The Mare: A Pertinent Model for Human Assisted Reproductive Technologies?
Abstract
Simple Summary
Abstract
1. Introduction
2. Comparative Anatomical, Physiological and Pathological Aspects of Reproduction in Mares and Women
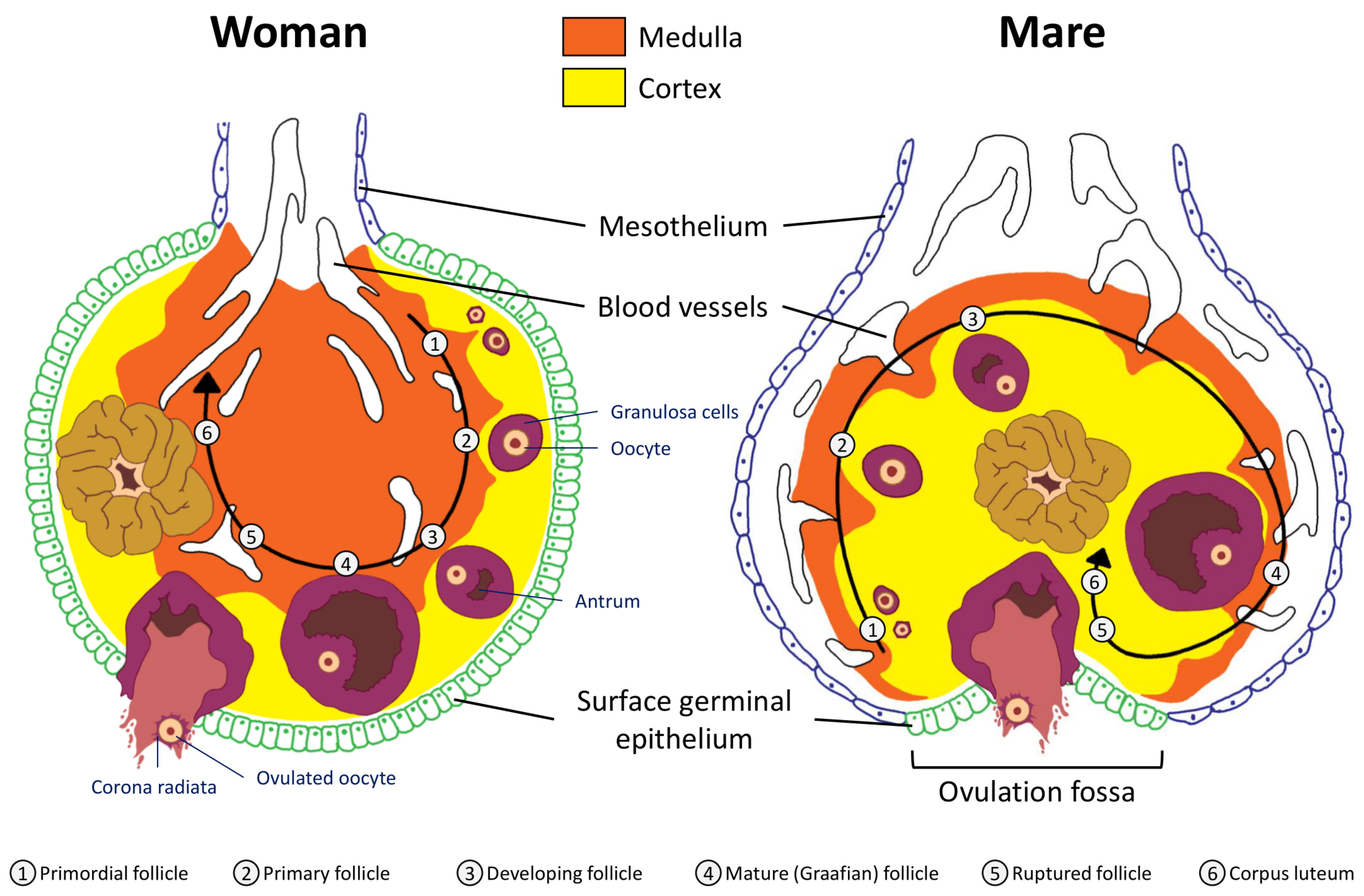
2.1. Anatomical Considerations: Uterus and Ovaries
2.2. Folliculogenesis
2.3. Ovulation
2.4. Preimplantation Embryos
2.5. Menopause
3. Comparison of Assisted Reproduction Techniques in Horses and Humans
3.1. Ovulation Stimulation
3.2. Oocyte Collection and Ovum Pick up (OPU)
3.3. Oocyte In Vitro Maturation (IVM)
3.4. Oocyte Manipulation
3.5. In Vitro Fertilization (IVF) and Intracytoplasmic Sperm Injection (ICSI)
3.6. Embryo Development and Transfer
3.7. Embryo Biopsy
3.8. Oocyte and Embryo Cryopreservation
4. Effects of Maternal Environment in Equine and Human
4.1. Effects of Maternal Age
4.2. Effects of Maternal Obesity
4.3. Effects of Maternal Excess Sport
5. Where Is the Mare a Good Model for ART in Humans?
5.1. Developmental Origins of Health and Disease
5.2. Follicular Cycle
5.3. Oocyte Retention in the Oviduct
5.4. Oocyte In Vitro Maturation (IVM)
5.5. Effects of Environment: Aging, Obesity and Excess Sport
5.6. Where Is the Mare Definitely Not a Good Model for ART in Humans?
5.7. Where Can the Woman Help the Mare?
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oldenbroek, K.; van der Waaij, L. Introduction to animal breeding. Textbook Animal Breeding and Genetics for BSc Students; Centre for Genetic Resources The Netherlands and Animal Breeding and Genomics Centre, 2015. Available online: https://wiki.groenkennisnet.nl/display/TAB/ (accessed on 22 June 2021).
- Viana, J.H. 2019 Statistics of embryo production and transfer in domestic farm animals. Divergent Trends IVD IVP Embryos. Embryo Technol. Newsl. 2020, 38, 7–26. [Google Scholar]
- Palmer, E.; Chavatte-Palmer, P. Contribution of reproduction management and technologies to genetic progress in horse breeding. J. Equine Vet. Sci. 2020, 103016. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Fertility Report 2015-Highlights; United Nations, 2017. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Feb/un_2015_worldfertilityreport_highlights.pdf (accessed on 22 June 2021).
- Attali, E.; Yogev, Y. The impact of advanced maternal age on pregnancy outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.W.; Demond, H.; Kelsey, G. Epigenetic regulation in development: Is the mouse a good model for the human? Hum. Reprod. Update 2018, 24, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Navarrete Santos, A.; Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 2012, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Anckaert, E.; Fair, T. DNA methylation reprogramming during oogenesis and interference by reproductive technologies: Studies in mouse and bovine models. Reprod. Fertil. Dev. 2015, 27, 739. [Google Scholar] [CrossRef]
- Carnevale, E.M.; Catandi, G.D.; Fresa, K. Equine aging and the oocyte: A potential model for reproductive aging in women. J. Equine Vet. Sci. 2020, 89, 103022. [Google Scholar] [CrossRef]
- Carnevale, E.M. The mare model for follicular maturation and reproductive aging in the woman. Theriogenology 2008, 69, 23–30. [Google Scholar] [CrossRef]
- Morris, L.H.; Maclellan, L.J. Factors affecting in vitro blastocyst production after intracytoplasmic sperm injection (ICSI). J. Equine Vet. Sci. 2020, 89, 103055. [Google Scholar] [CrossRef]
- Allen, W.R.; Wilsher, S. Half a century of equine reproduction research and application: A veterinary tour de force. Equine. Vet. J. 2018, 50, 10–21. [Google Scholar] [CrossRef]
- Giles, S.L.; Rands, S.A.; Nicol, C.J.; Harris, P.A. Obesity prevalence and associated risk factors in outdoor living domestic horses and ponies. PeerJ 2014, 2, e299. [Google Scholar] [CrossRef]
- Robin, C.A.; Ireland, J.L.; Wylie, C.E.; Collins, S.N.; Verheyen, K.L.P.; Newton, J.R. Prevalence of and risk factors for equine obesity in Great Britain based on owner-reported body condition scores: Prevalence of and risk factors for equine obesity in Great Britain. Equine Vet. J. 2015, 47, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Vick, M.M.; Sessions, D.R.; Murphy, B.A.; Kennedy, E.L.; Reedy, S.E.; Fitzgerald, B.P. Obesity is associated with altered metabolic and reproductive activity in the mare: Effects of metformin on insulin sensitivity and reproductive cyclicity. Reprod. Fertil. Dev. 2006, 18, 609. [Google Scholar] [CrossRef] [PubMed]
- Masko, M.; Domino, M.; Skierbiszewska, K.; Zdrojkowski, Ł.; Jasinski, T.; Gajewski, Z. Monitoring of the mare during the perinatal period at the clinic and in the stable. Equine Vet. Educ. 2020, 32, 654–663. [Google Scholar] [CrossRef]
- Monniaux, D.; Clément, F.; Dalbiès-Tran, R.; Estienne, A.; Fabre, S.; Mansanet, C.; Monget, P. The Ovarian Reserve of Primordial Follicles and the Dynamic Reserve of Antral Growing Follicles: What Is the Link? Biol. Reprod. 2014, 90. [Google Scholar] [CrossRef] [PubMed]
- Haadsma, M.L.; Groen, H.; Fidler, V.; Bukman, A.; Roeloffzen, E.M.A.; Groenewoud, E.R.; Broekmans, F.J.M.; Heineman, M.J.; Hoek, A. The predictive value of ovarian reserve tests for spontaneous pregnancy in subfertile ovulatory women. Hum. Reprod. 2008, 23, 1800–1807. [Google Scholar] [CrossRef]
- Driancourt, M.A.; Paris, A.; Roux, C.; Mariana, J.C.; Palmer, E. Ovarian follicular populations in pony and saddle-type mares. Reprod. Nutr. Dévelop. 1982, 22, 1035–1047. [Google Scholar] [CrossRef]
- Ginther, O.J. Reproductive Biology of the Mare: Basic And Applied Aspects, 2nd ed.; Equiservices: Cross-Plains, WI, USA, 1992. [Google Scholar]
- Messinis, I.E. From menarche to regular menstruation: Endocrinological background. Ann. N.Y. Acad. Sci. 2006, 1092, 49–56. [Google Scholar] [CrossRef]
- Burkhardt, J. Transition from anoestrus in the mare and the effects of artificial lighting. J. Agric. Sci. 1947, 37, 64–68. [Google Scholar] [CrossRef]
- Nishikawa, Y. Studies on Reproduction in Horses. Singularity and Artificial Control in Reproductive Phenomena; Japan Racing Association: Tokyo, Japan, 1959. [Google Scholar]
- Donadeu, F.; Pedersen, H. Follicle development in mares. Reprod. Domest. Anim. 2008, 43, 224–231. [Google Scholar] [CrossRef]
- Craig, J. Gonadotropin and intra-ovarian signals regulating follicle development and atresia: The delicate balance between life and death. Front. Biosci. 2007, 12, 3628. [Google Scholar] [CrossRef]
- Gougeon, A. Qualitative changes in medium and large antral follicles in the human ovary during the menstrual cycle. Ann. Biol. Anim. Bioch. Biophys. 1979, 19, 1461–1468. [Google Scholar] [CrossRef][Green Version]
- Hansen, K.R.; Knowlton, N.S.; Thyer, A.C.; Charleston, J.S.; Soules, M.R.; Klein, N.A. A new model of reproductive aging: The decline in ovarian non-growing follicle number from birth to menopause. Hum. Reprod. 2008, 23, 699–708. [Google Scholar] [CrossRef]
- Gougeon, A. Dynamics of follicular growth in the human: A model from preliminary results. Hum. Reprod. 1986, 1, 81–87. [Google Scholar] [CrossRef]
- Ginther, O.J.; Gastal, E.L.; Gastal, M.O.; Bergfelt, D.R.; Baerwald, A.R.; Pierson, R.A. Comparative study of the dynamics of follicular waves in Mares and women. Biol. Reprod. 2004, 71, 1195–1201. [Google Scholar] [CrossRef]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Characterization of ovarian follicular wave dynamics in women. Biol. Reprod. 2003, 69, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J. The mare: A 1000-pound guinea pig for study of the ovulatory follicular wave in women. Theriogenology 2012, 77, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A. Dynamics of follicle development in the human ovary. In Polycystic Ovary Syndrome; Chang, R.J., Ed.; Springer: New York, NY, USA, 1996; pp. 21–36. [Google Scholar] [CrossRef]
- Rapuri, P.B.; Gallagher, J.C.; Haynatzki, G. Endogenous levels of serum estradiol and sex hormone binding globulin determine bone mineral density, bone remodeling, the rate of bone loss, and response to treatment with estrogen in elderly women. J. Clin. Endocrinol. Metab. 2004, 89, 4954–4962. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, K.; Miyake, M.; Yoshikawa, T.; Kambegawa, A. Studies on serum oestrogen and progesterone levels during the oestrous cycle and early pregnancy in mares. Equine Vet. J. 1977, 9, 57–60. [Google Scholar] [CrossRef]
- Palmer, E. New results on follicular growth and ovulation in the mare. In Follicular Growth and Ovulation Rate in Farm Animals; Springer: Dordrecht, The Netherlands, 1987; pp. 237–255. [Google Scholar]
- Lebedeva, L.F.; Solodova, E.V. Technological approaches to the problem of double ovulation and twin pregnancies in mares. IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012036. [Google Scholar] [CrossRef]
- Morel, M.C.G.D.; Newcombe, J.R.; Swindlehurst, J.C. The effect of age on multiple ovulation rates, multiple pregnancy rates and embryonic vesicle diameter in the mare. Theriogenology 2005, 63, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Ginther, O.J. Twinning in mares: A review of recent studies. J. Equine Vet. Sci. 1982, 2, 127–135. [Google Scholar] [CrossRef]
- Santana, D.S.; Souza, R.T.; Surita, F.G.; Argenton, J.L.; Silva, C.M.; Cecatti, J.G. Twin pregnancy in Brazil: A Profile analysis exploring population information from the national birth e-registry on live births. BioMed Res. Int. 2018, 2018, e9189648. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, M.-C. Utero-ovarian relationships in placental mammals: Role of uterus and embryo in the regulation of progesterone secretion by the corpus luteum. Rev. Reprod. Nutr. Dévelop. 1983, 23, 793–816. [Google Scholar] [CrossRef] [PubMed]
- Stouffer, R.L.; Bishop, C.V.; Bogan, R.L.; Xu, F.; Hennebold, J.D. Endocrine and local control of the primate corpus luteum. Reprod. Biol. 2013, 13, 259–271. [Google Scholar] [CrossRef]
- Ginther, O.J. A 40-year odyssey into the mysteries of equine luteolysis. Theriogenology 2009, 72, 591–598. [Google Scholar] [CrossRef]
- Wallach, E.E.; Katz, E. The luteinized unruptured follicle other ovulatory dysfunctions. Fertil. Steril. 1988, 50, 839–850. [Google Scholar] [CrossRef]
- Check, J.H. Ovulation disorders: Part II. Anovulation associated with normal estrogen. Clin. Exp. Obstet. Gynecol. 2007, 34, 69–72. [Google Scholar]
- Kaiser, B.; Koene, M.; Swagemakers, J.; Bader, H.; Hoppen, H. Diagnosis, therapy and endocrinologic parameters of persistent follicles in mares in comparison with preovulatory follicles. Tierarztl. Prax. Ausg. G. Grosstiere/Nutztiere 1999, 27, 180–186. [Google Scholar]
- Ginther, O.J.; Gastal, E.L.; Gastal, M.O.; Beg, M.A. Incidence, endocrinology, vascularity, and morphology of hemorrhagic anovulatory follicles in Mares. J. Equine Vet. Sci. 2007, 27, 130–139. [Google Scholar] [CrossRef]
- Cuervo-Arango, J.; Newcombe, J.R. The effect of cloprostenol on the incidence of multiple ovulation and anovulatory hemorrhagic follicles in two mares: A case report. J. Equine Vet. Sci. 2009, 29, 533–539. [Google Scholar] [CrossRef]
- Hamilton, C.J.C.M.; Evers, J.L.H.; Hoogland, H.J. Ovulatory disorders and inflammatory adnexal damage: A neglected cause of the failure of fertility microsurgery. BJOG Int. J. OG 1986, 93, 282–284. [Google Scholar] [CrossRef]
- Qublan, H.; Amarin, Z.; Nawasreh, M.; Diab, F.; Malkawi, S.; Al-Ahmad, N.; Balawneh, M. Luteinized unruptured follicle syndrome: Incidence and recurrence rate in infertile women with unexplained infertility undergoing intrauterine insemination. Hum. Reprod. 2006, 21, 2110–2113. [Google Scholar] [CrossRef] [PubMed]
- Kerin, J.F.; Kirby, C.; Morris, D.; McEvoy, M.; Ward, B.; Cox, L.W. Incidence of the luteinized unruptured follicle phenomenon in cycling women**Supported in part by National Health and Medical Research Council (NH&MRC) grant 810549. Fertil. Steril. 1983, 40, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Dal, J.; Vural, B.; Caliskan, E.; Ozkan, S.; Yucesoy, I. Power Doppler ultrasound studies of ovarian, uterine, and endometrial blood flow in regularly menstruating women with respect to luteal phase defects. Fertil. Steril. 2005, 84, 224–227. [Google Scholar] [CrossRef]
- Kaya, H.; Oral, B. Effect of ovarian involvement on the frequency of luteinized unruptured follicle in endometriosis. Gynecol. Obs. Investig. 1999, 48, 123–126. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Brosens, I.A. The luteinized unruptured follicle syndrome. Fertil. Steril. 1983, 39, 249–250. [Google Scholar] [CrossRef]
- Kugu, K.; Taketani, Y.; Kohda, K.; Mizuno, M. Exaggerated prolactin response to thyrotropin-releasing hormone in infertile women with the luteinized unruptured follicle syndrome. Arch. Gynecol. Obstet. 1991, 249, 27–31. [Google Scholar] [CrossRef]
- Lefranc, A.-C.; Allen, W.R. Incidence and morphology of anovulatory haemorrhagic follicles in the mare. Pferdeheilkunde 2003, 19, 611–612. [Google Scholar] [CrossRef]
- Ginther, O.J.; Gastal, M.O.; Gastal, E.L.; Jacob, J.C.; Beg, M.A. Induction of haemorrhagic anovulatory follicles in mares. Reprod. Fertil. Dev. 2008, 20, 947. [Google Scholar] [CrossRef] [PubMed]
- Gastal, E.L.; Gastal, M.O.; Ginther, O.J. The suitability of echotexture characteristics of the follicular wall for identifying the optimal breeding day in mares. Theriogenology 1998, 50, 1025–1038. [Google Scholar] [CrossRef]
- Clewe, T.H.; Mastroianni, L.J. Mechanisms of ovum pickup. I. functional capacity of rabbit oviducts ligated near the fimbria. Obstet. Gynecol. Surv. 1958, 13, 551–552. [Google Scholar] [CrossRef]
- Corpa, J.M. Ectopic pregnancy in animals and humans. Reproduction 2006, 131, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.C.; Liu, I.K.M.; Bowers, J.; Lantz, K.C.; Schlafke, S.; Suarez, S. The ovulated ovum of the horse: Cytology of nonfertilized ova to pronuclear stage ova1. Biol. Reprod. 1987, 37, 453–466. [Google Scholar] [CrossRef]
- Betteridge, K.J.; Eaglesome, M.D.; Mitchellt, D.; Flood, P.F.; Beriault, R. Development of horse embryos up to twenty two days after ovulation: Observations on fresh specimens. J. Anat. 1982, 135, 191–209. [Google Scholar]
- Flood, P.F.; Betteridge, K.J.; Diocee, M.S. Transmission electron microscopy of horse embryos 3-16 days after ovulation. J. Reprod. Fertil. Suppl. 1982, 32, 319–327. [Google Scholar] [PubMed]
- Balaban, B.; Brison, D.; Calderon, G.; Catt, J.; Conaghan, J.; Cowan, L.; Ebner, T.; Gardner, D.; Hardarson, T. Alpha scientists in reproductive medicine and eshre special interest group of embryology. The istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef]
- Cruz, M.; Garrido, N.; Herrero, J.; Pérez-Cano, I.; Muñoz, M.; Meseguer, M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod. Biomed. Online 2012, 25, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Milewski, R.; Ajduk, A. Time-lapse imaging of cleavage divisions in embryo quality assessment. Reproduction 2017, 154, R37–R53. [Google Scholar] [CrossRef]
- Grøndahl, C.; Hyttel, P. Nucleologenesis and ribonucleic acid synthesis in preimplantation equine embryos1. Biol. Reprod. 1996, 55, 769–774. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.O.; Squires, E.L. Morphologic assessment of the equine embryo. J. Am. Vet. Med Assoc. 1988, 192, 401–406. [Google Scholar]
- Lane, M.; O’Donovan, M.K.; Squires, E.L.; Seidel, G.E.; Gardner, D.K. Assessment of metabolism of equine morulae and blastocysts. Mol. Reprod Dev. 2001, 59, 33–37. [Google Scholar] [CrossRef]
- Ball, B.A.; Little, T.V.; Weber, J.A.; Woods, G.L. Survival of Day-4 embryos from young, normal mares and aged, subfertile mares after transfer to normal recipient mares. Reproduction 1989, 85, 187–194. [Google Scholar] [CrossRef]
- Enders, A.C.; Schlafke, S.; Lantz, K.C.; Liu, I.K.M. Endoderm cells of the equine yolk sac from Day 7 until formation of the definitive yolk sac placenta. Equine Vet. J. 1993, 25, 3–9. [Google Scholar] [CrossRef]
- Motiei, M.; Vaculikova, K.; Cela, A.; Tvrdonova, K.; Khalili, R.; Rumpik, D.; Rumpikova, T.; Glatz, Z.; Saha, T. Non-invasive human embryo metabolic assessment as a developmental criterion. JCM 2020, 9, 4094. [Google Scholar] [CrossRef]
- Uyar, A.; Seli, E. Metabolomic assessment of embryo viability. Semin. Reprod. Med. 2014, 32, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F.; Gilbert, S.F. Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Battut, I.; Colchen, S.; Fieni, F.; Tainturier, D.; Bruyas, J.-F. Success rates when attempting to nonsurgically collect equine embryos at 144, 156 or 168 hours after ovulation. Equine Vet. J. 1998, 29, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.A.; Woods, G.L.; Vanderwall, D.K.; Weber, J.A. Embryo-initiated oviductal transport in mares. Reproduction 1992, 95, 535–538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freeman, D.A.; Weber, J.A.; Geary, R.T.; Woods, G.L. Time of embryo transport through the mare oviduct. Theriogenology 1991, 36, 823–830. [Google Scholar] [CrossRef]
- Weber, J.A.; Freeman, D.A.; Vanderwall, D.K.; Woods, G.L. Prostaglandin E2 secretion by oviductal transport-stage equine embryos. Biol. Reprod. 1991, 45, 540–543. [Google Scholar] [CrossRef]
- Weber, J.A.; Freeman, D.A.; Vanderwall, D.K.; Woods, G.L. Prostaglandin E2 hastens oviductal transport of equine embryos1. Biol. Reprod. 1991, 45, 544–546. [Google Scholar] [CrossRef]
- Weber, J.A.; Woods, G.L.; Freeman, D.A.; Vanderwall, D.K. Prostaglandin E2-specific binding to the equine oviduct. Prostaglandins 1992, 43, 61–65. [Google Scholar] [CrossRef]
- Weber, J.A.; Woods, G.L.; Lichtenwalner, A.B. Relaxatory effect of prostaglandin e2 on circular smooth muscle isolated from the equine oviductal isthmus1. Biol. Reprod. 1995, 52, 125–130. [Google Scholar] [CrossRef]
- Aguilar, J.J.; Woods, G.L.; Miragaya, M.H.; Olsen, L.M. Living fibroblast cells in the oviductal masses of mares. Equine Vet. J. 1997, S25, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.E. Consequences of accelerated ovum transport, including a re-evaluation of Estes’ operation. Reproduction 1979, 55, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Beyth, Y.; Polishuk, W.Z. Ovarian implantation into the Uterus (Estes Operation): Clinical and experimental evaluation. Fertil. Steril. 1979, 32, 657–660. [Google Scholar] [CrossRef]
- Neuhausser, W.M.; Vaughan, D.A.; Sakkas, D.; Hacker, M.R.; Toth, T.; Penzias, A. Non-inferiority of cleavage-stage versus blastocyst-stage embryo transfer in poor prognosis, IVF patients (PRECiSE trial): Study protocol for a randomized controlled trial. Reprod. Health 2020, 17, 16. [Google Scholar] [CrossRef]
- Pasqualini, R.; Quintans, C. Clinical practice of embryo transfer. Reprod. Biomed. Online 2002, 4, 83–92. [Google Scholar] [CrossRef]
- Swegen, A. Maternal recognition of pregnancy in the mare: Does it exist and why do we care? Reproduction 2021, 161, R139–R155. [Google Scholar] [CrossRef]
- Allen, W.R.; Wilsher, S. A Review of implantation and early placentation in the mare. Placenta 2009, 30, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, E.M.; Bergfelt, D.R.; Ginther, O.J. Follicular activity and concentrations of FSH and LH associated with senescence in mares. Anim. Reprod. Sci. 1994, 35, 231–246. [Google Scholar] [CrossRef]
- Vanderwall, D.K.; Woods, G.L.; Freeman, D.A.; Weber, J.A.; Rock, R.W.; Tester, D.F. Ovarian follicles, ovulations and progesterone concentrations in aged versus young mares. Theriogenology 1993, 40, 21–32. [Google Scholar] [CrossRef]
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 312, 366. [Google Scholar] [CrossRef]
- Macklon, N.S.; Stouffer, R.L.; Giudice, L.C.; Fauser, B.C.J.M. The science behind 25 Years of ovarian stimulation for in vitro fertilization. Endocr. Rev. 2006, 27, 170–207. [Google Scholar] [CrossRef] [PubMed]
- Al-Inany, H.G.; Youssef, M.A.; Ayeleke, R.O.; Brown, J.; Lam, W.S.; Broekmans, F.J. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Niederberger, C.; Pellicer, A.; Cohen, J.; Gardner, D.K.; Palermo, G.D.; O’Neill, C.L.; Chow, S.; Rosenwaks, Z.; Cobo, A.; Swain, J.E.; et al. Forty years of IVF. Fertil. Steril. 2018, 110, 185–324.e5. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.L.; McCue, P.M. Superovulation in mares. Anim. Reprod. Sci. 2007, 99, 1–8. [Google Scholar] [CrossRef]
- Roser, J.F.; Meyers-Brown, G. Enhancing Fertility in mares: Recombinant equine gonadotropins. J. Equine Vet. Sci. 2019, 76, 6–13. [Google Scholar] [CrossRef]
- Gleicher, N.; Friberg, J.; Fullan, N.; Giglia, R.; Mayden, K.; Kesky, T.; Siegel, I. Egg Retrieval for in vitro fertilisation by sonographically controlled vaginal culdocentesis. Lancet 1983, 322, 508–509. [Google Scholar] [CrossRef]
- Bennett, S.J.; Waterstone, J.J.; Cheng, W.C.; Parsons, J. Complications of transvaginal ultrasound-directed follicle aspiration: A review of 2670 consecutive procedures. J. Assist. Reprod. Genet. 1993, 10, 72–77. [Google Scholar] [CrossRef]
- Palmer, E.; Duchamp, G.; Bézard, J.; Magistrini, M.; King, W.A.; Betteridge, K.J. Non-surgical recovery of follicular fluid and oocytes of mares. J. Reprod. Fertil. Suppl. 1987, 35, 689–690. [Google Scholar]
- Lazzari, G.; Colleoni, S.; Crotti, G.; Turini, P.; Fiorini, G.; Barandalla, M.; Landriscina, L.; Dolci, G.; Benedetti, M.; Duchi, R.; et al. Laboratory production of equine embryos. J. Equine Vet. Sci. 2020, 89, 103097. [Google Scholar] [CrossRef]
- Morris, L.H.A. The development of in vitro embryo production in the horse. Equine Vet. J. 2018, 50, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Duchi, R.; Colleoni, S.; Lagutina, I.; Lazzari, G. Ovum pick up, intracytoplasmic sperm injection and somatic cell nuclear transfer in cattle, buffalo and horses: From the research laboratory to clinical practice. Theriogenology 2014, 81, 138–151. [Google Scholar] [CrossRef]
- Hawley, L.R.; Enders, A.C.; Hinrichs, K. Comparison of equine and bovine oocyte- cumulus morphology within the ovarian follicle. Biol. Reprod. Mono. 1995, 1, 243–252. [Google Scholar] [CrossRef]
- Hinrichs, K. The equine oocyte: Factors affecting meiotic and developmental competence. Mol. Reprod. Dev. 2010, 77, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A.; Ata, B.; Son, W.-Y.; Dahan, M.H.; Tan, S.L. Comparison of complication rates and pain scores after transvaginal ultrasound–guided oocyte pickup procedures for in vitro maturation and in vitro fertilization cycles. Fertil. Steril. 2014, 101, 705–709. [Google Scholar] [CrossRef]
- Souza, M.d.C.B.d.; Souza, M.M.d.; Antunes, R.d.A.; Tamm, M.A.; Silva, J.B.d.; Mancebo, A.C.A. Bladder hematoma: A complication from an oocyte retrieval procedure. JBRA Assist. Reprod. 2019. [Google Scholar] [CrossRef]
- Jacobson, C.C.; Choi, Y.-H.; Hayden, S.S.; Hinrichs, K. Recovery of mare oocytes on a fixed biweekly schedule, and resulting blastocyst formation after intracytoplasmic sperm injection. Theriogenology 2010, 73, 1116–1126. [Google Scholar] [CrossRef]
- Rock, J.; Menkin, M.F. In vitro fertilization and cleavage of human ovarian eggs. Science 1944, 100, 105–107. [Google Scholar] [CrossRef]
- Cha, K.Y.; Koo, J.J.; Ko, J.J.; Choi, D.H.; Han, S.Y.; Yoon, T.K. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program**Prize paper, presented at the 45th annual meeting of the american fertility society, San Francisco, California, November 11 to 16, 1989. Fertil. Steril. 1991, 55, 109–113. [Google Scholar] [CrossRef]
- Trounson, A.; Wood, C.; Kausche, A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil. Steril. 1994, 62, 353–362. [Google Scholar] [CrossRef]
- Gérard, N.; Robin, E. Cellular and molecular mechanisms of the preovulatory follicle differenciation and ovulation: What do we know in the mare relative to other species. Theriogenology 2019, 130, 163–176. [Google Scholar] [CrossRef]
- Hinrichs, K. Assisted reproductive techniques in mares. Reprod. Dom. Anim. 2018, 53, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Child, T.J.; Abdul-Jalil, A.K.; Gulekli, B.; Lin Tan, S. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil. Steril. 2001, 76, 936–942. [Google Scholar] [CrossRef]
- Le Du, A.; Kadoch, I.J.; Bourcigaux, N.; Doumerc, S.; Bourrier, M.-C.; Chevalier, N.; Fanchin, R.; Chian, R.-C.; Tachdjian, G.; Frydman, R.; et al. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: The French experience. Hum. Reprod. 2005, 20, 420–424. [Google Scholar] [CrossRef]
- Grynberg, M.; Poulain, M.; le Parco, S.; Sifer, C.; Fanchin, R.; Frydman, N. Similar in vitro maturation rates of oocytes retrieved during the follicular or luteal phase offer flexible options for urgent fertility preservation in breast cancer patients. Hum. Reprod 2016, 31, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Hourvitz, A.; Maman, E.; Brengauz, M.; Machtinger, R.; Dor, J. In vitro maturation for patients with repeated in vitro fertilization failure due to “oocyte maturation abnormalities”. Fertil. Steril. 2010, 94, 496–501. [Google Scholar] [CrossRef]
- Galvão, A.; Segers, I.; Smitz, J.; Tournaye, H.; De Vos, M. In vitro maturation (IVM) of oocytes in patients with resistant ovary syndrome and in patients with repeated deficient oocyte maturation. J. Assist. Reprod. Genet. 2018, 35, 2161–2171. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Qian, Y.; Mao, Y.; Ding, W. Pregnancies and births achieved from in vitro matured oocytes retrieved from poor responders undergoing stimulation in in vitro fertilization cycles. Fertil. Steril. 2003, 80, 447–449. [Google Scholar] [CrossRef]
- Grynberg, M.; Peltoketo, H.; Christin-Maître, S.; Poulain, M.; Bouchard, P.; Fanchin, R. First birth achieved after in vitro maturation of oocytes from a woman endowed with multiple antral follicles unresponsive to follicle-stimulating hormone. J. Clin. Endocrinol. Metab. 2013, 98, 4493–4498. [Google Scholar] [CrossRef] [PubMed]
- Benammar, A.; Fanchin, R.; Filali-Baba, M.; Vialard, F.; Fossard, C.; Vandame, J.; Pirtea, P.; Racowsky, C.; Ayoubi, J.-M.; Poulain, M. Utilization of in vitro maturation in cases with a FSH receptor mutation. J. Assist. Reprod. Genet. 2021. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.N.; Ho, V.N.A.; Ho, T.M.; Dang, V.Q.; Phung, T.H.; Giang, N.H.; Le, A.H.; Pham, T.D.; Wang, R.; Smitz, J.; et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: A randomized non-inferiority controlled trial. Hum. Reprod. 2020, 35, 2537–2547. [Google Scholar] [CrossRef]
- Mackens, S.; Mostinckx, L.; Drakopoulos, P.; Segers, I.; Santos-Ribeiro, S.; Popovic-Todorovic, B.; Tournaye, H.; Blockeel, C.; De Vos, M. Early pregnancy loss in patients with polycystic ovary syndrome after IVM versus standard ovarian stimulation for IVF/ICSI. Hum. Reprod. 2020, 35, 2763–2773. [Google Scholar] [CrossRef]
- Gliozheni, O.; Hambartsoumian, E.; Strohmer, H.; Petrovskaya, E.; Tishkevich, O.; Bogaerts, K.; Wyns, C.; Balic, D.; Antonova, I.; The European IVF-monitoring Consortium (EIM)‡ for the European Society of Human Reproduction and Embryology (ESHRE); et al. ART in Europe, 2016: Results generated from European registries by ESHRE. Hum. Reprod. Open 2020, 2020, hoaa032. [Google Scholar] [CrossRef]
- Hinrichs, K. Advances in holding and cryopreservation of equine oocytes and embryos. J. Equine Vet. Sci. 2020, 89, 102990. [Google Scholar] [CrossRef]
- Riera, F.L.; Roldán, J.E.; Espinosa, J.M.; Fernandez, J.E.; Ortiz, I.; Hinrichs, K. Application of embryo biopsy and sex determination via polymerase chain reaction in a commercial equine embryo transfer program in Argentina. Reprod. Fertil. Dev. 2019, 31, 1917. [Google Scholar] [CrossRef]
- Foss, R.; Ortis, H.; Hinrichs, K. Effect of potential oocyte transport protocols on blastocyst rates after intracytoplasmic sperm injection in the horse: Transport of equine oocytes for ICSI. Equine Vet. J. 2013, 45, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.J.; Johnson, M.H.; Braude, P.R.; Houliston, E. Cytoskeletal organization in fresh, aged and spontaneously activated human oocytes. Hum. Reprod. 1988, 3, 978–989. [Google Scholar] [CrossRef]
- Wang, W.-H. Meiotic spindle, spindle checkpoint and embryonic aneuploidy. Front. Biosci. 2006, 11, 620. [Google Scholar] [CrossRef]
- Chian, R.-C.; Buckett, W.M.; Tan, S.-L. In-vitro maturation of human oocytes. Reprod. Biomed. Online 2004, 8, 148–166. [Google Scholar] [CrossRef]
- Lane, M.; Gardner, D.K. Regulation of ionic homeostasis by mammalian embryos. Semin. Reprod. Med. 2000, 18, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Castelbaum, A.J.; Freedman, M.F. Is there a role for gamete intra-fallopian transfer and other tubal insemination procedures? Curr. Opin. Obs. Gynecol. 1998, 10, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.L.; Carnevale, E.M.; McCue, P.M.; Bruemmer, J.E. Embryo technologies in the horse. Theriogenology 2003, 59, 151–170. [Google Scholar] [CrossRef]
- Squires, E. Current reproductive technologies impacting equine embryo production. J. Equine Vet. Sci. 2020, 89, 102981. [Google Scholar] [CrossRef]
- Squires, E.L. Perspectives on the development and incorporation of assisted reproduction in the equine industry. Reprod. Fertil. Dev. 2019, 31, 1753. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Esteves, S.C.; Miyaoka, R.; Agarwal, A. An update on the clinical assessment of the infertile male. Clinics 2011, 66, 691–700. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International glossary on infertility and fertility care, 2017†‡§. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Roque, M.; Bedoschi, G.; Haahr, T.; Humaidan, P. Intracytoplasmic sperm injection for male infertility and consequences for offspring. Nat. Rev. Urol. 2018, 15, 535–562. [Google Scholar] [CrossRef]
- Dyer, S.; Chambers, G.M.; de Mouzon, J.; Nygren, K.G.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Adamson, G.D. International committee for monitoring assisted reproductive technologies world report: Assisted reproductive technology 2008, 2009 and 2010†. Hum. Reprod. 2016, 31, 1588–1609. [Google Scholar] [CrossRef]
- Grimstad, F.W.; Nangia, A.K.; Luke, B.; Stern, J.E.; Mak, W. Use of ICSI in IVF cycles in women with tubal ligation does not improve pregnancy or live birth rates. Hum. Reprod. 2016, 31, 1–6. [Google Scholar] [CrossRef]
- Chambers, G.M.; Wand, H.; Macaldowie, A.; Chapman, M.G.; Farquhar, C.M.; Bowman, M.; Molloy, D.; Ledger, W. Population trends and live birth rates associated with common ART treatment strategies. Hum. Reprod. 2016, 31, 2632–2641. [Google Scholar] [CrossRef]
- The Practice Committees of the American Society for Reproductive Medicine and Society for Assisted Reproductive Technology. Intracytoplasmic sperm injection (ICSI) for non-male factor infertility: A committee opinion. Fertil. Steril. 2012, 98, 1395–1399. [Google Scholar] [CrossRef]
- Palmer, E.; Bézard, J.; Magistrini, M.; Duchamp, G. In vitro fertilization in the horse. A Retrosp. Study J. Reprod. Fert. 1991, 44, 375–384. [Google Scholar]
- Choi, Y.H.; Okada, Y.; Hochi, S.; Braun, J.; Sato, K.; Oguri, N. In vitro fertilization rate of horse oocytes with partially removed zonae. Theriogenology 1994, 42, 795–802. [Google Scholar] [CrossRef]
- Smits, K.; Govaere, J.; Hoogewijs, M.; Piepers, S.; Van Soom, A. A pilot comparison of laser-assisted vs piezo drill icsi for the in vitro production of horse embryos: Icsi in horses: Laser Vs Piezo. Reprod. Domest. Anim. 2012, 47, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Oguri, N.; Tsutsumi, Y. Non-surgical egg transfer in mares. Reproduction 1974, 41, 313–320. [Google Scholar] [CrossRef][Green Version]
- Douglas, R.H. Review of induction of superovulation and embryo transfer in the equine. Theriogenology 1979, 11, 33–46. [Google Scholar] [CrossRef]
- Duranthon, V.; Chavatte-Palmer, P. Long term effects of ART: What do animals tell us? Mol. Reprod. Dev. 2018, 85, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, D.; Cohen, J.; Mortimer, S.T.; Fawzy, M.; McCulloh, D.H.; Morbeck, D.E.; Pollet-Villard, X.; Mansour, R.T.; Brison, D.R.; Doshi, A.; et al. Cairo consensus on the IVF laboratory environment and air quality: Report of an expert meeting. Reprod. Biomed. Online 2018, 36, 658–674. [Google Scholar] [CrossRef]
- De los Santos, M.J.; Apter, S.; Coticchio, G.; Debrock, S.; Lundin, K.; Plancha, C.E.; Prados, F.; Rienzi, L.; Verheyen, G.; Eshre Guideline Group on Good Practice in IVF Labs; et al. Revised Guidelines for good practice in IVF laboratories (2015)†. Hum. Reprod. 2016, 31, 685–686. [Google Scholar] [CrossRef]
- Swain, J. Optimal human embryo culture. Semin. Reprod. Med 2015, 33, 103–117. [Google Scholar] [CrossRef]
- Swain, J.E.; Carrell, D.; Cobo, A.; Meseguer, M.; Rubio, C.; Smith, G.D. Optimizing the culture environment and embryo manipulation to help maintain embryo developmental potential. Fertil. Steril. 2016, 105, 571–587. [Google Scholar] [CrossRef]
- Hinrichs, K. In vitro production of equine embryos: State of the art: In vitro production of equine embryos. Reprod. Domest. Anim. 2010, 45, 3–8. [Google Scholar] [CrossRef] [PubMed]
- The Vienna consensus: Report of an expert meeting on the development of ART laboratory performance indicators. Reprod. Biomed. Online 2017, 35, 494–510. [CrossRef] [PubMed]
- Carnevale, E.M.; Metcalf, E.S. Morphology, developmental stages and quality parameters of in vitro-produced equine embryos. Reprod. Fertil. Dev. 2019, 31, 1758. [Google Scholar] [CrossRef] [PubMed]
- Tremoleda, J.L.; Stout, T.A.E.; Lagutina, I.; Lazzari, G.; Bevers, M.M.; Colenbrander, B.; Galli, C. Effects of in vitro production on horse embryo morphology, cytoskeletal characteristics, and blastocyst capsule formation. Biol. Reprod. 2003, 69, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Harding, H.D.; Hartman, D.L.; Obermiller, A.D.; Kurosaka, S.; McLaughlin, K.J.; Hinrichs, K. The uterine environment modulates trophectodermal POU5F1 levels in equine blastocysts. Reproduction 2009, 138, 589–599. [Google Scholar] [CrossRef]
- Simopoulou, M.; Sfakianoudis, K.; Maziotis, E.; Tsioulou, P.; Grigoriadis, S.; Rapani, A.; Giannelou, P.; Asimakopoulou, M.; Kokkali, G.; Pantou, A.; et al. PGT-A: Who and when? A systematic review and network meta-analysis of RCTs. J. Assist. Reprod. Genet. 2021. [Google Scholar] [CrossRef]
- Troedsson, M.H.T.; Paprocki, A.M.; Koppang, R.W.; Syverson, C.M.; Griffin, P.G.; Klein, C.; Dobrinsky, J.R. Transfer success of biopsied and vitrified equine embryos. Anim. Reprod. Sci. 2010, 121, 295–296. [Google Scholar] [CrossRef]
- Sciorio, R. Use of time-lapse monitoring in medically assisted reproduction treatments: A mini-review. Zygote 2021, 29, 93–101. [Google Scholar] [CrossRef]
- Brooks, K.E.; Daughtry, B.L.; Metcalf, E.; Masterson, K.; Battaglia, D.; Gao, L.; Park, B.; Chavez, S.L. Assessing equine embryo developmental competency by time-lapse image analysis. Reprod. Fertil. Dev. 2019, 31, 1840. [Google Scholar] [CrossRef]
- Lewis, N.; Schnauffer, K.; Hinrichs, K.; Morganti, M.; Troup, S.; Argo, C. Morphokinetics of early equine embryo development in vitro using time-lapse imaging, and use in selecting blastocysts for transfer. Reprod. Fertil. Dev. 2019, 31, 1851. [Google Scholar] [CrossRef]
- Martino, N.A.; Marzano, G.; Mastrorocco, A.; Lacalandra, G.M.; Vincenti, L.; Hinrichs, K.; Dell’Aquila, M.E. Use of time-lapse imaging to evaluate morphokinetics of in vitro equine blastocyst development after oocyte holding for two days at 15°C versus room temperature before intracytoplasmic sperm injection. Reprod. Fertil. Dev. 2019, 31, 1862. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.; Burruel, V.; Kato, M.; de la Fuente, A.; Orellana, D.; Renaudin, C.; Dujovne, G. Equine non-invasive time-lapse imaging and blastocyst development. Reprod. Fertil. Dev. 2019, 31, 1874. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vargas, P.; Muñoz, M.; Domínguez, F. Identifying biomarkers for predicting successful embryo implantation: Applying single to multi-OMICs to improve reproductive outcomes. Hum. Reprod. Update 2020, 26, 264–301. [Google Scholar] [CrossRef]
- Handyside, A.H.; Kontogianni, E.H.; Hardy, K.; Winston, R.M.L. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature 1990, 344, 768–770. [Google Scholar] [CrossRef]
- Verlinsky, Y.; Ginsberg, N.; Lifchez, A.; Valle, J.; Moise, J.; Strom, C.M. Analysis of the first polar body: Preconception genetic diagnosis. Hum. Reprod. 1990, 5, 826–829. [Google Scholar] [CrossRef]
- Hinrichs, K.; Choi, Y.-H. Equine embryo biopsy, genetic testing, and cryopreservation. J. Equine Vet. Sci. 2012, 32, 390–396. [Google Scholar] [CrossRef]
- Herrera, C.; Morikawa, M.I.; Bello, M.B.; von Meyeren, M.; Eusebio Centeno, J.; Dufourq, P.; Martinez, M.M.; Llorente, J. Setting up equine embryo gender determination by preimplantation genetic diagnosis in a commercial embryo transfer program. Theriogenology 2014, 81, 758–763. [Google Scholar] [CrossRef]
- Choi, Y.H.; Penedo, M.C.T.; Daftari, P.; Velez, I.C.; Hinrichs, K. Accuracy of preimplantation genetic diagnosis in equine in vivo-recovered and in vitro-produced blastocysts. Reprod. Fertil. Dev. 2016, 28, 1382. [Google Scholar] [CrossRef]
- Barandalla, M.; Benedetti, M.; Colleoni, S.; Perota, A.; Galli, C.; Lazzari, G. Genetic diagnosis of Warmblood Fragile Foal Syndrome in equine in vitro produced preimplantation embryos. J. Equine Vet. Sci. 2020, 89, 103047. [Google Scholar] [CrossRef]
- Cimadomo, D.; Rienzi, L.; Capalbo, A.; Rubio, C.; Innocenti, F.; García-Pascual, C.M.; Ubaldi, F.M.; Handyside, A. The dawn of the future: 30 years from the first biopsy of a human embryo. Detail. Hist. Ongoing Revolut. Hum. Reprod. Update 2020, 26, 453–473. [Google Scholar] [CrossRef]
- Leaver, M.; Wells, D. Non-invasive preimplantation genetic testing (niPGT): The next revolution in reproductive genetics? Hum. Reprod. Update 2020, 26, 16–42. [Google Scholar] [CrossRef]
- Herrera, C.; Morikawa, M.I.; Castex, C.B.; Pinto, M.R.; Ortega, N.; Fanti, T.; Garaguso, R.; Franco, M.J.; Castañares, M.; Castañeira, C.; et al. Blastocele fluid from in vitro– and in vivo–produced equine embryos contains nuclear DNA. Theriogenology 2015, 83, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Leibo, S.P.; Sztein, J.M. Cryopreservation of mammalian embryos: Derivation of a method. Cryobiology 2019, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; Mohr, L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 1983, 305, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Downing, B.G.; Mohr, L.R.; Trounson, A.O.; Freeman, L.E.; Wood, C. Birth after transfer of cryopreserved embryos. Med. J. Aust. 1985, 142, 409–411. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Oguri, N.; Tsutsumi, Y.; Hachinohe, Y. Experiments in the freezing and storage of equine embryos. J. Reprod. Fertil. Suppl. 1982, 32, 399–403. [Google Scholar]
- Edgar, D.H.; Gook, D.A. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum. Reprod. Update 2012, 18, 536–554. [Google Scholar] [CrossRef] [PubMed]
- Squires, E.L.; McCue, P.M. Cryopreservation of equine embryos. J. Equine Vet. Sci. 2016, 41, 7–12. [Google Scholar] [CrossRef]
- Carnevale, E.M.; Squires, E.L. Comparison of ham’s F 1 0 W I T H CO2 or hepes buffer for storage of equine embryos at 5 C for 24 H l. J. Anim. Sci. 1987, 65, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef]
- Zaat, T.; Zagers, M.; Mol, F.; Goddijn, M.; van Wely, M.; Mastenbroek, S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst. Rev. 2021, 2, CD011184. [Google Scholar] [CrossRef]
- Galli, C.; Colleoni, S.; Duchi, R.; Lagutina, I.; Lazzari, G. Developmental competence of equine oocytes and embryos obtained by in vitro procedures ranging from in vitro maturation and ICSI to embryo culture, cryopreservation and somatic cell nuclear transfer. Anim. Reprod. Sci. 2007, 98, 39–55. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Hinrichs, K. Vitrification of in vitro -produced and in vivo -recovered equine blastocysts in a clinical program. Theriogenology 2017, 87, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, L.; Polyzos, N.P.; De Munck, N.; Blockeel, C.; Van de Velde, H.; Verheyen, G. A prospective randomized controlled trial investigating the effect of artificial shrinkage (collapse) on the implantation potential of vitrified blastocysts. Hum. Reprod. 2015, 30, 2509–2518. [Google Scholar] [CrossRef]
- Choi, Y.H.; Velez, I.C.; Riera, F.L.; Roldán, J.E.; Hartman, D.L.; Bliss, S.B.; Blanchard, T.L.; Hayden, S.S.; Hinrichs, K. Successful cryopreservation of expanded equine blastocysts. Theriogenology 2011, 76, 143–152. [Google Scholar] [CrossRef]
- Wilsher, S.; Rigali, F.; Kovacsy, S.; Allen, W.R. Successful vitrification of manually punctured equine embryos. Equine Vet. J. 2021, evj.13400. [Google Scholar] [CrossRef]
- Chen, S.-U.; Lien, Y.-R.; Chang, L.-J.; Tsai, Y.-Y.; Ho, H.-N.; Yang, Y.-S. Short communication: Cryopreserved sibling oocytes and intracytoplasmic sperm injection rescue unexpectedly poor fertilization in conventional in vitro fertilization. J. Assist. Reprod. Genet. 2004, 21, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E. Fertility preservation: A Comprehensive approach to the young woman with cancer. J. Natl. Cancer Inst. Monogr. 2005, 2005, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.A.; Swain, J.E. Oocyte cryopreservation: Searching for novel improvement strategies. J. Assist. Reprod. Genet. 2013, 30, 865–875. [Google Scholar] [CrossRef]
- Maclellan, L.J.; Carnevale, E.M.; Scoggin, C.F.; Bruemmer, J.E.; Squires, E.L. Pregnancies from vitrified equine oocytes collected from super-stimulated and non-stimulated mares. Theriogenology 2002, 58, 911–919. [Google Scholar] [CrossRef]
- Ortiz-Escribano, N.; Bogado Pascottini, O.; Woelders, H.; Vandenberghe, L.; De Schauwer, C.; Govaere, J.; Van den Abbeel, E.; Vullers, T.; Ververs, C.; Roels, K.; et al. An improved vitrification protocol for equine immature oocytes, resulting in a first live foal. Equine Vet. J. 2018, 50, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Canesin, H.S.; Brom-de-Luna, J.G.; Choi, Y.-H.; Ortiz, I.; Diaw, M.; Hinrichs, K. Blastocyst development after intracytoplasmic sperm injection of equine oocytes vitrified at the germinal-vesicle stage. Cryobiology 2017, 75, 52–59. [Google Scholar] [CrossRef]
- Ducheyne, K.D.; Rizzo, M.; Daels, P.F.; Stout, T.A.E.; De Ruijter-Villani, M. Vitrifying immature equine oocytes impairs their ability to correctly align the chromosomes on the MII spindle. Reprod. Fertil. Dev. 2019, 31, 1330. [Google Scholar] [CrossRef] [PubMed]
- Angel, D.; Canesin, H.S.; Brom-de-Luna, J.G.; Morado, S.; Dalvit, G.; Gomez, D.; Posada, N.; Pascottini, O.B.; Urrego, R.; Hinrichs, K.; et al. Embryo development after vitrification of immature and in vitro-matured equine oocytes. Cryobiology 2020, 92, 251–254. [Google Scholar] [CrossRef]
- Jeffcott, L.B.; Rossdale, P.D.; Freestone, J.; Frank, C.J.; Towers-Clark, P.F. An assessment of wastage in Thoroughbred racing from conception to 4 years of age. Equine Vet. J. 1982, 14, 185–198. [Google Scholar] [CrossRef]
- Langlois, B.; Blouin, C. Statistical analysis of some factors affecting the number of horse births in France. Reprod. Nutr. Dev. 2004, 44, 583–595. [Google Scholar] [CrossRef]
- Cuervo-Arango, J.; Claes, A.N.; Stout, T.A. A retrospective comparison of the efficiency of different assisted reproductive techniques in the horse, emphasizing the impact of maternal age. Theriogenology 2019, 132, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, E.M.; Ginther, O.J. Relationships of age to uterine function and reproductive efficiency in mares. Theriogenology 1992, 37, 1101–1115. [Google Scholar] [CrossRef]
- Carnevale, E.M.; Bergfelt, D.R.; Ginther, O.J. Aging effects on follicular activity and concentrations of FSH, LH, and progesterone in mares. Anim. Reprod. Sci. 1993, 31, 287–299. [Google Scholar] [CrossRef]
- Rizzo, M.; Ducheyne, K.D.; Deelen, C.; Beitsma, M.; Cristarella, S.; Quartuccio, M.; Stout, T.A.E.; Ruijter-Villani, M. Advanced mare age impairs the ability of in vitro-matured oocytes to correctly align chromosomes on the metaphase plate. Equine Vet. J. 2019, 51, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Campos-Chillon, F.; Farmerie, T.A.; Bouma, G.J.; Clay, C.M.; Carnevale, E.M. Effects of aging on gene expression and mitochondrial DNA in the equine oocyte and follicle cells. Reprod. Fertil. Dev. 2015, 27, 925. [Google Scholar] [CrossRef]
- Rambags, B.P.B.; Van Boxtel, D.C.J.; Tharasanit, T.; Lenstra, J.A.; Colenbrander, B.; Stout, T.A.E. Advancing maternal age predisposes to mitochondrial damage and loss during maturation of equine oocytes in vitro. Theriogenology 2014, 81, 959–965. [Google Scholar] [CrossRef]
- Cox, L.; Vanderwall, D.K.; Parkinson, K.C.; Sweat, A.; Isom, S.C. Expression profiles of select genes in cumulus–oocyte complexes from young and aged mares. Reprod. Fertil. Dev. 2015, 27, 914. [Google Scholar] [CrossRef] [PubMed]
- Doig, P.A.; Mcknight, J.D.; Miller, R.B. The use of endometrial biopsy in the infertile mare. Can. Vet. J. 1981, 22, 72–76. [Google Scholar]
- Siemieniuch, M.J.; Gajos, K.; Kozdrowski, R.; Nowak, M. Advanced age in mares affects endometrial secretion of arachidonic acid metabolites during equine subclinical endometritis. Theriogenology 2017, 103, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Nambo, Y.; Oikawa, M.-A.; Yoshihara, T.; Kuwano, A.; Katayama, Y. Age-related morphometrical changes of arteries of uterine wall in mares. J. Vet. Med. Ser. A 1995, 42, 383–387. [Google Scholar] [CrossRef]
- Oikawa, M.; Katayama, Y.; Yoshihara, T.; Kaneko, M.; Yoshikawa, T. Microscopical characteristics of uterine wall arteries in barren aged mares. J. Comp. Pathol. 1993, 108, 411–415. [Google Scholar] [CrossRef]
- Dubrovskaya, A.B.; Lebedeva, L.F.; Schukis, K.A. Comparative histomorphological characteristics of the endometrium of young and aged mares in estrus and diestrus. IOP Conf. Ser. Earth Environ. Sci. 2019, 341, 012067. [Google Scholar] [CrossRef]
- Pellestor, F.; Andréo, B.; Arnal, F.; Humeau, C.; Demaille, J. Maternal aging and chromosomal abnormalities: New data drawn from in vitro unfertilized human oocytes. Hum. Genet. 2003, 112, 195–203. [Google Scholar] [CrossRef]
- Hassold, T.; Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001, 2, 280–291. [Google Scholar] [CrossRef]
- Ball, B.A.; Little, T.V.; Hillman, R.B.; Woods, G.L. Pregnancy rates at Days 2 and 14 and estimated embryonic loss rates prior to day 14 in normal and subfertile mares. Theriogenology 1986, 26, 611–619. [Google Scholar] [CrossRef]
- Carnevale, E.M.; Ginther, O.J. Defective oocytes as a cause of subfertility in old mares. Biol. Reprod. 1995, 52, 209–214. [Google Scholar] [CrossRef]
- Ruggeri, E.; DeLuca, K.F.; Galli, C.; Lazzari, G.; DeLuca, J.G.; Carnevale, E.M. Cytoskeletal alterations associated with donor age and culture interval for equine oocytes and potential zygotes that failed to cleave after intracytoplasmic sperm injection. Reprod. Fertil. Dev. 2015, 27, 944. [Google Scholar] [CrossRef] [PubMed]
- Brinsko, S.P.; Ball, B.A.; Ellington, J.E. In vitro maturation of equine oocytes obtained from different age groups of sexually mature mares. Theriogenology 1995, 44, 461–469. [Google Scholar] [CrossRef]
- Rizzo, M.; Deelen, C.; Beitsma, M.; Stout, T.A.; De Ruijter-Villani, M. In vitro matured horse oocytes from aged mares show weakened centromeric cohesion and a higher incidence of aneuploidy. J. Equine Vet. Sci. 2020, 89, 103028. [Google Scholar] [CrossRef]
- Shilton, C.A.; Kahler, A.; Davis, B.W.; Crabtree, J.R.; Crowhurst, J.; McGladdery, A.J.; Wathes, D.C.; Raudsepp, T.; de Mestre, A.M. Whole genome analysis reveals aneuploidies in early pregnancy loss in the horse. Sci. Rep. 2020, 10, 13314. [Google Scholar] [CrossRef]
- Vialard, F.; Petit, C.; Bergere, M.; Gomes, D.M.; Martel-Petit, V.; Lombroso, R.; Ville, Y.; Gerard, H.; Selva, J. Evidence of a high proportion of premature unbalanced separation of sister chromatids in the first polar bodies of women of advanced age. Hum. Reprod. 2006, 21, 1172–1178. [Google Scholar] [CrossRef]
- Rizzo, M.; Stout, T.A.E.; Cristarella, S.; Quartuccio, M.; Kops, G.J.P.L.; De Ruijter-Villani, M. Compromised MPS1 activity induces multipolar spindle formation in oocytes from aged mares: Establishing the horse as a natural animal model to study age-induced oocyte meiotic spindle instability. Front. Cell Dev. Biol. 2021, 9, 657366. [Google Scholar] [CrossRef]
- Smits, M.A.J.; Wong, K.M.; Mantikou, E.; Korver, C.M.; Jongejan, A.; Breit, T.M.; Goddijn, M.; Mastenbroek, S.; Repping, S. Age-related gene expression profiles of immature human oocytes. Mol. Hum. Reprod. 2018, 24, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Hashimoto, S.; Yamanaka, M.; Nakano, T.; Satoh, M.; Nakaoka, Y.; Iwata, H.; Fukui, A.; Morimoto, Y.; Shibahara, H. Mitochondrial oxygen consumption rate of human embryos declines with maternal age. J. Assist. Reprod. Genet. 2020, 37, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Shen, H. Mitochondria: Emerging therapeutic strategies for oocyte rescue. Reprod. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Grøndahl, M.L.; Andersen, C.Y.; Bogstad, J.; Nielsen, F.C.; Meinertz, H.; Borup, R. Gene expression profiles of single human mature oocytes in relation to age. Hum. Reprod. 2010, 25, 957–968. [Google Scholar] [CrossRef]
- Llonch, S.; Barragán, M.; Nieto, P.; Mallol, A.; Elosua-Bayes, M.; Lorden, P.; Ruiz, S.; Zambelli, F.; Heyn, H.; Vassena, R.; et al. Single human oocyte transcriptome analysis reveals distinct maturation stage-dependent pathways impacted by age. Aging Cell 2021, 20, e13360. [Google Scholar] [CrossRef] [PubMed]
- Spacek, S.G.; Carnevale, E.M. Impact of equine and bovine oocyte maturation in follicular fluid from young and old mares on embryo production in vitro. J. Equine Vet. Sci. 2018, 68, 94–100. [Google Scholar] [CrossRef]
- Hendriks, W.K.; Colleoni, S.; Galli, C.; Paris, D.B.B.P.; Colenbrander, B.; Roelen, B.A.J.; Stout, T.A.E. Maternal age and in vitro culture affect mitochondrial number and function in equine oocytes and embryos. Reprod. Fertil. Dev. 2015, 27, 957. [Google Scholar] [CrossRef]
- Derisoud, E.; Jouneau, L.; Dubois, C.; Archilla, C.; Jaszczyszyn, Y.; Legendre, R.; Daniel, N.; Peynot, N.; Dahirel, M.; Auclair-Ronzaud, J.; et al. Maternal age affects equine Day 8 embryo gene expression both in trophoblast and inner cell mass. BioRxiv 2021, 438786. [Google Scholar] [CrossRef]
- Coi, A.; Santoro, M.; Garne, E.; Pierini, A.; Addor, M.-C.; Alessandri, J.-L.; Bergman, J.E.H.; Bianchi, F.; Boban, L.; Braz, P.; et al. Epidemiology of achondroplasia: A population-based study in Europe. Am. J. Med. Genet. A 2019, 179, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Sandin, S.; Schendel, D.; Magnusson, P.; Hultman, C.; Surén, P.; Susser, E.; Grønborg, T.; Gissler, M.; Gunnes, N.; Gross, R.; et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol. Psychiatry 2016, 21, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Shinde, D.N.; Elmer, D.P.; Calabrese, P.; Boulanger, J.; Arnheim, N.; Tiemann-Boege, I. New evidence for positive selection helps explain the paternal age effect observed in achondroplasia. Hum. Mol. Genet. 2013, 22, 4117–4126. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and overweight. Fact Sheets. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 22 June 2021).
- Gallus, S.; Lugo, A.; Murisic, B.; Bosetti, C.; Boffetta, P.; La Vecchia, C. Overweight and obesity in 16 European countries. Eur. J. Nutr. 2015, 54, 679–689. [Google Scholar] [CrossRef]
- Jensen, R.B.; Danielsen, S.H.; Tauson, A.-H. Body condition score, morphometric measurements and estimation of body weight in mature Icelandic horses in Denmark. Acta. Vet. Scand. 2016, 58, 59. [Google Scholar] [CrossRef]
- Wyse, C.A.; McNie, K.A.; Tannahil, V.J.; Murray, J.K.; Love, S. Prevalence of obesity in riding horses in Scotland. Vet. Rec. 2008, 162, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, C.D.; Pleasant, R.S.; Geor, R.J.; Elvinger, F. Prevalence of Overconditioning in Mature Horses in Southwest Virginia during the Summer. J. Vet. Intern. Med. 2012, 26, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Harker, I.J.; Harris, P.A.; Barfoot, C.F. The body condition score of leisure horses competing at an unaffiliated championship in the UK. J. Equine Vet. Sci. 2011, 31, 253–254. [Google Scholar] [CrossRef]
- Frank, N.; Geor, R.J.; Bailey, S.R.; Durham, A.E.; Johnson, P.J. American college of veterinary internal, m. equine metabolic syndrome. J. Vet. Intern. Med./Am. Coll. Vet. Intern. Med. 2010, 24, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 960, pp. 1–17. [Google Scholar] [CrossRef]
- Grieger, J.A.; Hutchesson, M.J.; Cooray, S.D.; Bahri Khomami, M.; Zaman, S.; Segan, L.; Teede, H.; Moran, L.J. A review of maternal overweight and obesity and its impact on cardiometabolic outcomes during pregnancy and postpartum. Ther. Adv. Reprod. Health 2021, 15, 2633494120986544. [Google Scholar] [CrossRef] [PubMed]
- Gesink Law, D.C.; Maclehose, R.F.; Longnecker, M.P. Obesity and time to pregnancy. Hum. Reprod. 2006, 22, 414–420. [Google Scholar] [CrossRef]
- Incedal Irgat, S.; Bakirhan, H. The effect of obesity on human reproductive health and foetal life. Hum. Fertil. 2021, 1–12. [Google Scholar] [CrossRef]
- Sessions, D.R.; Reedy, S.E.; Vick, M.M.; Murphy, B.A.; Fitzgerald, B.P. Development of a model for inducing transient insulin resistance in the mare: Preliminary implications regarding the estrous cycle12. J. Anim. Sci. 2004, 82, 2321–2328. [Google Scholar] [CrossRef]
- Gentry, L.R.; Thompson, D.L.J.; Gentry, G.T.J.; Davis, K.A.; Godke, R.A.; Cartmill, J.A. The relationship between body condition, leptin, and reproductive and hormonal characteristics of mares during the seasonal anovulatory period. J. Anim. Sci. 2002, 80, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- D’Fonseca, N.M.M.; Gibson, C.M.E.; Hummel, I.; van Doorn, D.A.; Roelfsema, E.; Stout, T.A.E.; van den Broek, J.; de Ruijter-Villani, M. Overfeeding extends the period of annual cyclicity but increases the risk of early embryonic death in shetland pony mares. Animals 2021, 11, 361. [Google Scholar] [CrossRef]
- Waller, C.A.; Thompson, D.L.; Cartmill, J.A.; Storer, W.A.; Huff, N.K. Reproduction in high body condition mares with high versus low leptin concentrations. Theriogenology 2006, 66, 923–928. [Google Scholar] [CrossRef]
- Provost, M.P.; Acharya, K.S.; Acharya, C.R.; Yeh, J.S.; Steward, R.G.; Eaton, J.L.; Goldfarb, J.M.; Muasher, S.J. Pregnancy outcomes decline with increasing body mass index: Analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008–2010 society for assisted reproductive technology registry. Fertil. Steril. 2016, 105, 663–669. [Google Scholar] [CrossRef]
- Sermondade, N.; Huberlant, S.; Bourhis-Lefebvre, V.; Arbo, E.; Gallot, V.; Colombani, M.; Fréour, T. Female obesity is negatively associated with live birth rate following IVF: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Wang, N.; Wang, M.; Liu, Y.; Niu, Z.; Ding, Z. TMT-based proteomic and bioinformatic analyses of human granulosa cells from obese and normal-weight female subjects. Reprod. Biol. Endocrinol. 2021, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Sessions-Bresnahan, D.R.; Heuberger, A.L.; Carnevale, E.M. Obesity in mares promotes uterine inflammation and alters embryo lipid fingerprints and homeostasis†. Biol. Reprod. 2018, 99, 761–772. [Google Scholar] [CrossRef]
- Vick, M.M.; Adams, A.A.; Murphy, B.A.; Sessions, D.R.; Horohov, D.W.; Cook, R.F.; Shelton, B.J.; Fitzgerald, B.P. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse1,2. J. Anim. Sci. 2007, 85, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Pennington, P.M.; Splan, R.K.; Jacobs, R.D.; Chen, Y.; Singh, R.P.; Li, Y.; Gucek, M.; Wagner, A.L.; Freeman, E.W.; Pukazhenthi, B.S. Influence of Metabolic Status and Diet on Early Pregnant Equine Histotroph Proteome: Preliminary Findings. J. Equine Vet. Sci. 2020, 88, 102938. [Google Scholar] [CrossRef]
- Sessions-Bresnahan, D.R.; Carnevale, E.M. The effect of equine metabolic syndrome on the ovarian follicular environment1. J. Anim. Sci. 2014, 92, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Sessions-Bresnahan, D.R.; Schauer, K.L.; Heuberger, A.L.; Carnevale, E.M. Effect of obesity on the preovulatory follicle and lipid fingerprint of equine oocytes1. Biol. Reprod. 2016, 94. [Google Scholar] [CrossRef]
- Boutari, C.; Pappas, P.D.; Mintziori, G.; Nigdelis, M.P.; Athanasiadis, L.; Goulis, D.G.; Mantzoros, C.S. The effect of underweight on female and male reproduction. Metabolism 2020, 107, 154229. [Google Scholar] [CrossRef] [PubMed]
- Kishali, N.F.; Imamoglu, O.; Katkat, D.; Atan, T.; Akyol, P. Effects of menstrual cycle on sports performance. Int. J. Neurosci. 2006, 116, 1549–1563. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: A systematic review and meta-analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef]
- Heather, A.K.; Thorpe, H.; Ogilvie, M.; Sims, S.T.; Beable, S.; Milsom, S.; Schofield, K.L.; Coleman, L.; Hamilton, B. Biological and socio-cultural factors have the potential to influence the health and performance of elite female athletes: A cross sectional survey of 219 elite female athletes in aotearoa new zealand. Front. Sports Act. Living 2021, 3, 601420. [Google Scholar] [CrossRef]
- Sundgot-Borgen, J.; Sundgot-Borgen, C.; Myklebust, G.; Sølvberg, N.; Torstveit, M.K. Elite athletes get pregnant, have healthy babies and return to sport early postpartum. BMJ Open Sport Exerc. Med. 2019, 5, e000652. [Google Scholar] [CrossRef]
- Mortensen, C.J.; Choi, Y.H.; Hinrichs, K.; Ing, N.H.; Kraemer, D.C.; Vogelsang, S.G.; Vogelsang, M.M. Embryo recovery from exercised mares. Anim. Reprod. Sci. 2009, 110, 237–244. [Google Scholar] [CrossRef]
- Kelley, D.E.; Gibbons, J.R.; Smith, R.; Vernon, K.L.; Pratt-Phillip, S.E.; Mortensen, C.J. Exercise affects both ovarian follicular dynamics and hormone concentrations in mares. Theriogenology 2011, 76, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Vernon, K.L.; Kelley, D.E.; Gibbons, J.R.; Mortensen, C.J. Impact of moderate exercise on ovarian blood flow and early embryonic outcomes in mares1. J. Anim. Sci. 2012, 90, 3770–3777. [Google Scholar] [CrossRef]
- Pessoa, M.A.; Cannizza, A.P.; Reghini, M.F.S.; Alvarenga, M.A. Embryo Transfer efficiency of quarter horse athletic mares. J. Equine Vet. Sci. 2011, 31, 703–705. [Google Scholar] [CrossRef]
- Pinto, M.R.; Miragaya, M.H.; Burns, P.; Douglas, R.; Neild, D.M. Strategies for increasing reproductive efficiency in a commercial embryo transfer program with high performance donor mares under training. J. Equine Vet. Sci. 2017, 54, 93–97. [Google Scholar] [CrossRef]
- Sairanen, J.; Katila, T.; Virtala, A.-M.; Ojala, M. Effects of racing on equine fertility. Anim. Reprod. Sci. 2011, 124, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Anton, J.E.; Vernon, K.L.; Kelley, D.E.; Gibbons, J.R.; Birrenkott, G.; Mortensen, C.J. Exercising the pregnant mare from day 16 to day 80 of gestation. J. Equine Vet. Sci. 2014, 34, 415–420. [Google Scholar] [CrossRef]
- Kilgenstein, H.J.; Schöniger, S.; Schoon, D.; Schoon, H.-A. Microscopic examination of endometrial biopsies of retired sports mares: An explanation for the clinically observed subfertility? Res. Vet. Sci. 2015, 99, 171–179. [Google Scholar] [CrossRef]
- Farin, C.E.; Farmer, W.T.; Farin, P.W. Pregnancy recognition and abnormal offspring syndrome in cattle. Reprod. Fertil. Dev. 2010, 22, 75. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Kalbfleisch, T.S.; Rice, E.S.; DePriest, M.S.; Walenz, B.P.; Hestand, M.S.; Vermeesch, J.R.; O′Connell, B.L.; Fiddes, I.T.; Vershinina, A.O.; Saremi, N.F.; et al. Improved reference genome for the domestic horse increases assembly contiguity and composition. Commun. Biol. 2018, 1, 197. [Google Scholar] [CrossRef]
- Okólski, A.; Bézard, J.; Duchamp, G.; Driancourt, M.-A.; Goudet, G.; Palmer, E. Successive Puncture of the dominant follicle followed by ovulation and fertilization: A new experimental model for the study of follicular maturation in the mare1. Biol. Reprod. 1995, 52, 385–392. [Google Scholar] [CrossRef]
- Palmer, E.; Duchamp, G.; Cribiu, E.P.; Mahla, R.; Boyazoglu, S.; Bézard, J. Follicular fluid is not a compulsory carrier of the oocyte at ovulation in the mare. Equine Vet. J. 1997, 29, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Goudet, G.; Bezard, J.; Duchamp, G.; Palmer, E. Transfer of immature oocytes to a preovulatory follicle: An alternative to in vitro maturation in the mare? Equine Vet. J. 1997, 25, 54–59. [Google Scholar] [CrossRef]
- Bashir, S.T.; Gastal, M.O.; Tazawa, S.P.; Tarso, S.G.S.; Hales, D.B.; Cuervo-Arango, J.; Baerwald, A.R.; Gastal, E.L. The mare as a model for luteinized unruptured follicle syndrome: Intrafollicular endocrine milieu. Reproduction 2016, 151, 271–283. [Google Scholar] [CrossRef]
- Panelli, D.M.; Phillips, C.H.; Brady, P.C. Incidence, diagnosis and management of tubal and nontubal ectopic pregnancies: A review. Fertil. Res. Pr. 2015, 1, 15. [Google Scholar] [CrossRef]
- Allen, W.R.; Wilsher, S.; Morris, L.; Crowhurst, J.S.; Hillyer, M.H.; Neal, H.N. Laparoscopic application of PGE2 to re-establish oviducal patency and fertility in infertile mares: A preliminary study. Equine Vet. J. 2010, 38, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Katila, T.; Reilas, T.; Nivola, K.; Peltonen, T.; Virtala, A.-M. A 15-year survey of reproductive efficiency of Standardbred and Finnhorse trotters in Finland-descriptive results. Acta Vet. Scand. 2010, 11. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Veiga-Lopez, A. Sheep models of polycystic ovary syndrome phenotype. Mol. Cell. Endocrinol. 2013, 373, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Bourgneuf, C.; Bailbé, D.; Lamazière, A.; Dupont, C.; Moldes, M.; Farabos, D.; Roblot, N.; Gauthier, C.; Mathieu d’Argent, E.; Cohen-Tannoudji, J.; et al. The Goto-Kakizaki rat is a spontaneous prototypical rodent model of polycystic ovary syndrome. Nat. Commun. 2021, 12, 1064. [Google Scholar] [CrossRef]

| Woman | Mare | ||
|---|---|---|---|
| Similarities | Physiology | Formation of primordial follicles during fetal life | |
| Two or three follicular waves per ovarian cycle | |||
| Mono-ovulatory cycles, LUF syndrome | |||
| Timing of early developmental stages until the blastocyst stage | |||
| Easy and efficient in vitro embryo culture up to the blastocyst stage in humans, a bit less efficient in horses | |||
| ART procedure | ICSI is widely used in both species | ||
| Blastocoel collapsing is used for freezing embryos | |||
| Development of several tools for embryo selection in ART procedures, including embryo biopsy and time-lapse | |||
| Shared interest in non-invasive preimplantation genetic testing | |||
| Impact of environment | Reduction of fertility with age | ||
| Frequency of embryonic aneuploidies | |||
| Menopause for woman; ovarian senescence for mares | |||
| Potential impact of excess sport on spontaneous reproduction | |||
| Effects of obesity on cycles, inflammation, follicular fatty acids and triglycerides, lower oocyte and embryo quality | |||
| Differences | Physiology | Preovulatory follicles: 2 cm diameter | Large follicles (×2.1 human) |
| ZP surrounding the embryo | Presence of a capsule in addition to the ZP | ||
| Uterine prostaglandin secretion not needed for luteolysis | Uterine prostaglandin secretion needed for luteolysis | ||
| Human blastocyst only reaches a maximum size of 200 μm and a few hundred cells before implantation | Horse blastocyst is larger on Day 7 (200–800 μm) | ||
| Hatching and implantation on Day 7–10 | No hatching, implantation on Days 35–40 | ||
| PCOS | No PCOS | ||
| ART procedure | Oocyte collection limited to OPU OPU mostly performed after ovarian stimulation | Oocytes can be collected from live (by OPU) or dead mares OPU only performed in non-stimulated mares Oocyte embedded in the follicular wall making it necessary to scrape-off the follicular wall | |
| Possibility of ovarian stimulation, IVM, IVF, ICSI | Possibility of only IVM and ICSI; IVF not efficient | ||
| Effective embryo freezing at all stages of development | Effective embryo freezing on embryos < 300 µm in size and in vitro produced embryos; methods need to be improved | ||
| Oocyte vitrification is mastered | The technique of oocyte freezing needs to be improved and is not used commercially | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benammar, A.; Derisoud, E.; Vialard, F.; Palmer, E.; Ayoubi, J.M.; Poulain, M.; Chavatte-Palmer, P. The Mare: A Pertinent Model for Human Assisted Reproductive Technologies? Animals 2021, 11, 2304. https://doi.org/10.3390/ani11082304
Benammar A, Derisoud E, Vialard F, Palmer E, Ayoubi JM, Poulain M, Chavatte-Palmer P. The Mare: A Pertinent Model for Human Assisted Reproductive Technologies? Animals. 2021; 11(8):2304. https://doi.org/10.3390/ani11082304
Chicago/Turabian StyleBenammar, Achraf, Emilie Derisoud, François Vialard, Eric Palmer, Jean Marc Ayoubi, Marine Poulain, and Pascale Chavatte-Palmer. 2021. "The Mare: A Pertinent Model for Human Assisted Reproductive Technologies?" Animals 11, no. 8: 2304. https://doi.org/10.3390/ani11082304
APA StyleBenammar, A., Derisoud, E., Vialard, F., Palmer, E., Ayoubi, J. M., Poulain, M., & Chavatte-Palmer, P. (2021). The Mare: A Pertinent Model for Human Assisted Reproductive Technologies? Animals, 11(8), 2304. https://doi.org/10.3390/ani11082304








