High Drug Resistance in Feline Mammary Carcinoma Cell Line (FMCm) and Comparison with Human Breast Cancer Cell Line (MCF-7)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Treatments
2.3. Cell Viability Assays
2.4. Statistical Analysis
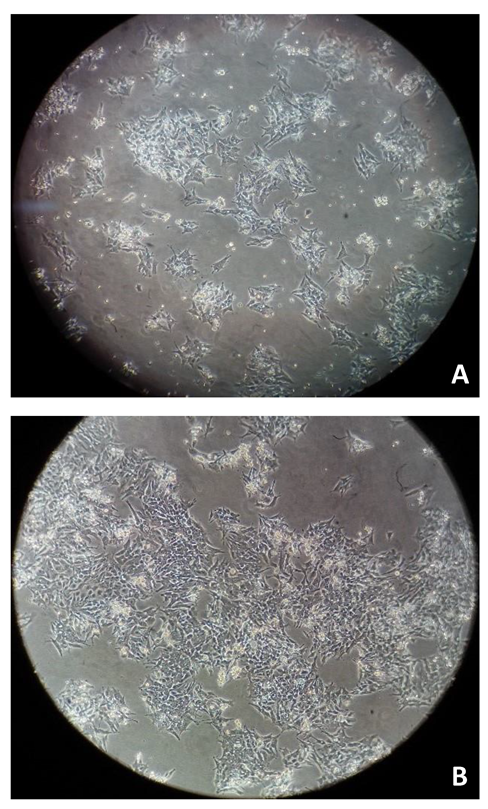
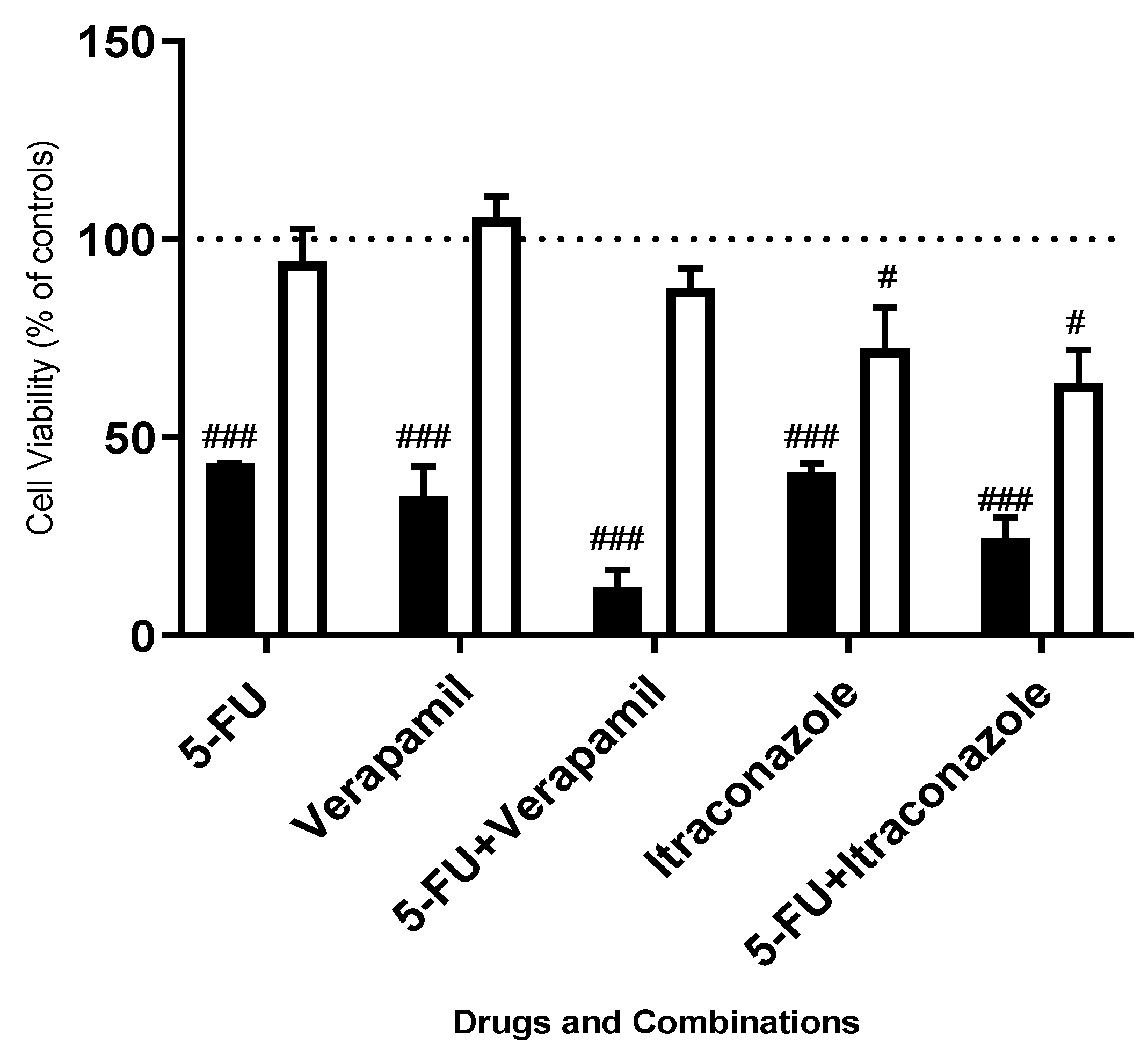
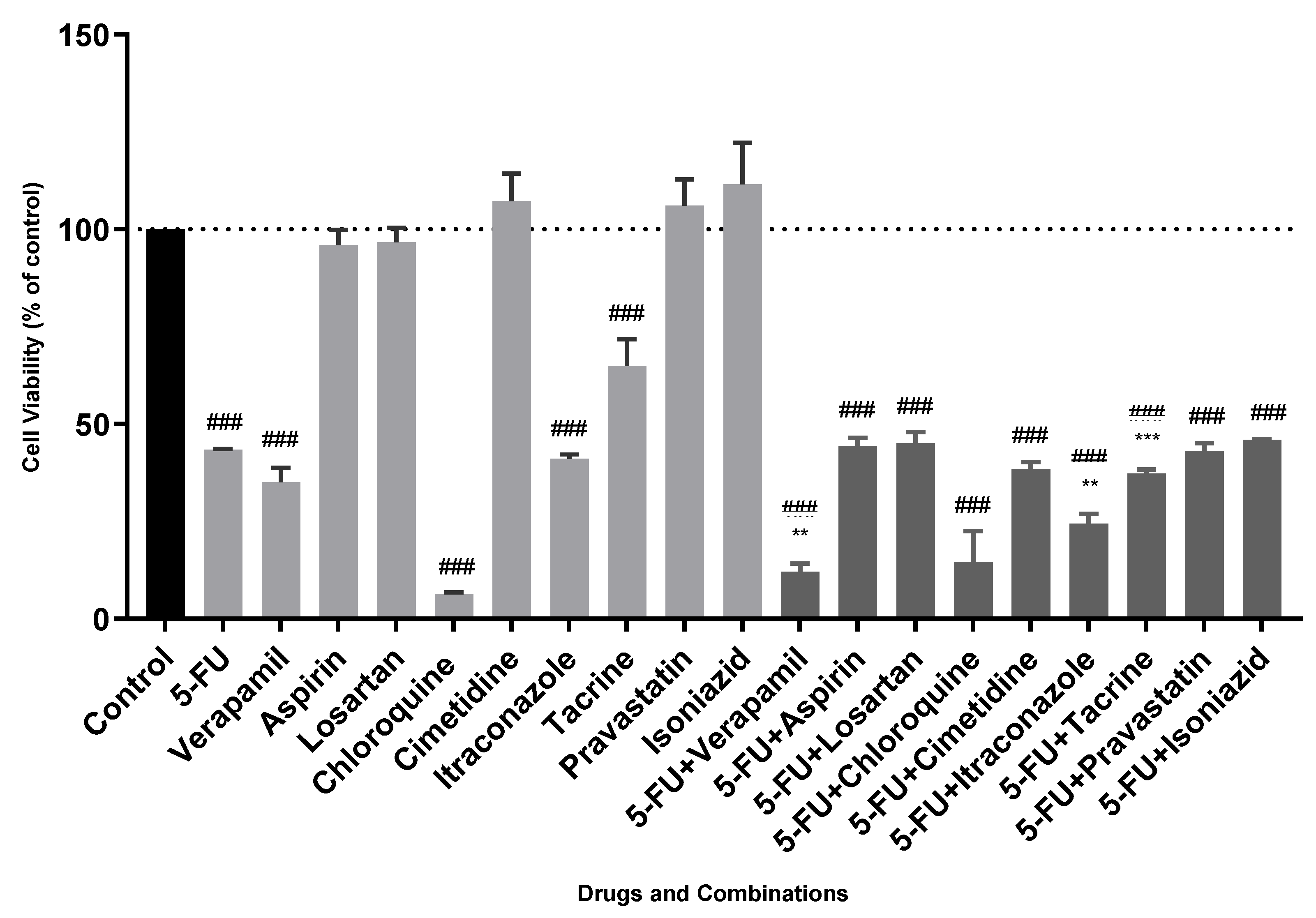
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, L.; Li, W.; Chen, C.S. Breast cancer animal models and applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Beltrán Hernández, I.; Kromhout, J.Z.; Teske, E.; Hennink, W.E.; Van Nimwegen, S.A.; Oliveira, S. Molecular targets for anticancer therapies in companion animals and humans: What can we learn from each other? Theranostics 2021, 11, 3882–3897. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; De Zan, G.; Cardazzo, B.; Bargelloni, L.; Castagnaro, M. Feline mammary tumours in comparative oncology. J. Dairy Res. 2005, 72, 98–106. [Google Scholar] [CrossRef]
- Feline Mammary Tumors|Veterinary Medical Center. Available online: https://vet.osu.edu/vmc/companion/our-services/oncology-and-hematology/common-tumor-types/feline-mammary-tumors (accessed on 27 May 2021).
- Almeida, F.; Gameiro, A.; Correia, J.; Ferreira, F. Histone Deacetylase Inhibitors and Microtubule Inhibitors Induce Apoptosis in Feline Luminal Mammary Carcinoma Cells. Animals 2021, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Figueira, A.C.; Gomes, C.; Mendes, N.; Amorim, I.; De Matos, A.J.F.; Dias-Pereira, P.; Gärtner, F. An in vitro and in vivo characterization of the cadherin-catenin adhesion complex in a feline mammary carcinoma cell line. Clin. Diagn. Pathol. 2016, 1. [Google Scholar] [CrossRef]
- Correia, A.; Silva, D.; Correia, A.; Vilanova, M.; Gärtner, F.; Vale, N. Study of new therapeutic strategies to combat breast cancer using drug combinations. Biomolecules 2018, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.; Konishi, M.; Ebner, N.; Springer, J. Repurposing of approved cardiovascular drugs. J. Transl. Med. 2016, 14, 269. [Google Scholar] [CrossRef]
- Bertolini, F.; Sukhatme, V.P.; Bouche, G. Drug repurposing in oncology—Patient and health systems opportunities. Nat. Rev. Clin. Oncol. 2015, 12, 732–742. [Google Scholar] [CrossRef]
- Han, K.; Jeng, E.E.; Hess, G.T.; Morgens, D.W.; Li, A.; Bassik, M.C. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions. Nat. Biotechnol. 2017, 35, 463–474. [Google Scholar] [CrossRef]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, 149. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, F.; Ma, X.; Cao, Z.W.; Li, Y.X.; Chen, Y.Z. Mechanisms of drug combinations: Interaction and network perspectives. Nat. Rev. Drug Discov. 2009, 8, 111–128. [Google Scholar] [CrossRef]
- Comprehensive Cancer Information-National Cancer Institute. Available online: https://www.cancer.gov/ (accessed on 27 May 2021).
- DrugBank Online|Database for Drug and Drug Target Info. Available online: https://go.drugbank.com/ (accessed on 23 July 2021).
- Li, P.; Zhong, D.; Gong, P. Synergistic effect of paclitaxel and verapamil to overcome multi-drug resistance in breast cancer cells. Biochem. Biophys. Res. Commun. 2019, 516, 183–188. [Google Scholar] [CrossRef]
- Cheng, R.; Liu, Y.-J.; Cui, J.-W.; Yang, M.; Liu, X.-L.; Li, P.; Wang, Z.; Zhu, L.-Z.; Lu, S.-Y.; Zou, L.; et al. Aspirin regulation of c-myc and cyclinD1 proteins to overcome tamoxifen resistance in estrogen receptor-positive breast cancer cells. Oncotarget 2017, 8, 30252. [Google Scholar] [CrossRef] [PubMed]
- Coulson, R.; Liew, S.H.; Connelly, A.A.; Yee, N.S.; Deb, S.; Kumar, B.; Vargas, A.C.; O’Toole, S.A.; Parslow, A.C.; Poh, A.; et al. The angiotensin receptor blocker, Losartan, inhibits mammary tumor development and progression to invasive carcinoma. Oncotarget 2017, 8, 18640–18656. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Abadía, B.; Morán-Zendejas, R.; Rangel-Flores, J.M.; Rodríguez-Menchaca, A.A. Chloroquine inhibits tumor-related Kv10.1 channel and decreases migration of MDA-MB-231 breast cancer cells in vitro. Eur. J. Pharmacol. 2019, 855, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Neoplasma. 2003, Volume 50, pp. 148–151. Available online: http://www.elis.sk/index.php?page=shop.product_details&flypage=flypage.tpl&product_id=889&category_id=31&option=com_virtuemart&vmcchk=1&Itemid=1 (accessed on 23 July 2021).
- Wang, X.; Wei, S.; Zhao, Y.; Shi, C.; Liu, P.; Zhang, C.; Lei, Y.; Zhang, B.; Bai, B.; Huang, Y.; et al. Anti-proliferation of breast cancer cells with itraconazole: Hedgehog pathway inhibition induces apoptosis and autophagic cell death. Cancer Lett. 2017, 385, 128–136. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, D.; Yang, Z.; Yang, J.; Zhang, R.; Yang, X.; Wang, M.; Wang, Y. Repurposing antitubercular agent isoniazid for treatment of prostate cancer. Biomater. Sci. 2018, 7, 296–306. [Google Scholar] [CrossRef]
- Sánchez, C.A.; Rodriguez, E.; Varela, E.; Zapata, E.; Páez, A.; Massó, F.A.; Montaño, L.F.; Maure-Lóopez, R. Statin-induced inhibition of MCF-7 breast cancer cell proliferation is related to cell cycle arrest and apoptotic and necrotic cell death mediated by an enhanced oxidative stress. Cancer Investig. 2008, 26, 698–707. [Google Scholar] [CrossRef]
- Silva, S.; Alves, C.; Duarte, D.; Costa, A.; Sarmento, B.; Almeida, A.J.; Gomes, P.; Vale, N. Model Amphipathic Peptide Coupled with Tacrine to Improve Its Antiproliferative Activity. Int. J. Mol. Sci. 2020, 22, 242. [Google Scholar] [CrossRef]
- Uyama, R.; Hong, S.H.; Nakagawa, T.; Yazawa, M.; Kadosawa, T.; Mochizuki, M.; Tsujimoto, H.; Nishimura, R.; Sasaki, N. Establishment and characterization of eight feline mammary adenocarcinoma cell lines. J. Vet. Med. Sci. 2005, 67, 1273–1276. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Hassan, B.B.; Elshafae, S.M.; Supsavhad, W.; Simmons, J.K.; Dirksen, W.P.; Sokkar, S.M.; Rosol, T.J. Feline Mammary Cancer: Novel Nude Mouse Model and Molecular Characterization of Invasion and Metastasis Genes. Vet. Pathol. 2017, 54, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Albergaria, A.; Sousa, B.; Correia, A.L.; Bracke, M.; Seruca, R.; Schmitt, F.C.; Paredes, J. Extracellular cleavage and shedding of P-cadherin: A mechanism underlying the invasive behaviour of breast cancer cells. Oncogene 2010, 29, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Sousa, B.; Carreto, L.; Mendes, N.; Nobre, A.R.; Ricardo, S.; Albergaria, A.; Cameselle-Teijeiro, J.F.; Gerhard, R.; Söderberg, O.; et al. P-cadherin functional role is dependent on E-cadherin cellular context: A proof of concept using the breast cancer model. J. Pathol. 2013, 229, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Y.; Sun, X.; Feng, Y.; Du, Y.; Liu, F.; Yu, C.; Jin, F. Chloroquine (CQ) exerts anti-breast cancer through modulating microenvironment and inducing apoptosis. Int. Immunopharmacol. 2017, 42, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Diasio, R.B.; Harris, B.E. Clinical Pharmacology of 5-Fluorouracil. Clin. Pharmacokinet. 1989, 16, 215–237. [Google Scholar] [CrossRef]
- Wohlhueter, R.M.; McIvor, R.S.; Plagemann, P.G.W. Facilitated transport of uracil and 5-fluorouracil, and permeation of orotic acid into cultured mammalian cells. J. Cell. Physiol. 1980, 104, 309–319. [Google Scholar] [CrossRef]
- Vieira, A.F.; Paredes, J. P-cadherin and the journey to cancer metastasis. Mol. Cancer 2015, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, A.M.; Na, T.Y.; Gumbiner, B.M. E-cadherin in contact inhibition and cancer. Oncogene 2018, 37, 4769–4780. [Google Scholar] [CrossRef]
- Verbaanderd, C.; Maes, H.; Schaaf, M.B.; Sukhatme, V.P.; Pantziarka, P.; Sukhatme, V.; Agostinis, P.; Bouche, G. Repurposing Drugs in Oncology (ReDO)—chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience 2017, 11. [Google Scholar] [CrossRef]

| Drug | Indication | Pharmacodynamics | Mechanism of Action | Examples of Evidence in Breast Cancer |
|---|---|---|---|---|
| Verapamil | Angina Cardiac arrhythmias Cardiomyopathies Hypertension | Blocks calcium channels. | Inhibits calcium channels of L-type by binding to a specific area of their alpha-1 subunit. | Verapamil is a P-Glycoprotein inhibitor and leads to enhanced sensitivity levels of breast cancer cells to other drugs, such as paclitaxel [15]. |
| Aspirin | Pain Fever Inflammation Reduction of risk of myocardial infarction | Destabilizes the production of prostaglandins by targeting cyclooxygenase (COX)-1 and -2. | By binding COX enzymes, disrupts the conversion of arachidonic acid to thromboxane A2. | In MCF-7 cells, aspirin in combination with tamoxifen led to cell cycle arrest (in G0/G1 phase of cell cycle), decreasing the levels of cyclinD1 [16]. |
| Losartan | Hypertension Reduction of the risk of stroke | Blocks Angiotensin II receptor. | Impedes angiotensin II binding to the receptor AT1 (Angiotensin Receptor Type I) in tissues like the adrenal gland. | By targeting AT1, this drug inhibits breast cancer cells proliferation and the progression of the cancer to an invasive phenotype [17]. |
| Chloroquine | Infections of P. vivax, P. malariae, P. ovale, and P. falciparum. Off label for rheumatic diseases | Inhibits heme polymerase. | Inhibits heme polymerase, disrupting the conversion of heme to hemazoin, process that kills the parasite. | By blocking KCNH1 channels, this drug decreases migration of MDA-MB-231 breast cancer cells [18]. |
| Cimetidine | Acid-reflux disorders Peptic ulcer disease Heartburn Acid indigestion | Antagonizes Histamine H2-receptor. | Binds to an H2-receptor in gastric cells, blocking histamine effects. | In breast cancer cells, Cimetidine can decrease cell adhesion, presenting antimetastatic effects [19]. |
| Itraconazole | Fungal infections, such as aspergillosis and onychomycosis | Inhibits the fungal enzyme cytochrome P450 14α-demethylase. | Interacts with 14-α demethylase, necessary to convert lanosterol to ergosterol and maintain fungal viability. | Itraconazole inhibits the Hedgehog pathway, leading to elevated levels of cell death [20]. |
| Isoniazid | Mycobacterial infections, such as M. tuberculosis | Inhibits the production of bacterial mycolic acids. | In, M. tuberculosis, inhibits InhA (enoyl reductase). | Few studies in breast cancer. However, being an inhibitor of monoamine oxidase A, it may improve outcomes in prostate cancer, where elevated levels of this enzyme correlate with aggressiveness [21]. |
| Pravastatin | Hyperlipidemia, Prevention of some coronary events | Decreases the plasmatic concentration of low-density lipoprotein (LDL) cholesterol. | Inhibits HMG-CoA reductase, reducing cholesterol levels. | Statins promote cell cycle arrest and increased reactive oxygen species (ROS) production, enhancing oxidative stress [22]. |
| Tacrine | Alzheimer’s Disease | Enhances cholinergic function. | Inhibits cholinesterase enzymes, leading to prolonged action of acetylcholine. | The conjugation of this drug with model amphipathic peptide (MAP) (leads to high toxicity levels to MCF-7 cells [23]. |
| Drug | IC50 (μM) |
|---|---|
| 5-FU | 11.8 ± 1.8 |
| Verapamil | 29.5 ± 4.5 |
| Itraconazole | 2.1 ± 0.6 |
| Chloroquine | 16.0 ± 2.3 |
| Molecule | Description |
|---|---|
| P-cadherin | Cell-to-cell adhesion glycoprotein. Overexpression is connected to tumor-enhancing effects in breast cancer and other types of cancer, such as ovarian [32]. |
| E-cadherin | Mediates cell adhesion and junction formations, being a part of the adherents junctions. In a general way, loss/decrease of its expression is linked with tumor progression and metastatic lesions [33]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.S.; Matos, R.; Gärtner, F.; Amorim, I.; Vale, N. High Drug Resistance in Feline Mammary Carcinoma Cell Line (FMCm) and Comparison with Human Breast Cancer Cell Line (MCF-7). Animals 2021, 11, 2321. https://doi.org/10.3390/ani11082321
Correia AS, Matos R, Gärtner F, Amorim I, Vale N. High Drug Resistance in Feline Mammary Carcinoma Cell Line (FMCm) and Comparison with Human Breast Cancer Cell Line (MCF-7). Animals. 2021; 11(8):2321. https://doi.org/10.3390/ani11082321
Chicago/Turabian StyleCorreia, Ana Salomé, Rita Matos, Fátima Gärtner, Irina Amorim, and Nuno Vale. 2021. "High Drug Resistance in Feline Mammary Carcinoma Cell Line (FMCm) and Comparison with Human Breast Cancer Cell Line (MCF-7)" Animals 11, no. 8: 2321. https://doi.org/10.3390/ani11082321
APA StyleCorreia, A. S., Matos, R., Gärtner, F., Amorim, I., & Vale, N. (2021). High Drug Resistance in Feline Mammary Carcinoma Cell Line (FMCm) and Comparison with Human Breast Cancer Cell Line (MCF-7). Animals, 11(8), 2321. https://doi.org/10.3390/ani11082321







