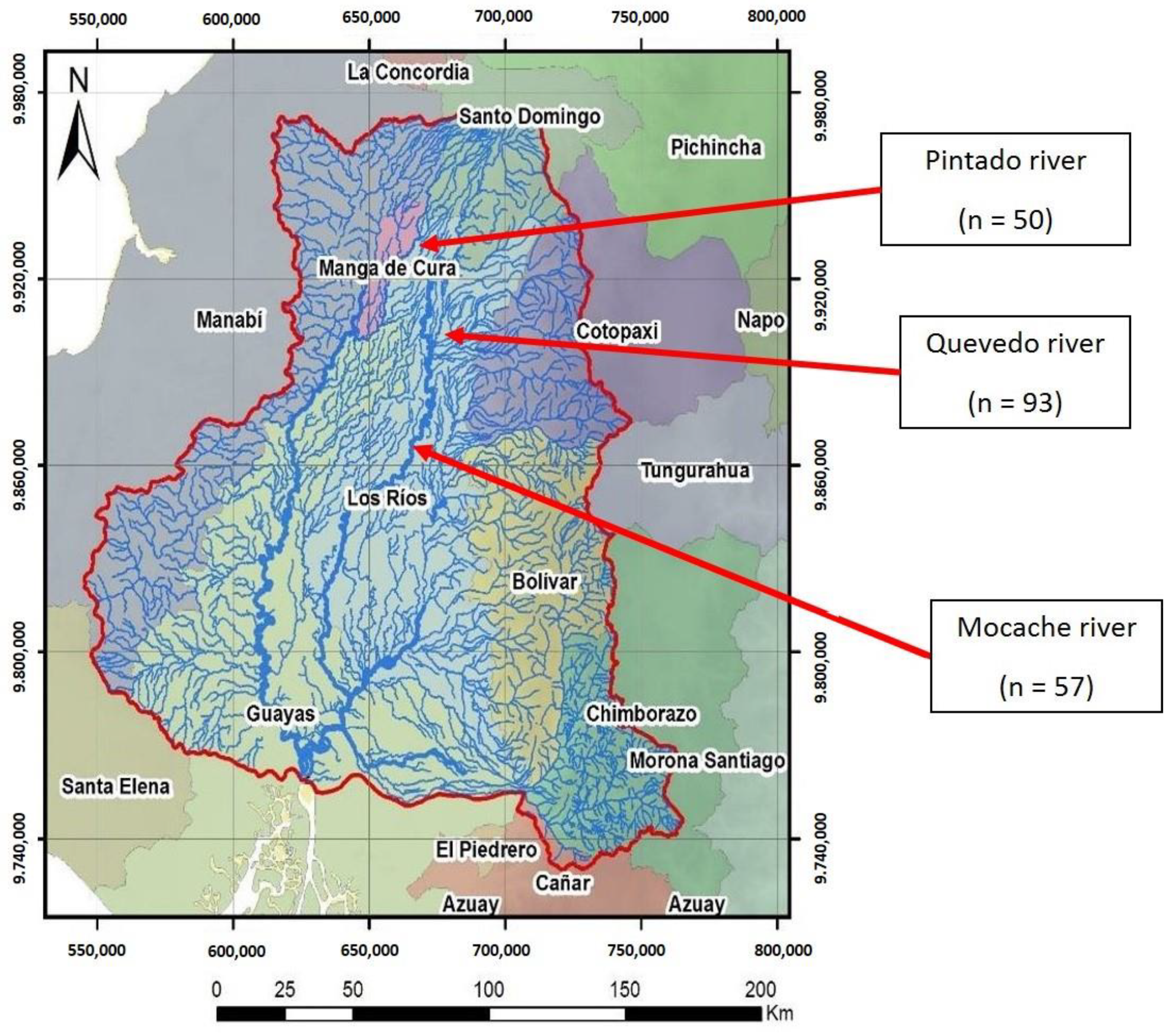
Developing countries are home to most fish species in the world, although a large proportion of fish species remain unassessed due to insufficient scientific studies. Ecuador, with 951 native freshwater species, is considered to be a biodiversity reserve [29]. The fluvial network of Ecuador is complex and diverse, and the Guayas river hydrographic basin (CHG), covering 53,299 km2 (Figure 1), is the largest Pacific Ocean basin in South America [30].
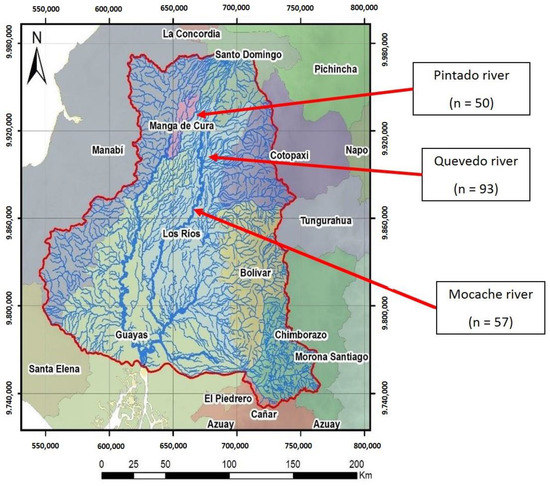
Figure 1.
Guayas hydrographic basin (CHG). Site 1: Pintado river; Site 2: Quevedo river; Site 3: Mocache river (n = sample size).
Brycon dentex Günther 1860 (pez dama), from the Bryconidae family, is a native species that is widely distributed in western Ecuador in different rivers of the CHG [31,32,33], in the Tahuín dam near Peru [34], and in the North of Perú [35]. In a previous study, our research group conducted a preliminary morphology characterization of Brycon dentex [36]. Morphologically, this species has a single dorsal fin and two bifurcations in the caudal fin, a total length of 51 cm, and a powerful upper jaw. It is considered an omnivorous species [33,37]. Sampling in natural environments has shown that it can reach sexual maturity at lengths of 20–26 cm [37]. Its conservation status is of “least concern” (LC), and it is included in the IUCN Red List.
The subject of our study, Brycon dentex, is widely spread across a broad geographical range of ecological conditions accompanied by equally diverse morphological variations in Ecuador. It is included on the IUCN Red List as LC, although there are insufficient data for characterization. Brycon dentex has an omnivorous mode of feeding and a strong ability to rapidly adapt to different environmental conditions. In addition to characterizing Brycon dentex, it is of great interest to relate its morphological variability to biodiversity maintenance. The variation among the stocks of river populations could be a consequence of phenotypic plasticity in response to unusual hydrological conditions [2,17].
Ferrito et al. [38] and Mir et al. [39] conducted similar studies on other freshwater species, and Dasgupta et al. [40] stated that morphological discrimination in various populations are strongly influenced by habitat differences. Growth variations also occur in response to different habitats [41]. Under the hypothesis that the morphological differences between populations of native freshwater species could be used as bioindicators, the causes of these differences were classified into anthropogenic and habitat modifications [42]. Knowledge in this area can be used as a tool for both the smart and ecological management of resources [43,44]. In addition, there is a lack of knowledge about the morphological characterization of Brycon dentex and the variation in the traits of different rivers in the Guayas basin. In this context, this study aimed to contribute to the attainment of an adequate management ecosystem equilibrium and the characterization of animal genetic resources.
Therefore, we investigated whether three wild populations of Brycon dentex in the CHG have undergone significant morphological diversification and whether these differences can be related to fishing management and environmental conditions. Knowledge of the phenotypic variations in relation to environmental modifications could be used to identify key factors for policy makers in terms of the development of both diversity conservation programs and sustainable fishing practices.
The general hypothesis of this study was that fishing policies, resource management, and environmental conditions influence the differentiation of populations due to the phenotypic plasticity of Brycon dentex. Subsequently, three hypotheses were formulated, as displayed below:
Hypothesis 1.
Fishing and resource management practices (such as fishing methods and pressure, respect of the closure periods, land use, endogeneity level, and competition with native and non-native species) influence the morphological variation of Brycon dentex in the CHG. The null hypothesis (H0) was that there would be an equality of means between the populations of Brycon dentex in the Pintado and Mocache rivers (H0: μ1 = μ3). In contrast, the alternative hypothesis was that significant differences would exist due to fishing management practices (H1: μ1 ≠ μ3).
Hypothesis 2.
The physical environmental conditions influence the morphological variation of Brycon dentex. The null hypothesis (H0) was that there would be an equality of means between the populations of Brycon dentex in the Quevedo and Mocache rivers (H0: μ2 = μ3). In contrast, the alternative hypothesis was that significant differences would exist due to a change in environmental conditions (H1: μ2 ≠ μ3).
Hypothesis 3.
The fishing management and environmental conditions influence the morphological variation of Brycon dentex in the CHG. The relationships among three populations were analyzed through discriminant analysis, where three groups were identified: Population 1 (Pintado river) with a medium fishing pressure and a low flow velocity, Population 2 (Quevedo river) with a high fishing pressure and white water or water containing a high concentration of oxygen, and Population 3 (Mocache river) with a high fishing pressure and a low flow velocity.
2. Materials and Methods
2.1. Data Collection and Study Area
A stratified sample of 200 adult specimens from three populations of wild Brycon dentex—from the Pintado (population 1; sample size = 50), Quevedo (population 2; sample size = 93), and Mocache (population 3; sample size = 57) rivers—was chosen. The non-representative specimens of Brycon dentex with small size, fish of other species, mutilated, and young individuals were immediately returned to the river. These areas are in the CHG (Figure 1). The Pintado river is located in the northwest of Manabí province in the “La manga del Cura” area, and it flows into the Daule-Peripa dam. The Pintado river is born as a continuation of the Pupusá river and borders the canton of Carmen with very slow waters, high turbidity, and little oxygenation. The water characteristics of the Pintado River are shown in Table 1. These values are similar to those of the Mocache river (pH, electric conductivity, and temperature). The river delimits a protected natural area of tropical humid forest inhabited by native farmers called “montuvios”. The fishing pressure on native species is medium–low, and resource management practices favor “land sharing or wildlife-friendly agriculture”, so farmers in this area apply low-intensity, more environmentally friendly agricultural practices [45]. The Quevedo river, which originates in the foothills of the Andes mountain range in the mid–high area of the basin, contains white, fast, and highly oxygenated waters and has a length of 163 km. The Mocache river is located in the mid–low area of the Guayas delta basin and contains slow water, a low oxygen level, and a high level of dissolved solids. Both rivers (Quevedo and Mocache), located in the province of Manabí, have high fishing pressure for native species [46].

Table 1.
Water characteristics in the three rivers of Guayas basin.
The physical attributes (pH, temperature, color, conductivity, turbidity, dissolved oxygen, and total dissolved solids), chemical indicators (chlorides, alkalinity, nitrates, and ammonium), and biological indicators (phytoplankton) of the water in the three rivers show values within the recommended ranges for freshwater life [5,7,31]. Table 1 shows the main water quality characteristics in the three rivers sampled.
The most commonly used types of fishing gear are hand-held lines with hooks (10% of catches); cloth or tape mode nets (20%) with a length of 100–150 m, a height of 4–5 m, and a mesh light diameter of ¾–1 inch; trammels (30%) with a mesh size of between 2 and 3½ inches; casts (35%) with a mesh size of between ½ and 1 inch; and others such as harpoon hooks (5%). Manual riverbank throw nets, trammel nets, and fishing spears are the most frequently used fishing methods in the Pintado river. By contrast, trawl lines, riverbank manual throw nets, trammel nets, throw nets, and fishhooks are the most commonly used methods in the Quevedo and Mocache rivers.
Balsas and canoes are ancestrally used by fishermen, while bongos (15–70 cv; measurement of power where 1 cavallo vapore (cv) = 0.98632 horsepower (hp)) boats (25–50 cv), and lanchas (30–180 cv) use motors from low to high strengths and are autonomous. Previous research by Pacheco-Bedoya [50], FAO [51], MAGAP [52], and Ochoa Ubilla et al. [53] widely analyzed the diverse traditional fishing tackle methods utilized in the CHG. Rural communities were found to use highly diverse fishing vessels. In the Pintado river, the bongo and boat were identified as the most commonly used fishing vessels. In the Quevedo and Mocache rivers, the balsa, canoe, bongo, boat, and lancha were identified as the most commonly used.
Brycondentex specimens (weight > 56 g; body length > 12.38 mm) were caught by fishermen between January and March 2019. The capture, transport, and stunning of specimens were conducted following the recommendations of Gonzalez-Martinez et al. [13]. A veterinary practitioner supervised the animals’ welfare in each research step.
2.2. Body Measurements
Morphometric trait data were collected using an ichthyometer with graduated digital calipers and tape with a precision of 0.01 mm. To avoid errors, the same researcher measured all fish starting from the left side, except for the widths and perimeters, following the conventional method described by Diodatti et al. [54]. A total of twenty-six morphometric measurements based on 23 landmarks and five meristic counts were obtained (Figure 2 and Table 2), in agreement with the methodology used to assess native species in Ecuador [11,55,56].

Figure 2.
Locations of 23 anatomical landmark points showed in the left view of the Brycon dentex. Landmark points: 1: highest cranial point of the upper pre-maxilla; 2: highest cranial point of the lower pre-maxilla; 3: commissure of the mouth; 4: anterior edge of the eye; 5: posterior edge of the eye; 6: end of the operculum; 7: origin of the pectoral fin; 8: end of the pectoral fin radius; 9: origin of the first dorsal fin; 10: end of the dorsal fin; 11: origin of the second dorsal fin; 12: end of the second dorsal fin; 13: dorsal origin of the caudal fin; 14: ventral origin of the caudal fin; 15: highest cranial point of the caudal peduncle; 16: highest caudal point in the superior part of the caudal peduncle; 17: highest caudal point in the inferior part of the caudal peduncle; 18: end of the anal fin; 19: origin of the anal fin; 20: anal opening; 21: origin of the pelvic fin; 22: end of the pelvic fin radius; 23: nape, highest caudal point of the head.

Table 2.
Morphometric measurements and meristic counts used to assess Brycon dentex in this study.
2.3. Fulton Condition Coefficient (K)
The Fulton condition coefficient (K) is a widely used indicator of animal welfare in fisheries and general fish biology studies to quantify variations in fish populations [57]. It was calculated with the equation K = 100 × (BW/SL3), where BW is the total weight (g) and SL is the standard length (cm).
2.4. Statistical Analysis
The morphometric characters were standardized in accordance with the work of Elliot et al. [58]. The efficiency of the transformation-adjusted size was evaluated by testing the correlation significance between each transformed variable and the standard length. The Kolmogorov–Smirnov and Bartlett tests were performed before the analyses to verify the normality and equality of the data variance (homoscedasticity). The KMO sampling adequacy test showed a value of 0.6, while the Bartlett test showed a satisfactory probability value (p < 0.001), thus indicating that the analysis was suitable [13,14]. The total length was obtained by calculating the arithmetic mean of both total lengths due to the bifurcated caudal fin of this species (Figure 2). Morphometric characteristics (original and adjusted) were compared by Student t-tests (hypotheses 1 and 2), whilst meristic counts were compared with the Kruskal–Wallis test. Sex was not considered as a fixed effect, since no significant differences between males and females have been found previously. To contrast Hypothesis 3, the DISCRIM procedure was used to perform a canonical discriminant analysis of size-adjusted geometric morphometric data using the three populations as the grouping variable. The probabilities of entering and staying in the model were both set at p < 0.05. The selection of the most discriminant variables was conducted by applying the F-Snedecor, Wilks’ lambda, and 1-Tolerance methods. The correct assignment percentage was considered, and the Mahalanobis distances are represented graphically as clusters. Statistica 12.0 for Windows software was used to perform the statistical analyses (StatSoft, Tulsa, OK, USA).
3. Results
The fish in the Brycon dentex population were shown to have an average body weight of 154.47 g; standard and total lengths of 15.65 and 21.46 cm, respectively; and an average head length of 5.65 cm (Table 3). The morphometric traits had a medium level of homogeneity with low coefficients of variation. The Pearson correlation coefficients between morphometric measures were high and significant (p < 0.05) (data not presented). The highest weight and K condition factor were obtained in the Pintado river (172.61 g and 5.10, respectively), and the lowest were obtained in the Quevedo river (137.95 and 3.53 g, respectively) (Table 3).

Table 3.
Descriptive statistics for body measurements (original data) in three populations of Brycon dentex (Mean ± SE (CV, %)).
In relation to Hypothesis 1, the results of the morphometric comparisons between two wild populations from the Pintado and Mocache rivers with different fishing management practices are shown in Table 3 (A × C). There were no significant differences in body weight or the condition factor between the two populations (p > 0.05). Eleven of the 25 morphometric measures showed significant differences (p < 0.05) for the populations from the Pintado and Mocache rivers (Pre-PvL, Pre-AL, DFRL, AFL, UJL, AC3, P1, P3, LC1, LC2, and LC3). Regarding Hypothesis 2, Table 3 (B × C) shows the influence of environmental conditions on morphological differentiation in populations of Brycon dentex. Significant differences between the Quevedo and Mocache rivers were found for 10 morphometric variables: TL, SL, HL, Pre-OL, Pre-DL, Pre-PcL, Pre-PvL, Pre-AL, AFL, and UJL.
Meristic traits based on the number of dorsal, pectoral, pelvic, anal, and caudal fin rays showed mean values of 9.98, 10.41, 7.81, 29.39, and 19.01, respectively (data not presented). Coefficients of variation were low. The river did not significantly (p > 0.05) affect the meristic traits, with close values shown for the three populations.
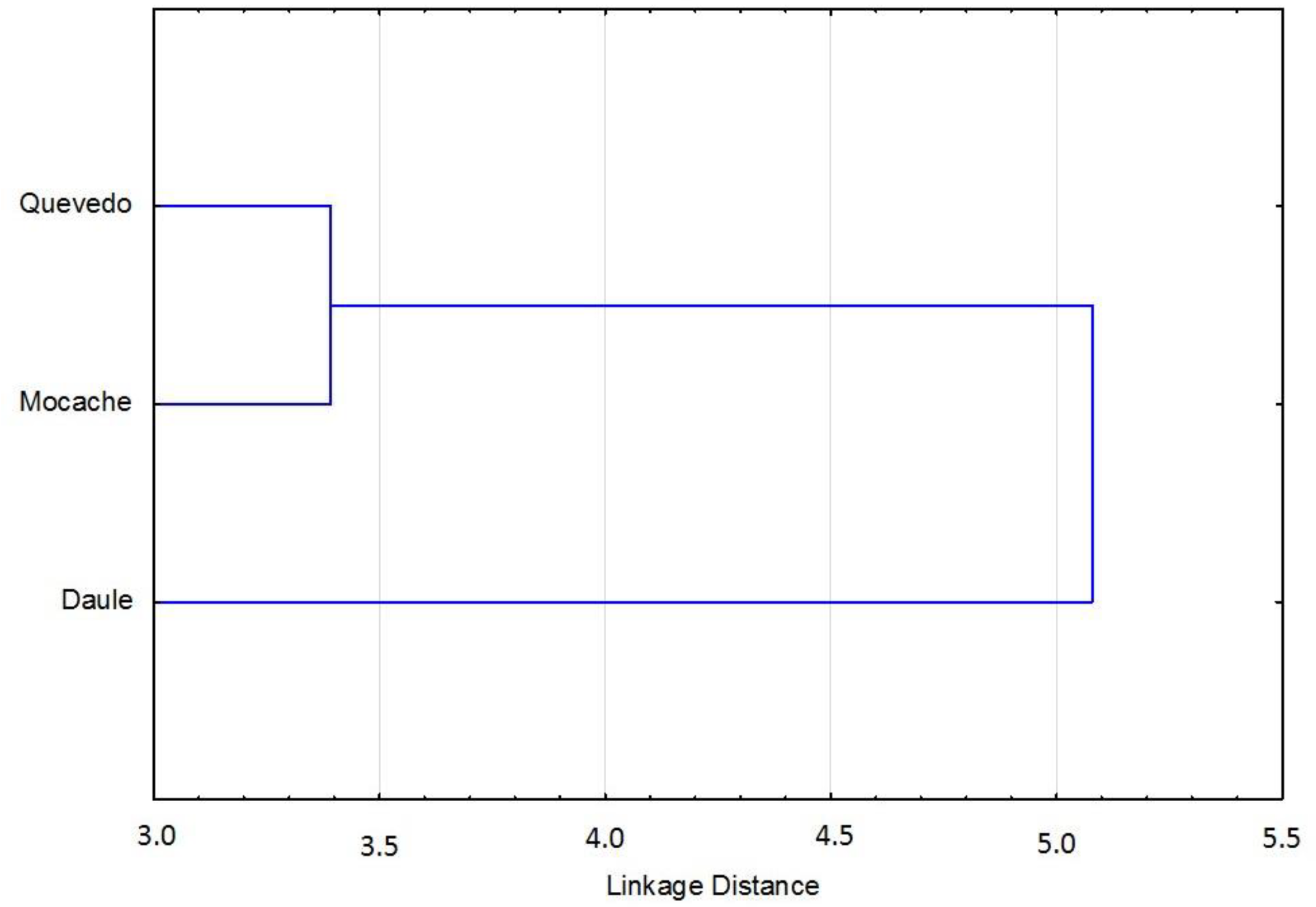
The discriminant function, obtained with 24 measurements, showed that were 14 (58.33%) variables were accepted in the model and 10 measurements (41.67%) were significant (p < 0.05). According to the results of the F-Snedecor, Wilks’ lambda, and 1-Toler methods, there were six major discriminant variables in the model (25%): the anal fin length (AFL), body perimeter 3 (P3), body depth 3 (AC3), total length (TL), upper jaw length (UJL), and pre-pelvic fin length (Pre-PvL) (Table 4). The relationships among discriminant variables showed a specific morphology model for each group of Brycon dentex, with a percentage of correct assignment of 68.84. The morpho-structural differences among the three analyzed fish populations were visually obtained from morphometric measurements through a graphical representation of Mahalanobis distances (Figure 3). There was a first cluster grouping of specimens from the Quevedo and Mocache rivers, and there was a second cluster made up of specimens from the Pintado river.

Table 4.
Discriminant functions for truss measurements in three populations of Brycon dentex.
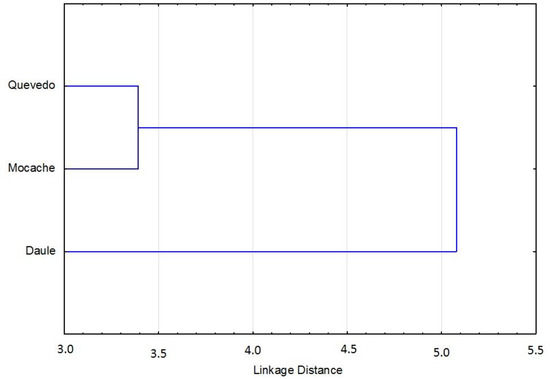
Figure 3.
Clusters of Mahalanobis distances of Brycon dentex from three rivers.
4. Discussion
With a total average length of 21.46 cm, the Brycon dentex individuals caught in our study were smaller than those recorded by Revelo [59] (23.7 cm) and Revelo and Laaz [33] (23.3 cm). According to Ochoa Ubilla et al. [53], the fishing gear used and the fishing pressure to which a river is subjected to determine both the frequency of capture and the size of the captured fish.
The results of this study showed significant differences between rivers; however, a low standard error and low variability within each river were found. This indicates the presence of homogeneity due to a potentially shared morphological plasticity and parallel adaptation to similar habitat types [22]. The availability and diversity of fish are indicators of the degrees of human intervention and habitat modification [60]. There are many factors that can be used to understand the morphometric variations among the three Brycon dentex populations, including food availability, strong competition with other non-native species (Oreochromis spp.), overfishing through several fishing methods (riverbank manual throw nets, trammel nets, and fishing spears), unsuitable fishing policies, land use, agricultural practices, and the destruction of Ecuador river habitats [6,8,45,61,62]. Moreover, food availability and pollution are conditioned by different abiotic parameters, including current flow, turbidity, and dissolved oxygen concentration [61]. In the Quevedo river, Prado et al. [61] identified a high level of diversity for phytoplankton and a low diversity for zooplankton. However, the Mocache and Pintado rivers presented greater variability and a greater proportion of zooplankton, insects from Baetis spp., and larvae from Astyanax spp. [61]. Furthermore, in the Mocache river, the presence of Polymyxus coronalis has been related to eutrophic waters.
Hypothesis 1, that fishing policies and resource management influence the morphological differentiation of Brycon dentex populations between the Pintado and Mocache rivers, was accepted for 11 of the 25 variables. For the traits linked to the body development of both populations (depth, perimeter, and width of body), significant differences were found. The morphological differences in BW, K, TL, and SL between the Pintado and Mocache rivers were not significant. Contrary to expectations, there were no differences in the size and structure of the fish between these rivers (Hypothesis 1), most likely because the local fishing regimes are closer than initially considered [29,47,59]. Brycon dentex populations in the Pintado river were found to have greater body depths, and specimens from the Mocache river had greater perimeters and widths. In the Pintado river, the fishing pressure was low, as this area acts as a reservoir of native freshwater species and encourages the use of environmentally friendly agricultural practices.
The differences between specimens in the rivers were influenced by the high fishing pressure in some areas, the overfishing of native species in the Mocache river, and the introduction of non-native species [29,46,59,63]. The role of non-natives species was not tested in this study, although it has been studied widely. According to Canonico et al. [64], the introduction of tilapia has had a large effect on native biodiversity, because tilapias are fast-growing and tolerant of a range of environmental conditions. This species readily adapts to changes in salinity levels and oxygen availability, can feed at different trophic levels, and (under certain circumstances) can tolerate overcrowding. In addition, tilapias are reproductively active for long periods—for most of the year in some places. They have short reproductive cycles and have been observed to spawn year-round in the wild with a higher frequency than most fish. They are also competitors with native species for food and space, and, therefore, native fish have fewer resources for somatic growth than at non-invaded sites. Hypothesis 2, which states that the differences in environmental conditions between the Quevedo and Mocache rivers influence the size and structure of Brycon dentex, was accepted in 12 out of 25 variables. Body weight and the condition factor (K), which indicates the nutritional status of fish, were higher in the Mocache river population (165.82 g and 4.93, respectively). In contrast, the total length, standard length, and eye diameter were higher in specimens in the Quevedo river. Specimens of greater size and with a greater hydrodynamic structure were found in the Quevedo river. These factors enhance their ability to survive and give them speed during flight from predators [65]. In terms of variability interpretation, there are multifactorial causes [66], such as food availability [33], seasonality [67], and the interaction between the two [68]. Therefore, the different morphologies found for populations in the Quevedo and Mocache rivers might be due to the fact that the Mocache river is slower, shallower, has greater turbidity, and has a higher level of photosynthesis and oxidative reactions [6,31]. Phytoplankton and zooplankton were identified as the most dominant foods [7]. Food availability seems to be the most relevant factor in the Mocache river, because this fish is an opportunistic feeder that switches from one diet to another according to food availability [19].
The three rivers showed differences in the morphology of Brycon dentex with factors associated with the fragmentation and deterioration of the ecosystem and a high fishing pressure existing in areas with the highest vulnerability level. According to Ochoa Ubilla et al. [53], the native species fishing pressure in the Quevedo and Mocache rivers is very high due to the large number of fishing cooperatives and the high fishing effort during the fishery season. The use of illegal fishing gear has also increased, thus contributing to the rivers’ deterioration [37,59]. Our findings are in agreement with those of Youson et al. [69] and Escanta-Molina and Jimenez-Prado [3], who related morphological differentiation to increases in temperature, suspended solids, and water turbidity, as well as decreases in pH, dissolved oxygen, and alkalinity. Ekaete [70] related morphology to the temperature and the amount of oxygen that fish absorb through their gills, the type of vegetation cover in the river, and the availability of food.
Hypothesis 3 was partially accepted. Though the discriminant model was accepted, Mahalanobis distances only differentiated two groups. Six morphologic variables with a high discriminant power were selected. The three analyzed Brycon dentex populations were discriminated by the generated morphometric models. Melvin et al. [71] and Ujjania and Kohli [43] pointed out the importance of using genetic and environmental interactions to complement the morphology approach, although it is not always easy to explain the causes of morphological differences between populations [72]. Surprisingly, the use of Mahalanobis distances only allowed us to differentiate two groups based on the morpho-structural model: Cluster 1 (Quevedo and Mocache rivers) with high fishing pressure and Cluster 2 (Pintado river) with medium–low fishing pressure.
AFL, UJL, and Pre-PvL variables were common in the differentiation of both factors. The AC3 and P3 traits were found to be associated with body development and the fishing management factor. The TL variable was linked with size, structure, and the water characteristics in each river. Native species tend to maintain a constant body mass and adaptively respond to anthropogenic and environmental conditions. Brycon dentex specimens in the Quevedo river (fast and highly oxygenated waters) are larger and narrower than those in the other rivers. On the contrary, fish in warm, slow, and less oxygenated waters (Mocache and Pintado rivers) respond by modifying their body development (width and deep). These results agree with those of Cavalcanti et al. [73] and Olaya Carbó et al. [74]. The CHG is made up of several fragile river ecosystems that are permanently subjected to risk factors such as the modification, fragmentation, and destruction of habitats; the introduction of non-native species (Oreochromis spp.); overfishing; environmental contamination (herbicides, heavy metals, etc.); the development of large-scale intensive forestry practices; a loss of modification of the natural hydrological regime, including basin river connectivity; and, finally, climate change [6,30,75,76]. Uncontrolled exploitation is a process that could lead to dangerous situations in terms of fish extinction [3,31,33,37,59]. Anthropogenic activities directly influence fishing policies, land use, and the indirect method of modifying the limnological characteristics of different ecosystems [77]. The adequate regulation of human activity, which contributes to the risk factors mentioned above, is essential for the conservation of these ecosystems [10,60]. These factors have great importance in resource conservation for the development of fisheries and sectorial policies [29,37,78].
Native freshwater populations are very sensitive to environmental changes and quickly adapt to morphological changes [79]. Therefore, the discriminant model built for Brycon dentex could be used as an indicator of habitat conservation and endogeneity degree [3,15,75]. The characterization carried out in this study was preliminary, since it only considered the morphological aspects and investigated variations among different habitats in terms of fish morphology. The ideal scenario would be to conduct studies with molecular markers to identify genetic relationships among populations from different habitats and specimens captured at different points, but, at the moment, little study on native species from Ecuador has been done. Morphometric methodology is a direct, simple, and low-cost method, so its in situ use is recommended in rural communities and developing countries [13]. It also could be used to assess fishing pressure and the effectiveness of sustainable policies [33].
The interactions between factors were not considered in this research, nor were the action mechanisms existing for each factor. Additionally, the characterization of Brycon dentex lacked a genetic analysis of the species.
5. Conclusions
The three populations of Brycon dentex in the CHG showed morphological differences due to fishing management and environmental conditions. Both factors are considered to be direct drivers of diversity maintenance.
The different fishing policies and resource management practices present in the studied rivers have generated morphological differences that are associated with body development. Under the different environmental conditions present in the rivers, differences in traits related to the size and structure of fish have primarily arisen.
The analyzed populations could be discriminated using the generated morphometric model, showing that discriminant analysis is a useful way to differentiate populations. Six morphometric measurements were found to have the greatest discriminant power and were deemed to be appropriate for population discrimination. These variables were the anal fin length, body perimeter 3, body depth 3, total length, upper jaw length, and pre-pelvic fin length.
The Brycon dentex morphometric model could be a useful framework for the conservation of the species, so it could indicate habitat status by quickly detecting changes in fish shape. A very small number of easily obtained morphometric traits in each population could be used to determine the biodiversity status, and this information could also be used in the implementation of fishing management policies in Ecuador’s rivers. This model could be extended for use in other rivers in Latin America.
Author Contributions
Conceptualization and methodology, all authors; formal analysis, software, data curation, and data processing, A.G.-M. and A.G.; statistical analysis, A.G.-M., C.B. and A.G.; validation and investigation, A.G. and A.G.-M.; supervision and project administration, A.G., J.R., C.D.-P.-H. and M.G.; data acquisition, J.R. and M.G. All authors were involved in developing, writing, commenting on, editing, and reviewing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful for the funding provided to the research project by the competitive scientific and technological research funds of FOCICYT of University Tecnica Estatal of Quevedo (Ecuador).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Quevedo State Technical University.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available on request from the corresponding author.
Acknowledgments
We want to thank RED-RIIDPA, the open research and innovation network for the development of alternative aquaculture productions between the University Técnica Estatal of Quevedo (Ecuador), the University of Cordoba (Spain), and the Agricultural Polytechnic of Manabi (Ecuador).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schluter, D. The Ecology of Adaptative Radiation; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Ndiwas, T.C.; Nyingi, D.W.; Claude, J.; Agnése, J.F. Morphological variation of wild populations of Nile tilapia (Oreochromis niloticus) living in extreme environmental conditions in the Kenyan Rift-Valley. Environ. Biol Fish. 2016, 99, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Escanta-Molina, R.; Jiménez-Prado, P. Uso de la morfometría geométrica para establecer contrastes biológicos y ambientales en poblaciones de peces del río Teaone. Rev. Cient. Hallazgos 2019, 4, 55–69. [Google Scholar]
- Collyer, M.L.; Stockwell, C.A.; Adams, D.C.; Reiser, M.H. Phenotypic plasticity and contemporary evolution in introduced populations: Evidence from translocated populations of white sands pupfish (Cyrpinodon tularosa). Ecol. Res. 2007, 22, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Gagnaire, P.A.; Normandeau, E.; Pavey, S.A.; Bernatchez, L. Mapping phenotypic, expression and transmission ratio distortion QTL using RAD markers in the Lake Whitefish (Coregonus clupeaformis). Mol. Ecol. 2013, 22, 3036–3048. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mieles, G.; Irvine, K.; Griensven, A.V.; Arias-Hidalgo, M.; Torres, A.; Mynett, A.E. Relationships between aquatic biotic communities and water quality in a tropical river–wetland system (Ecuador). Environ. Sci. Policy 2013, 34, 115–127. [Google Scholar] [CrossRef]
- Aguilar, C.; González-Sansón, G.; Cabrera, Y.; Ruiz, A.; Allen Curry, R. Inter-habitat variation in density and size composition of reef fishes from the Cuban Northwestern shelf. Rev. Biol. Trop. 2014, 62, 589–602. [Google Scholar]
- Kerezsy, A.; Arthington, A.H.; Balcombe, S.R. Fish Distribution in Far Western Queensland, Australia: The Importance of Habitat, Connectivity and Natural Flows. Diversity 2014, 6, 380–395. [Google Scholar] [CrossRef] [Green Version]
- Arceo-Carranza, D.; Gamboa, E.; Teutli-Hernández, C.; Badillo-Alemán, M.; Herrera-Silveira, J.A. Los peces como indicador de restauración de áreas de manglar en la costa norte de Yucatán. Rev. Mex. Biodivers. 2016, 87, 489–496. [Google Scholar] [CrossRef] [Green Version]
- FAO. El Estado de la Biodiversidad Para la Alimentación y la Agricultura; Comisión de Recursos Genéticos Para la Alimentación y la Agricultura; Evaluaciones: Rome, Italy, 2019. [Google Scholar]
- FAO. Realización de Encuestas y Seguimiento de los Recursos Zoogenéticos; Directrices FAO, Producción y Sanidad Animal: Rome, Italy, 2012. [Google Scholar]
- FAO. Plan de Acción Mundial Sobre los Recursos Zoogenéticos y la Declaración de Interlaken; FAO: Rome, Italy, 2007. [Google Scholar]
- Gonzalez-Martinez, A.; De-Pablos-Heredero, C.; González, M.; Rodriguez, J.; Barba, C.; García, A. Usefulness of discriminant analysis in the morphometric differentiation of six native freshwater species from Ecuador. Animals 2021, 11, 111. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Lopez, M.; Molero, H.M.; Rodriguez, J.; González, M.; Barba, C.; García, A. Morphometric and Meristic Characterization of Native Chame Fish (Dormitator latifrons) in Ecuador Using Multivariate Analysis. Animals 2020, 10, 1805. [Google Scholar] [CrossRef]
- Cocha-Alulema, A.P. Análisis de la Variación Morfológica de Hoplias Malabaricus (Bloch 1794), Asociada al Tipo de Hábitat Utilizando Morfometría Geométrica. BSc, Facultad de Ciencias Biológicas, Universidad Central del Ecuador: Quito, Ecuador, 2018. Available online: http://www.dspace.uce.edu.ec/handle/25000/15123 (accessed on 14 June 2021).
- Dauda, A.; Abbaya, H.Y.; Ebegbulem, V.N. Application of multifactorial discriminant analysis of morphostructural differentiation of sheep. J. Genet. Genet. Eng. 2018, 2, 11–16. [Google Scholar]
- Siddik, M.A.B.; Hanif, M.A.; Chaklader, M.R.; Nahar, A.; Mahmud, S. Fishery biology of gangetic whiting Sillaginopsis panijus (Hamilton, 1822) endemic to Ganges delta, Bangladesh, Egypt. J. Aquat. Res. 2015, 41, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Hanif, M.A.; Siddik, M.A.B.; Islam, M.A.; Chaklader, M.R.; Nahar, A. Multivariate morphometric variability in sardine, Amblygaster clupeoides (Bleeker, 1849), from the Bay of Bengal coast, Bangladesh. JoBAZ 2019, 80, 53. [Google Scholar] [CrossRef]
- Bilikis Iyabo, U. Length weight relationship, condition factor and diet composition of brycinus nurse (characiformes: Alestidae) in a tropical flood river basin. Cont. J. Fish. Aquat. Sci. 2014, 8, 25–34. [Google Scholar]
- Mazon Paredes, E.; Herrera Rodriguez, M.; Mazon Paredes, C.; Garcia Martinez, A.; Delgado Pertinez, M.; Guzman Guerrero, J.L. Bromatological composition of palm kernel meal according to its origin and production periods potential use of palm kernel meal in animal feed. J. Oil Palm Res. 2020, 32, 639–646. [Google Scholar]
- Teimori, A.; Schulz-Mirbach, T.; Esmaeili, H.R.; Reichenbacher, B. Geographical differentiation of Aphanius dispar (Teleostei: Cyprinodontidae) from southern Iran. J. Zool. Syst. Evol. Res. 2012, 50, 289–304. [Google Scholar] [CrossRef]
- Aguirre, W.W.; Shervette, V.R.; Navarrete, R.; Calle, P.; Agorastos, S. Morphological and Genetic Divergence of Hoplias microlepis (Characiformes: Erythrinidae) in Rivers and Artificial Impoundments of Western Ecuador. Copeia 2013, 2, 312–323. [Google Scholar] [CrossRef]
- Sobczuk, D.; Komosa, M. Morphological differentiation of polish Arabian horses-multivariate analysis. Bull. Vet. Inst. Pulawy 2012, 56, 623–629. [Google Scholar] [CrossRef] [Green Version]
- N’goran, K.E.; Kouassi, N.C.; Loukou, N.E.; Dayo, G.S.M.; Yapi-Gnaore, C.V. Multivariate analysis for morphological caracteristics of N’Dama cattle breed in two agroecological zones of Côte d’Ivoire. Eur. Sci. J. 2018, 14, 602–621. [Google Scholar]
- Hanif, M.A.; Siddik, M.A.B.; Chaklader, M.R.; Pham, H.D.; Kleindienst, R. Length-weight relationships of three catfish species from a tributary of the Dhaleshwari River, Bangladesh. J. Appl. Ichthyol. 2017, 33, 1261–1262. [Google Scholar] [CrossRef]
- Islam, M.A.; Siddik, M.A.B.; Hanif, M.A.; Chaklader, M.R.; Nahar, A.; Ilham, I. Length–weight relationships of four small indigenous fish species from an inland artisanal fishery, Bangladesh. J. Appl. Ichthyol. 2017, 33, 851–852. [Google Scholar] [CrossRef]
- Chaklader, M.R.; Siddik, M.A.B.; Hanif, M.A.; Nahar, A.; Sultan, M.; Piria, M. Morphometric and meristic variation of endangered Pabda catfish, Ompok pabda (Hamilton-Buchanan, 1822) from southern coastal waters of Bangladesh. Pak. J. Zool. 2016, 48, 681–687. [Google Scholar]
- Yılmaz, S.; Yazıcıoğlu, O.; Erbaşaran, M.; Esen, S.; Zengin, M.; Polat, N. Length-weight relationship and relative condition factor of white bream, Blicca bjoerkna (L., 1758), from Lake Ladik, Turkey. J. Black Sea Medit. Environ. 2012, 18, 380–387. [Google Scholar]
- Barriga, R. Peces de los Afluentes de la Costa de Ecuador. In Cuencas Pericontinentales de Colombia, Ecuador, Perú y Venezuela; Serie Editorial Recursos Hidrobiológicos y Pesqueros Continentales de Colombia; Instituto de Investigación de Recursos Biológicos Alexander Von Humboldt: Bogotá, Colombia, 2015. [Google Scholar]
- Rodríguez, J.; González, A.; Angón, E.; Vivas, R.; Barba, C.; González, M.A.; Peña, F.; García, A. Efecto del tamaño de las reproductoras en la producción de alevines de Cichlasoma festae en condiciones semicontroladas en Ecuador. ITEA 2020, 116, 93–105. [Google Scholar] [CrossRef]
- Jiménez-Prado, P.; Aguirre, W.; Laaz-Moncayo, E.; Navarrete-Amaya, R.; Nugra-Salazar, F.; Rebolledo-Monsalve, E.; Zárate-Hugo, E.; Torres-Noboa, A.; Valdiviezo-Rivera, J. Guía de Peces Para Aguas Continentales en la Vertiente Occidental del Ecuador; Pontificia Universidad Católica del Ecuador Sede Esmeraldas (PUCESE); Universidad del Azuay (UDA) y Museo Ecuatoriano de Ciencias Naturales (MECN) del Instituto Nacional de Biodiversidad: Esmeraldas, Ecuador, 2015; p. 416. [Google Scholar]
- Barnhill, B.; Lopez, E.; Les, A. Estudio Sobre Biología de los Peces del Río Vinces; Instituto Nacional de Pesca de Ecuador, Boletín Científico Técnico, 1974; Volume 3. Available online: http://institutopesca.gob.ec/wp-content/uploads/2017/07/BCT.-VOL.-3-1.pdf (accessed on 14 June 2021).
- Revelo, W.; Laaz, E. Catálogo de peces de aguas continentales de la provincia de los Ríos-Ecuador. Bol. Espec. 2012, 3, 1–56. [Google Scholar]
- Valdiviezo-Rivera, J.; Garzón-Santomaro, C.; Inclán-Luna, D.; Mena_Jaén, J.; González-Romero, D. Ecosistemas Dulceacuícolas de la Provincia de El Oro: Peces y Macroinvertebrados Acuáticos como Indicadores Biológicos del Páramo al Manglar; Publicación Miscelánea N° 10: Serie de Publicaciones GADPEO-INABIO: Quito, Ecuador, 2018. [Google Scholar]
- Ortega, H.; Hidalgo, M.; Correa, E.; Espino, J.; Chocano, L.; Trevejo, G.; Meza Cortijo, A.M.; Quispe, R. Lista Anotada de los Peces de Aguas Continentales del Perú: Estado Actual del Conocimiento, Distribución, Usos y Aspectos de Conservación; Ministerio del Ambiente, Dirección General de Diversidad Biológica-Museo de Historia Natural, UNMSM: Lima, Peru, 2011; p. 48. [Google Scholar]
- Triviño, J.L. Características Morfométricas, Merísticas, Físicas y Químicas Del Pescado Ratón Silvestre (Leporinus Ecuadorensis) en la Zona de Babahoyo-2017; Proyecto de Investigación; Universidad Técnica Estatal de Quevedo: Quevedo, Ecuador, 2017. [Google Scholar]
- Revelo, W. Aspectos biológicos y pesqueros de las principales especies de peces en el sistema hídrico de la provincia de los Ríos, durante 2009. Bol. Cient. Tec. 2010, 20, 53–84. Available online: https://aquadocs.org/bitstream/handle/1834/4790/2.%20PUBLICACION%202009%20AGUA%20DULCE.pdf?sequence=1 (accessed on 14 June 2021).
- Ferrito, V.; Mannino, M.C.; Pappalardo, A.M.; Tigano, C. Morphological variation among populations of Aphanius fasciatus Nardo, 1827 (Teleostei, Cyprinodontidae) from the Mediterranean. J. Fish Biol. 2007, 70, 1–20. [Google Scholar] [CrossRef]
- Mir, F.A.; Mir, J.I.; Chandra, S. Phenotypic variation in the Snowtrout Schizothorax richardsonii (Gray, 1832) (Actinopterygii: Cypriniformes: Cyprinidae) from the Indian Himalayas. Contrib. Zool. 2013, 82, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, S.; Moqbul Hossain, M.D.; Huq, M.; Wheeler, D. Facing the hungry tide: Climate change, livelihood threats, and household responses in coastal Bangladesh. Clim. Change Econ. 2016, 7, 1650007. [Google Scholar] [CrossRef]
- Hossain, M.; Nahiduzzaman, M.; Saha, D.; Khanam, M.; Alam, M. Landmark-based morphometric and meristic variations of the endangered carp, Kalibaus labeocalbasu, from stocks of two isolated Rivers, the Jamuna and Halda and a hatchery. Zool. Stud. 2010, 49, 556–563. [Google Scholar]
- García, S.M.; Kolding, J.; Rice, J.; Rochet, M.J.; Zhou, S.; Arimoto, T.; Beyer, J.E.; Borges, L.; Bundy, A.; Dunn, D.; et al. Reconsidering the consequences of selective fisheries. Science 2012, 335, 1045–1047. [Google Scholar] [CrossRef] [Green Version]
- Ujjania, N.C.; Kohli, M.P.S. Landmark-based morphometric analysis for selected species of indian major carp (catla catla.; ham. 1822). Int. J. Food Agric. Vet. Sci. 2011, 1, 64–74. [Google Scholar]
- Quiceno-Cuartas, P.; Palacio-Baena, J.; Escobar-Cardona, J.; Werding, B. Parámetros de crecimiento individual de Petrolisthes caribensis (Werding, 1983) y Petrolisthes galathinus (Bosc, 1902) (Decapoda: Porcellanidae) en el golfo de Morrosquillo, Caribe colombiano. Actual. Biológicas 2014, 36, 163–171. [Google Scholar]
- Torres, B.; Vasseur, L.; López, R.; Lozano, P.; García, Y.; Arteaga, Y.; Bravo, C.; Barba, C.; Garcia, A. Structure and above ground biomass along an elevation small-scale gradient: Case study in an Evergreen Andean Amazon forest, Ecuador. Agrofor. Syst. 2020, 94, 1235–1245. [Google Scholar] [CrossRef]
- Gonzáles, A.; Acosta, J.; Andrade, S. Evaluación de las inundaciones de la cuenca baja del Guayas, datos y manejo. CLIRSEN. In Proceedings of the XI Congreso Ecuatoriano de la Ciencia del Suelo, Quito, Ecuador, 29–31 October 2008. [Google Scholar]
- Gobierno Provincial de Manabí. Informe Ambiental del Area Provincial de Recreación Ecológica Cascadas El Armadillo—El Pintado; Gobierno Provincial de Manabí: Portoviejo, Ecuador, 2018. [Google Scholar]
- Robin, R.R. Caracterización de la Calidad del agua para Consumo Doméstico del río Quevedo en el Cantón Quevedo, Provincia de los Ríos; BSc, University of Guayaquil: Guayaquil, Ecuador, 2015. [Google Scholar]
- Loor Castillo, E.P. Calidad del agua para la producción piscícola en el proceso de captación en la Comunidad de Pajarito del Cantón Mocache; Research Proyect, Statal University of Quevedo: Quevedo, Ecuador, 2018. [Google Scholar]
- Pacheco Bedoya, J.L. Aspectos Biológicos y Pesqueros de las Principales Especies Capturadas en el Río Babahoyo y Afluentes en el Cantón Samborondón de la Provincia del Guayas. Available online: http://www.institutopesca.gob.ec/wp-content/uploads/2018/01/Aspectos.-Biol%C3%B3gicos-y-Pesqueros-R%C3%ADo-Babahoyo-y-Afluentes-Cant%C3%B3n-Samborond%C3%B3n-2015-2.pdf (accessed on 11 May 2021).
- FAO. Perfiles Sobre la Pesca y la Acuicultura por Países La Republica de Ecuador; FAO; Fisheries and Aquaculture Department: Rome, Italy, 2013. [Google Scholar]
- MAGAP. Aspectos Pesqueros de las Principales Especies Capturadas en el Embalse Parque Lago Chongón; MAGAP, Instituto Nacional de Pesca: Guayaquil, Ecuador, 2008–2013.
- Ochoa Ubilla, B.Y.; Mendoza Nieto, K.X.; Vivas Moreira, R.; Zambrano, J.U.; Ferrer-Sánchez, Y. Ecuador Structure of catch sizes and length-weight ratio of native fish in the Abras de Mantequilla wetland, Ecuador. Cient. Tecnol. UTEQ 2016, 9, 19–27. (In Spanish) [Google Scholar]
- Diodatti, F.C.; Fonseca de Freitas, R.T.; Freato, T.A.; Pérez Ribeiro, P.A.; Solis Murgas, L.D. Parámetros morfométricos en el rendimiento de los componentes corporales de tilapia del Nilo (Oreochromis niloticus). An. Vet. Murcia 2008, 24, 45–55. [Google Scholar]
- González, M.A.; Rodríguez, J.M.; Angón, E.; Martínez, A.; García, A.; Peña, F. Characterization of morphological and meristic traits and their variations between two different populations (wild and cultured) of Cichlasoma festae, a species native to tropical Ecuadorian rivers. Arch. Anim. Breed. 2016, 59, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Caez, J.; González, A.; González, M.A.; Angón, E.; Rodríguez, J.M.; Peña, F.; Barca, C.; García, A. Application of multifactorial discriminant analysis in the morphostructural differentiation of wild and cultured populations of Vieja Azul (Andinoacara rivulatus). Turk. J. Zool. 2019, 43, 516–530. [Google Scholar] [CrossRef]
- Nash, R.R.M.; Valencia, A.H.; Geffen, A.J. The origin of Fulton’s condition factor-setting the record straight. Fisheries 2006, 31, 236–238. [Google Scholar]
- Elliott, N.G.; Haskard, K.; Koslow, J.A. Morphometric analysis of orange roughy (Hoplostethus atlanticus) off the continental slope of southern Australia. J. Fish Biol. 1995, 46, 202–220. [Google Scholar] [CrossRef]
- Revelo, W.; Castro, R. Aspectos Biológicos y Pesqueros de las Principales Especies de Peces en el Sistema Hídrico de la Provincia de los Ríos, Durante 2010; Instituto Nacional de Pesca: Guayaquil, Ecuador, 2010; p. 28. [Google Scholar]
- Choi, J.-Y.; Kim, S.-K. Effects of Aquatic Macrophytes on Spatial Distribution and Feeding Habits of Exotic Fish Species Lepomis macrochirus and Micropterus salmoides in Shallow Reservoirs in South Korea. Sustainability 2020, 12, 1447. [Google Scholar] [CrossRef] [Green Version]
- Prado, M.; Bucheli, R.; Calderón, G. Composición, distribución y abundancia del plancton en sistemas fluviales de la provincia de Los Ríos-Ecuador. Bol. Cient. Tec. 2010, 20, 1–51. [Google Scholar]
- Zhang, H.; Kang, M.; Wu, J.; Wang, C.; Li, J.; Du, H.; Yang, H.; Wei, O.V. Increasing River Temperature Shifts Impact the Yangtze Ecosystem: Evidence from the Endangered Chinese Sturgeon. Animals 2019, 9, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barriga, R. Lista de peces de agua dulce e intermareales del Ecuador. Rev. Politec. 2012, 30, 83–119. [Google Scholar]
- Canonico, G.C.; Arthington, A.; McCrary, J.K.; Thieme, M.L. The effects of introduced tilapias on native biodiversity. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 15, 463–483. [Google Scholar] [CrossRef]
- Aguirre, W.E.; Navarrete, R.; Malato, G.; Calle, P.; Loh, M.K.; Vital, W.F.; Granda, J.C. Body shape variation and population genetic structure of Rhoadsia altipinna (Characidae: Rhoadsiinae) in southwestern Ecuador. Copeia 2016, 104, 554–569. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichth. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Trudel, M.; Tucker, S.; Morris, J.; Higgs, D.; Welch, D. Indicators of energetic status in juvenile coho and chinook salmon. N. Am. J. Fish. Manag. 2005, 25, 374–390. [Google Scholar] [CrossRef]
- Rennie, M.D.; Verdon, R. Evaluation of condition indices for the lake whitefish, Coregonus clupeaformis. N. Am. J. Fish. Manag. 2008, 28, 1270–1293. [Google Scholar] [CrossRef]
- Youson, J.H.; Holmes, J.A.; Guchardi, J.; Seelye, J.G.; Beaver, R.E.; Gersmehl, J.E.; Sower, S.A.; Beamish, F.W.H. The importance of condition factor and the influence of water temperature and photoperiod in metamorphosis of sea lampreys. Petromyzon marinus. Can. J. Fish Aquat. Sci. 1993, 50, 2448–2456. [Google Scholar] [CrossRef]
- Ekaete, A. Preliminary studies of the condition factors in five tropical fish species of a coastal state, Lagos Nigeria. Researcher 2013, 5, 1–5. [Google Scholar]
- Melvin, G.D.; Dadswell, M.J.; McKenzie, A. Usefulness of meristic and morphometric characters in discriminating populations of American shad (Alosa sapidissima) (Ostreichthys: Clupeidae) inhabiting a marine environment. Can. J. Fish. Aquat. Sci. 1992, 49, 266–280. [Google Scholar] [CrossRef]
- Caldecutt, W.C.; Adams, D.C. Morphometrics of trophic osteology in the three spine stickleback, Gasterosteus aculeatus. Copeia 1998, 4, 827–838. [Google Scholar] [CrossRef]
- Cavalcanti, M.J.; Rabello Monteiro, L.; Duarte Lopes, P.R. Landmark-based Morphometric Analysis in Selected Species of Serranid Fishes (Perciformes: Teleostei). Zoo. Stud. 1999, 38, 287–294. [Google Scholar]
- Olaya Carbó, P. Estado ecológico del sistema estuarino del Río Guayas, Cantón Durán, Ecuador: Simulación numérica de su dinámica fluvial y principios ecológicos para el diseño de actuaciones de restauración y/o recuperación. Master’s Thesis. Universidad de Alcalá, Universidad Complutense de Madrid, Universidad Rey Juan Carlos, Universidad Politécnica de Madrid, Madrid, Spain, 2016. [Google Scholar]
- Ansah, Y.B.; Frimpong, E.A.; Hallerman, E.M. Genetically-Improved Tilapia Strains in Africa: Potential Benefits and Negative Impacts. Sustainability 2014, 6, 3697–3721. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Lendech, N.; Martínez-Falcón, A.P.; Schmitter-Soto, J.J.; Mejía-Mojica, H.; Sorani-Dalbón, V.; Cruz-Ruíz, G.I.; Mercado-Silva, N. Ichthyological Differentiation and Homogenization in the Pánuco Basin, Mexico. Diversity 2020, 12, 187. [Google Scholar] [CrossRef]
- De Sosa, E.; De Paula, F. Spatial and temporal hydrochemical variation of a third order river network in a quasi pristine coastal watershed, at southern Bahia, Brazil. Ann. Braz. Acad. Sci. 2013, 85, 1357–1370. [Google Scholar]
- Laaz Moncayo, E.; Torres Noboa, A. Lista de Peces continentales de la Cuenca del Río Guayas. 2014. Available online: https://condor.depaul.edu/waguirre/fishwestec/lista_peces_guayasv2.pdf (accessed on 9 June 2021).
- Brraich, O.S.; Akhter, S. Morhometric characters and meristic Counts of a Fish, Crossocheilus latius latius (Hamilton-Buchanan) from Ranjit Sagar Wetland, India. Int. J. Fish. Aquat. Stud. 2015, 2, 260–265. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).