L-Asparaginase, Doxorubicin, Vincristine, and Prednisolone (LHOP) Chemotherapy as a First-Line Treatment for Dogs with Multicentric Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Evaluation
2.2. Treatment Protocol
2.3. Response Assessment
2.4. Toxicity
2.5. Historical Comparison Group That Received CHOP Chemotherapy Protocol
2.6. Statistical Analysis
3. Results
3.1. LHOP Protocol
3.2. CHOP Protocol
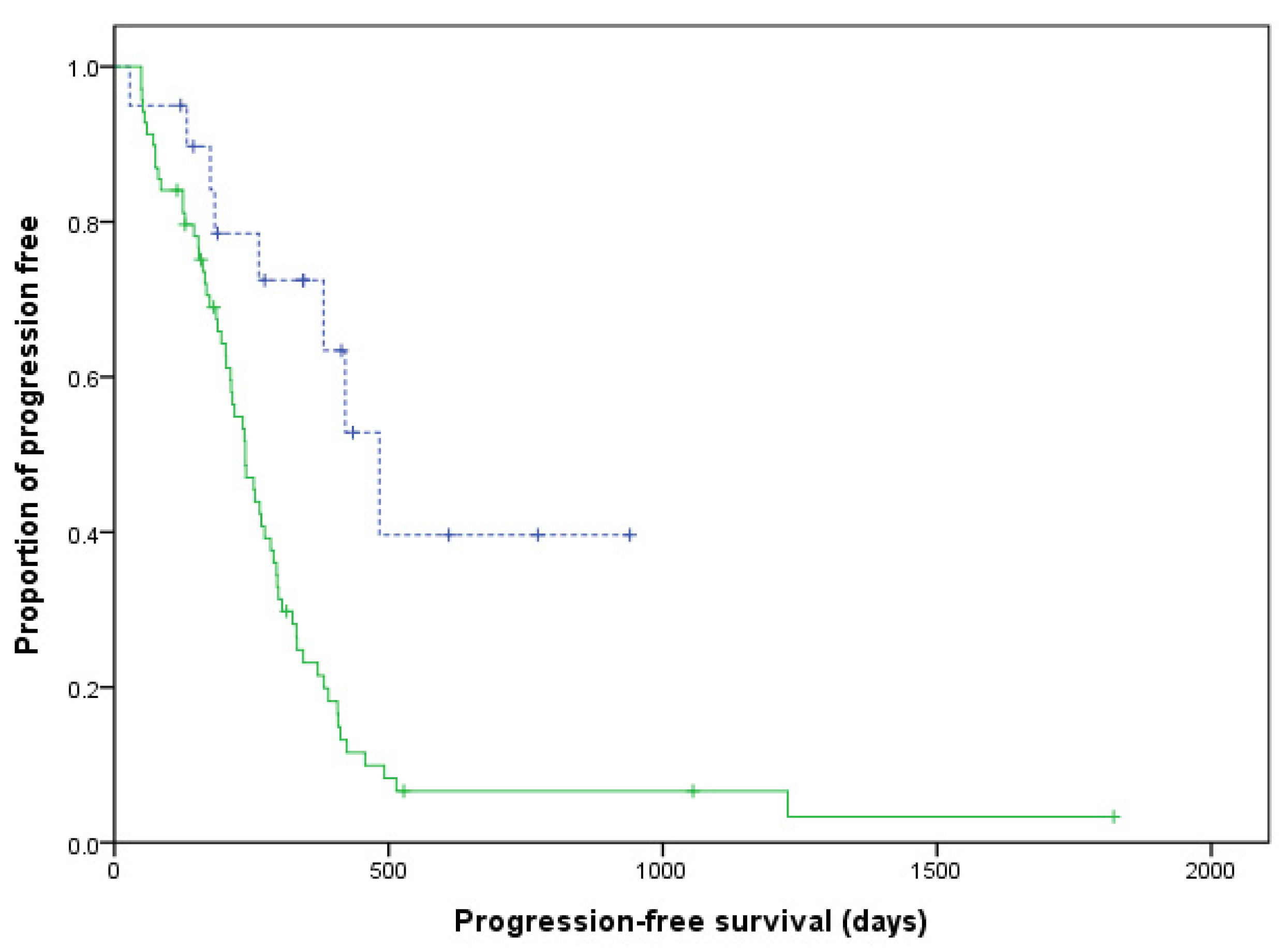
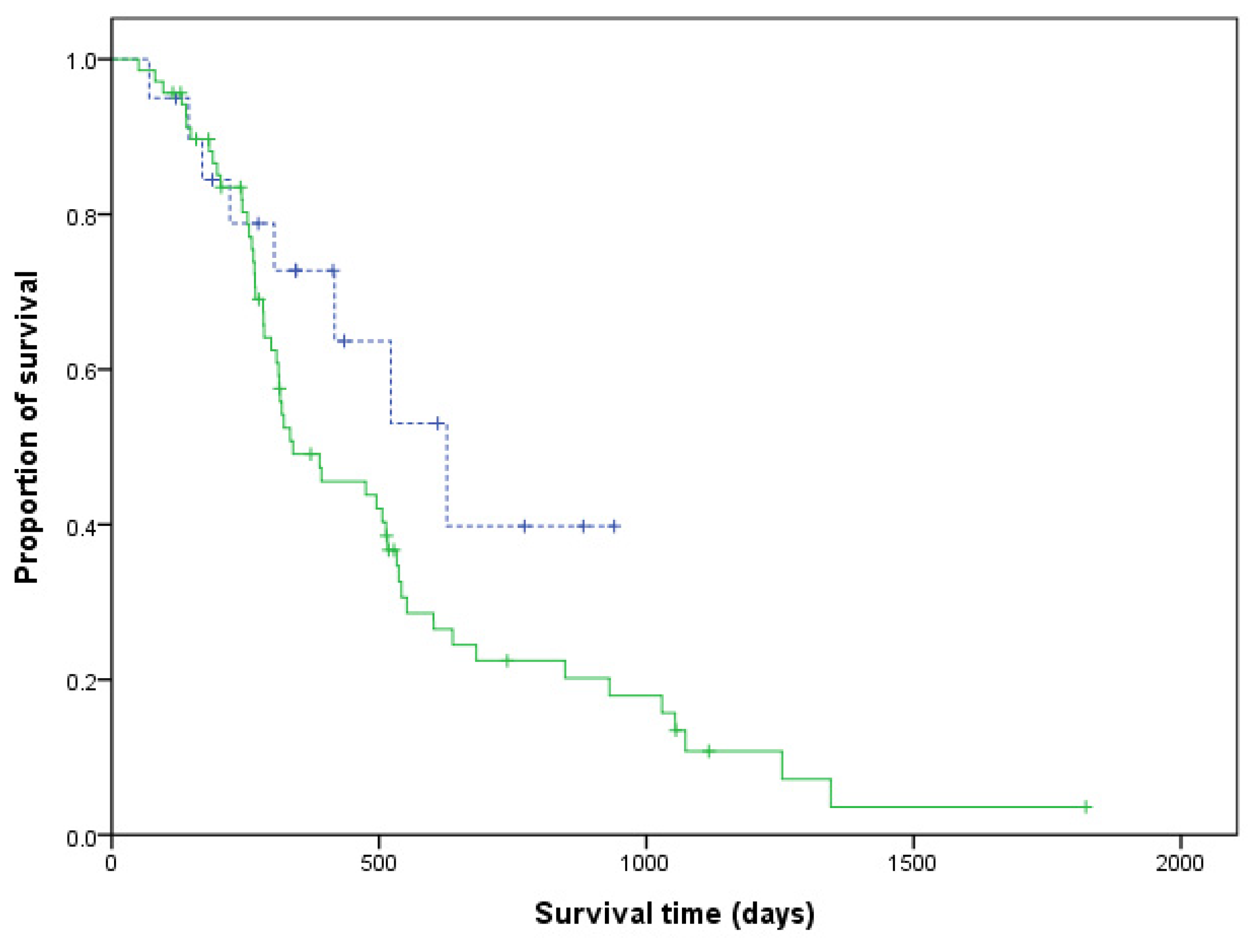
3.3. Comparison between the LHOP and CHOP Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L.N. Canine neoplasia in the uk: Estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef]
- Dorn, C.R.; Taylor, D.O.; Schneider, R. The epidemiology of canine leukemia and lymphoma. Bibl. Haematol. 1969, 36, 403–415. [Google Scholar]
- Burton, J.; Garrett-Mayer, E.; Thamm, D. Evaluation of a 15-week chop protocol for the treatment of canine multicentric lymphoma. Vet. Comp. Oncol. 2013, 11, 306–315. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, V.S.; Thamm, D.H.; Kurzman, I.D.; Turek, M.M.; Vail, D.M. Does l-asparaginase influence efficacy or toxicity when added to a standard chop protocol for dogs with lymphoma? J. Vet. Intern. Med. 2005, 19, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Garrett, L.D.; Thamm, D.H.; Chun, R.; Dudley, R.; Vail, D.M. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J. Vet. Intern. Med. 2002, 16, 704–709. [Google Scholar] [CrossRef]
- Sato, M.; Yamazaki, J.; Goto-Koshino, Y.; Takahashi, M.; Fujino, Y.; Ohno, K.; Tsujimoto, H. Evaluation of cytoreductive efficacy of vincristine, cyclophosphamide, and doxorubicin in dogs with lymphoma by measuring the number of neoplastic lymphoid cells with real-time polymerase chain reaction. J. Vet. Intern. Med. 2011, 25, 285–291. [Google Scholar] [CrossRef]
- Wang, S.L.; Lee, J.J.; Liao, A.T. Assessment of temporal association of relapse of canine multicentric lymphoma with components of the chop protocol: Is cyclophosphamide the weakest link? Vet. J. 2016, 213, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K. L-asparaginase for treatment of lymphoid neoplasia in dogs. J. Am. Vet. Med. Assoc. 1989, 194, 1626–1630. [Google Scholar]
- Cawley, J.R.; Wright, Z.M.; Meleo, K.; Post, G.S.; Clifford, C.A.; Vickery, K.R.; Vail, D.M.; Bergman, P.J.; Thamm, D.H. Concurrent use of rabacfosadine and l-asparaginase for relapsed or refractory multicentric lymphoma in dogs. J. Vet. Intern. Med. 2020, 34, 882–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, L.N. TNM Classification of Tumours in Domestic Animals, 1st ed.; World Health Organization: Geneva, Switzerland, 1980; pp. 46–47. [Google Scholar]
- Vail, D.M.; Michels, G.M.; Khanna, C.; Selting, K.A.; London, C.A. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1. 0)–a veterinary cooperative oncology group (vcog) consensus document. Vet. Comp. Oncol. 2010, 8, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Saba, C.F.; Thamm, D.H.; Vail, D.M. Combination chemotherapy with l-asparaginase, lomustine, and prednisone for relapsed or refractory canine lymphoma. J. Vet. Intern. Med. 2007, 21, 127–132. [Google Scholar] [CrossRef] [PubMed]
- AK, L.B.; Atherton, M.; Bentley, R.; Boudreau, C.; Burton, J.; Curran, K.; Dow, S.; Giuffrida, M.; Kellihan, H.; Mason, N. Veterinary cooperative oncology group-common terminology criteria for adverse events (vcog-ctcae v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar]
- Gustafson, D.L.; Bailey, D.B. Withrow and Macewen’s Small Animal Clinical Oncology, 6th ed.; Elsevier: St. Louis, MO, USA, 2020; pp. 182–208. [Google Scholar]
- Greenstein, S.; Ghias, K.; Krett, N.L.; Rosen, S.T. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin. Cancer Res. 2002, 8, 1681–1694. [Google Scholar]
- Baxter, J.D.; Harris, A.W.; Tomkins, G.M.; Cohn, M. Glucocorticoid receptors in lymphoma cells in culture: Relationship to glucocorticoid killing activity. Science 1971, 171, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.J. Effects of antimitotic agents on tubulin-nucleotide interactions. Pharmacol. Ther. 1991, 52, 127–147. [Google Scholar] [CrossRef]
- Colvin, M.; Brundrett, R.B.; Kan, M.-N.N.; Jardine, I.; Fenselau, C. Alkylating properties of phosphoramide mustard. Cancer Res. 1976, 36, 1121–1126. [Google Scholar]
- Tewey, K.M.; Chen, G.L.; Nelson, E.M.; Liu, L.-F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase ii. J. Biol. Chem. 1984, 259, 9182–9187. [Google Scholar] [CrossRef]
- Taatjes, D.J.; Gaudiano, G.; Resing, K.; Koch, T.H. Alkylation of DNA by the anthracycline, antitumor drugs adriamycin and daunomycin. J. Med. Chem. 1996, 39, 4135–4138. [Google Scholar] [CrossRef]
- Doroshow, J.H. Role of hydrogen peroxide and hydroxyl radical formation in the killing of ehrlich tumor cells by anticancer quinones. Proc. Natl. Acad. Sci. USA 1986, 83, 4514–4518. [Google Scholar] [CrossRef] [Green Version]
- Susaneck, S. Doxorubicin therapy in the dog. J. Am. Vet. Med. Assoc. 1983, 182, 70–72. [Google Scholar]
- Jeffreys, A.B.; Knapp, D.W.; Carlton, W.W.; Thomas, R.M.; Bonney, P.L.; Degortari, A.; Lucroy, M.D. Influence of asparaginase on a combination chemotherapy protocol for canine multicentric lymphoma. J. Am. Anim. Hosp. Assoc. 2005, 41, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Dorr, R.T.; Fritz, W.L. Cancer Chemotherapy Handbook; Elsevier north-holland: New York, NY, USA, 1980; pp. 230–239. [Google Scholar]
- Warry, E.; Hansen, R.J.; Gustafson, D.L.; Lana, S.E. Pharmacokinetics of cyclophosphamide after oral and intravenous administration to dogs with lymphoma. J. Vet. Intern. Med. 2011, 25, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Story, M.D.; Voehringer, D.W.; Stephens, L.C.; Meyn, R.E. L-asparaginase kills lymphoma cells by apoptosis. Cancer Chemother. Pharmacol. 1993, 32, 129–133. [Google Scholar] [CrossRef]
- Mealey, K.; Fidel, J. P-glycoprotein mediated drug interactions in animals and humans with cancer. J. Vet. Intern. Med. 2015, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schleis, S.E.; Rizzo, S.A.; Phillips, J.C.; LeBlanc, A.K. Asparaginase-associated pancreatitis in a dog. Can. Vet. J. 2011, 52, 1009. [Google Scholar]
- Wright, Z.; Steiner, J.; Suchodolski, J.; Rogers, K.; Barton, C.; Brown, M. A pilot study evaluating changes in pancreatic lipase immunoreactivity concentrations in canines treated with l-asparaginase (asnase), vincristine, or both for lymphoma. Can. J. Vet. Res. 2009, 73, 103. [Google Scholar] [PubMed]
- Kidd, J.A.; Ross, P.; Buntzman, A.S.; Hess, P.R. Development of an elisa to detect circulating anti-asparaginase antibodies in dogs with lymphoid neoplasia treated with escherichia coli l-asparaginase. Vet. Comp. Oncol. 2015, 13, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerius, K.D.; Ogilvie, G.K.; Fettman, M.J.; Walton, J.A.; Richardson, K.; Powers, B.E.; McNiel, E.A.; Rogers, Q. Comparison of the effects of asparaginase administered subcutaneously versus intramuscularly for treatment of multicentric lymphoma in dogs receiving doxorubicin. J. Am. Vet. Med. Assoc. 1999, 214, 353–356. [Google Scholar]
- Weir, E.C.; Greenlee, P.; Matus, R.; Brooks, M.; Morris, C.; Insogna, K. Hypercalcemia in canine lymphosarcoma is associated with the T cell subtype and with secretion of a PTH-like factor. J. Bone Miner. Res. 1988, 3, S106. [Google Scholar]
| Week | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 11 | 12 | 13 | 14 | 16 | 17 | 18 | 19 | |
| Vincristine (0.7 mg/m2 IV) | ˙ | ˙ | ˙ | ˙ | ˙ | ˙ | ˙ | ˙ | |||||||||
| L-asparaginase (400 U/kg IM) | ˙ | ˙ | ˙ | ˙ | |||||||||||||
| Doxorubicin (30 mg/m2 IV) | ˙ | ˙ | ˙ | ˙ | |||||||||||||
| Prednisolone (PO q24 h) | 2 mg/kg × 7 d, then 1.5 mg/kg × 7 d, then 1.0 mg/kg × 7 d, then 0.5 mg/kg × 7 d, then stop | ||||||||||||||||
| Adverse Events | |||||
|---|---|---|---|---|---|
| Dog | Injection Times | Lethargy | Anorexia | Vomiting | Diarrhea |
| 1 | 4 | 1 (Grade 1) | 1 (Grade 1) 1 (Grade 2) | - | - |
| 2 | 4 | - | - | - | - |
| 3 | 4 | - | 1 (Grade 1) 1 (Grade 2) | - | - |
| 4 | 6 | - | 1 (Grade 1) | - | - |
| 5 | 4 | - | 1 (Grade 1) | - | - |
| 6 | 4 | 1 (Grade 1) | 1 (Grade 1) | - | - |
| 7 | 4 | - | 1 (Grade 1) | - | - |
| 8 | 8 | 1 (Grade 1) | 2 (Grade 1) | - | - |
| 9 | 2 | - | - | - | - |
| 10 | 2 | - | - | - | - |
| 11 | 4 | 2 (Grade 1) | 2 (Grade 1) | - | 1 (Grade 1) 1 (Grade 2) |
| 12 | 4 | 1 (Grade 1) | 1 (Grade 1) | - | 1 (Grade 1) |
| 13 | 4 | 2 (Grade 1) | 1 (Grade 2) | 2 (Grade 1) 1 (Grade 2) | 1 (Grade 1) |
| 14 | 4 | - | 1 (Grade 1) | - | - |
| 15 | 4 | - | - | - | - |
| 16 | 4 | 1 (Grade 1) | 1 (Grade 1) 1 (Grade 2) | - | - |
| 17 | 4 | - | - | - | - |
| 18 | 1 | 1 (Grade 1) | - | - | - |
| 19 | 3 | 1 (Grade 1) | 1 (Grade 1) | - | - |
| 20 | 8 | 2 (Grade 1) | 1 (Grade 1) 1 (Grade 2) | - | - |
| Total | 82 | 13 (Grade 1) | 15 (Grade 1) 5 (Grade 2) | 2 (Grade 1) 1 (Grade 2) | 3 (Grade 1) 1 (Grade 2) |
| LHOP (n = 20) | CHOP (n = 69) | p-Value | |
|---|---|---|---|
| Age (years) | 9.5 | 8.2 | 0.107 |
| Body weight (kg) | 11.3 | 16 | 0.051 |
| Sex | |||
| Female | 12 (60%) | 34 (49.3%) | 0.453 |
| Male | 8 (40%) | 35 (50.7%) | |
| Clinical stage V | 2 (10%) | 7 (10.1%) | 1 |
| Substage b | 7 (35%) | 18 (26.1%) | 0.573 |
| T cell | 3 (15%, 3/20) | 3 (5.9%, 3/51) | 0.340 |
| Hypercalcaemia | 0 (0%) | 1 (1.4%) | 1 |
| Overall response (CR + PR) | 20 (100%) | 66 (95.7%) | 1 |
| Median progression free survival (days) | 344 | 234 | 0.001 |
| Median survival time (days) | 344 | 314 | 0.131 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-J.; Liao, A.T.; Wang, S.-L. L-Asparaginase, Doxorubicin, Vincristine, and Prednisolone (LHOP) Chemotherapy as a First-Line Treatment for Dogs with Multicentric Lymphoma. Animals 2021, 11, 2199. https://doi.org/10.3390/ani11082199
Lee J-J, Liao AT, Wang S-L. L-Asparaginase, Doxorubicin, Vincristine, and Prednisolone (LHOP) Chemotherapy as a First-Line Treatment for Dogs with Multicentric Lymphoma. Animals. 2021; 11(8):2199. https://doi.org/10.3390/ani11082199
Chicago/Turabian StyleLee, Jih-Jong, Albert Taiching Liao, and Shang-Lin Wang. 2021. "L-Asparaginase, Doxorubicin, Vincristine, and Prednisolone (LHOP) Chemotherapy as a First-Line Treatment for Dogs with Multicentric Lymphoma" Animals 11, no. 8: 2199. https://doi.org/10.3390/ani11082199
APA StyleLee, J.-J., Liao, A. T., & Wang, S.-L. (2021). L-Asparaginase, Doxorubicin, Vincristine, and Prednisolone (LHOP) Chemotherapy as a First-Line Treatment for Dogs with Multicentric Lymphoma. Animals, 11(8), 2199. https://doi.org/10.3390/ani11082199






