Regulation of Nutritional Metabolism in Transition Dairy Goats: Energy Balance, Liver Activity, and Insulin Resistance in Response to Berberine Supplementation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Design, Animal Management, and Treatments
2.3. Data Collection, Calculations, and Analyses
2.3.1. Dry Matter Intake, Body Weight, and Body Condition Score
2.3.2. Energy Balance
2.3.3. Milk Yield and Milk Components
2.3.4. Biomarkers of Energy Balance and IR
2.3.5. Biomarkers and Quantification of Liver Function
2.4. Statistical Analysis
3. Results
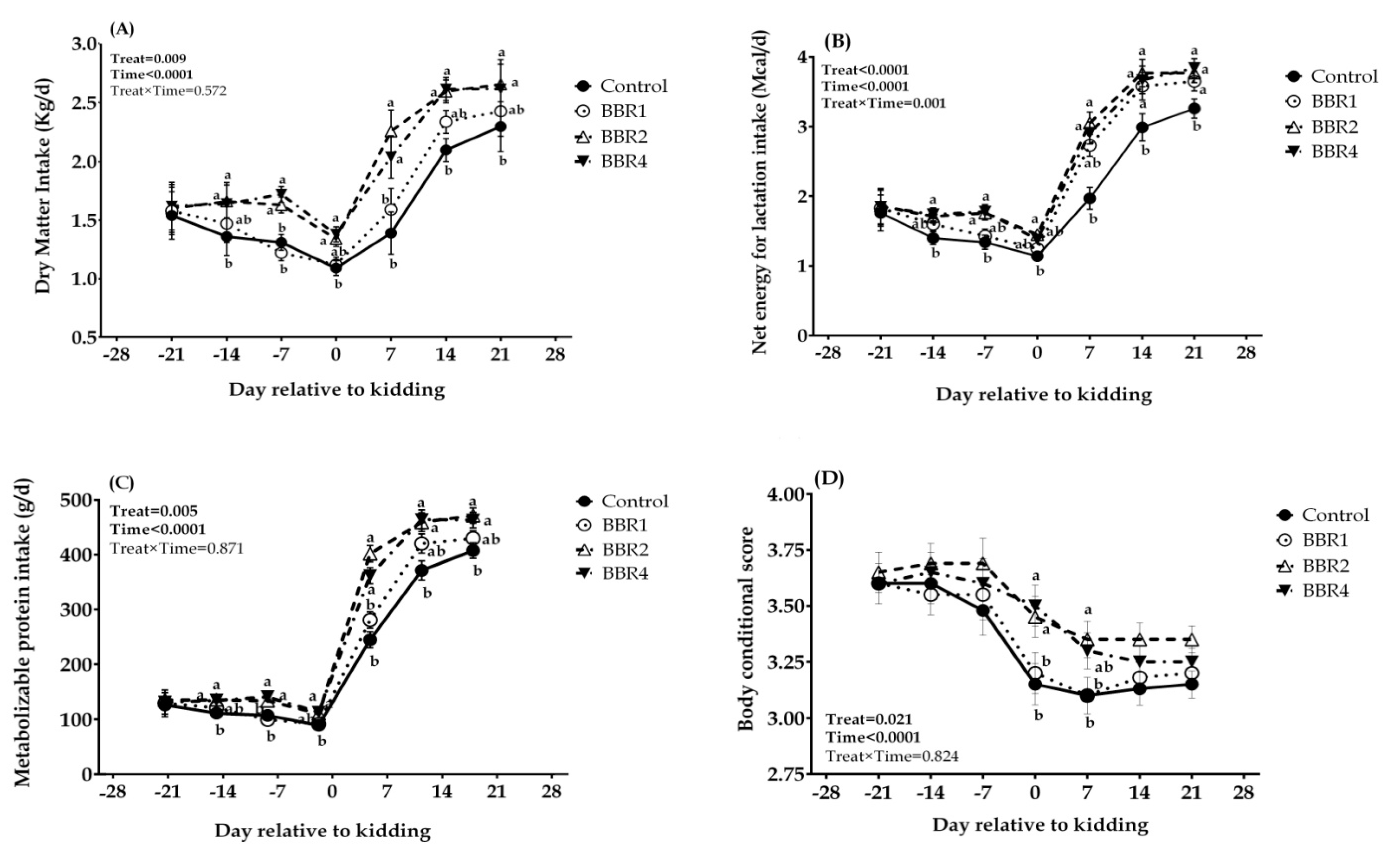
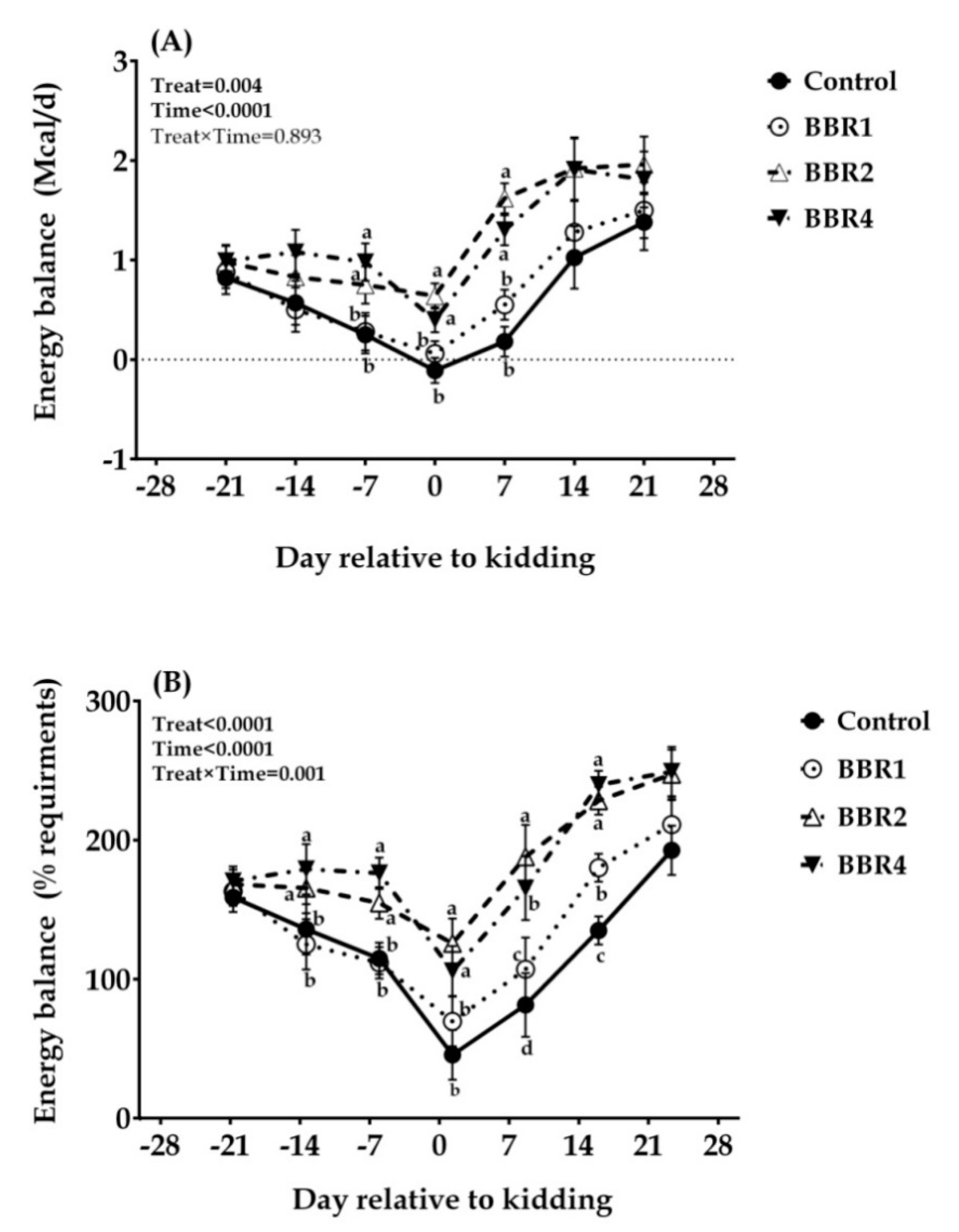
3.1. Dry Matter Intake, Body Condition Score, and Energy Balance
3.2. Milk Yield and Milk Components
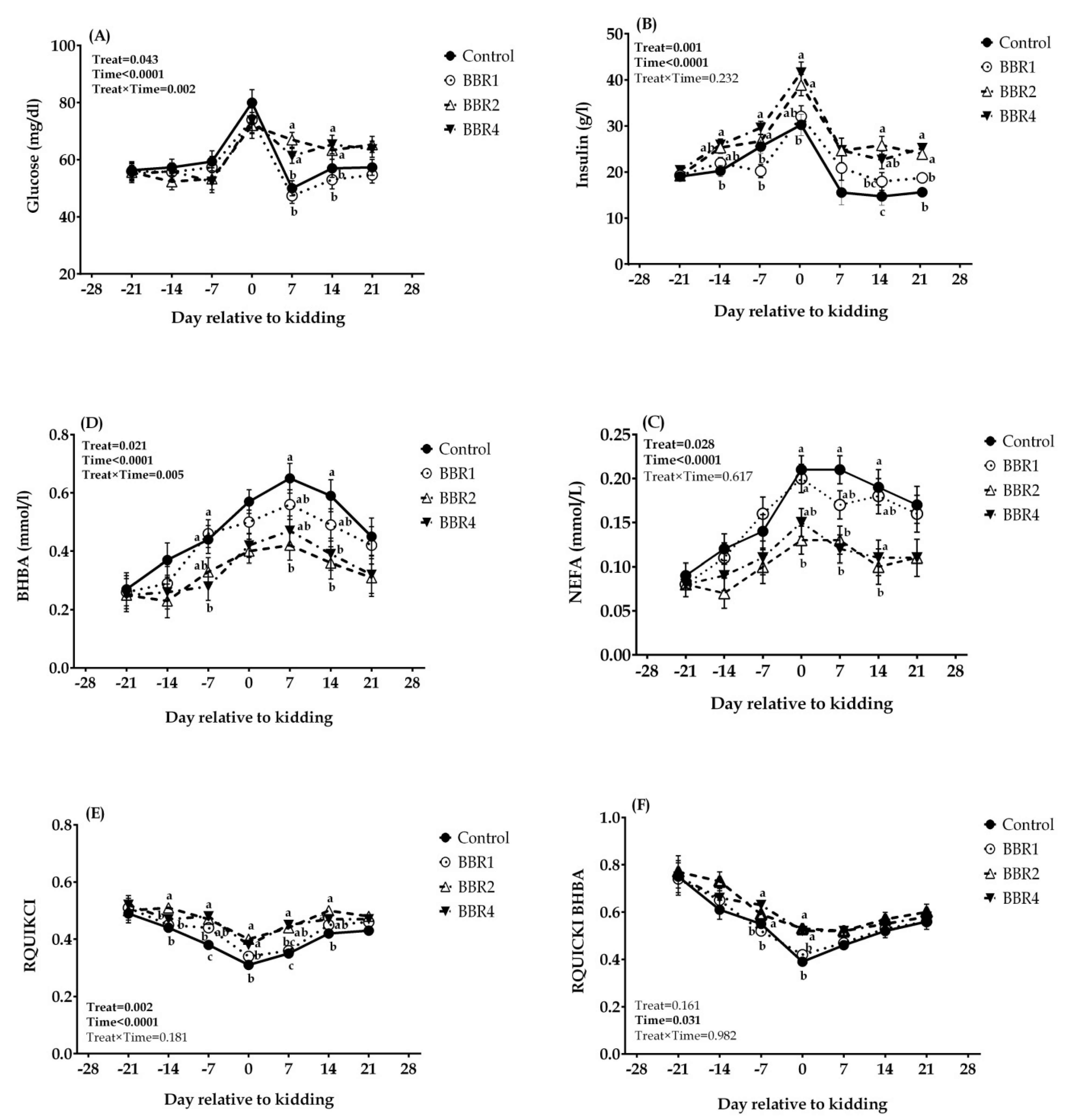
3.3. Biomarkers of Energy Balance and Insulin Resistance
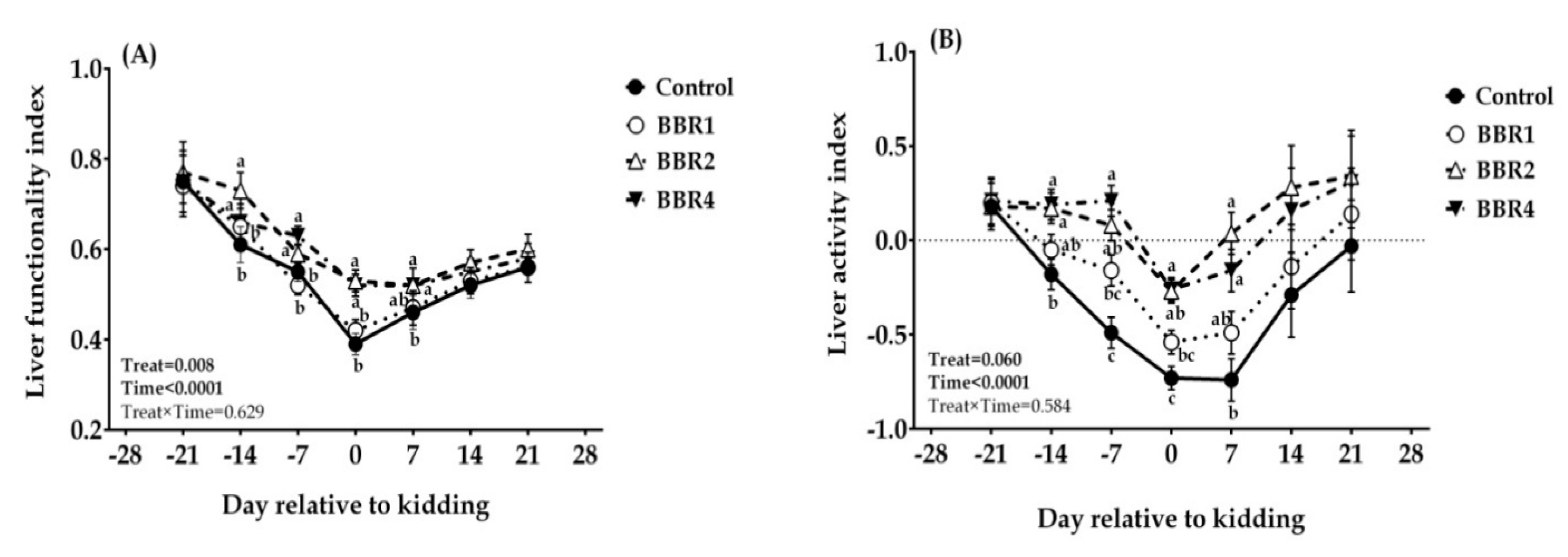
3.4. Biomarkers and Quantification of Liver Function
4. Discussion
4.1. Dry Matter Intake, Body Condition Score, and Energy Balance
4.2. Milk Yield and Components
4.3. Biomarkers of Energy Balance and IR
4.4. Biomarkers and Quantification of Liver Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, A.; Khan, M.J.; Graugnard, D.E.; Vailati-Riboni, M.; Rodriguez-Zas, S.L.; Osorio, J.S.; Loor, J.J. Prepartal energy intake alters blood polymorphonuclear leukocyte transcriptome during the peripartal period in Holstein cows. Bioinform. Biol. Insights 2017, 11, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E. Use of the liver activity index and other metabolic variables in the assessment of metabolic health in dairy herds. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 413–431. [Google Scholar] [CrossRef]
- Dann, H.M.; Litherland, N.B.; Underwood, J.P.; Bionaz, M.; D’Angelo, A.; Mcfadden, J.W. Diets during far-off and close-up dry periods affect periparturient metabolism and lactation in multiparous cows. J. Dairy Sci. 2006, 89, 3563–3577. [Google Scholar] [CrossRef]
- Trevisi, E.; Riva, F.; Filipe, J.F.S.; Massara, M.; Minuti, A.; Bani, P.; Amadori, M. Innate immune responses to metabolic stress can be detected in rumen fluids. Res. Vet. Sci. 2018, 117, 65–73. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Kalscheur, K.F.; Drackley, J.K. Symposium review: Nutrition strategies for improved health, production, and fertility during the transition period. J. Dairy Sci. 2020, 103, 5684–5693. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Zamuner, F.; DiGiacomo, K.; Cameron, A.W.N.; Leury, B.J. Associations between nonesterified fatty acids, β-hydroxybutyrate, and glucose in periparturient dairy goats. J. Dairy Sci. 2020, 103, 6672–6678. [Google Scholar] [CrossRef]
- Bell, A.W. Regulation of organic nutrient metabolism duringtransition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- Zamuner, F.; Cameron, A.W.N.; Carpenter, E.K.; Leury, B.J.; Di Giacomo, K. Endocrine and metabolic responses to glucose, insulin, and adrenocorticotropin infusions in early-lactation dairy goats of high and low milk yield. J. Dairy Sci. 2020, 103, 12045–12058. [Google Scholar] [CrossRef] [PubMed]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr. Physiol. 2011, 9, 1–58. [Google Scholar] [CrossRef]
- Kim, C.; Newton, K.M.; Knopp, R.H. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care 2002, 25, 1862–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. Periparturient immunosuppression and strategies to improve dairy cow health during the periparturient period. Res. Vet. Sci. 2016, 108, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef] [Green Version]
- Grummer, R.R. Nutritional and management strategies for the prevention of fatty liver in dairy cattle. Vet. J. 2008, 176, 10–20. [Google Scholar] [CrossRef]
- Alemardan, A.; Asadi, W.; Rezaei, M.; Tabrizi, L.; Mohammadi, S. Cultivation of Iranian seedless barberry (Berberis integerrima ‘Bidaneh’): A medicinal shrub. Ind. Crops. Prod. 2013, 50, 276–287. [Google Scholar] [CrossRef]
- Chow, Y.L.; Sogame, M.; Sato, F. 13-Methylberberine, a berberine analogue with stronger anti-adipogenic effects on mouse 3T3-L1 cells. Sci. Rep. 2016, 6, 38129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, W.L.; Zhu, L.; Jiang, J.G. Active ingredients from natural botanicals in the treatment of obesity. Obes. Rev. 2014, 15, 957–967. [Google Scholar] [CrossRef]
- Chen, Q.M.; Xie, M.Z. Studies on the hypoglycemic effect of Coptis chinensis and berberine. Acta Pharm. Sin. 1986, 21, 401–406. [Google Scholar]
- Pirillo, A.; Catapano, A.L. Berberine, a plant alkaloid with lipid-and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef]
- Cok, A.; Plaisier, C.; Salie, M.J.; Oram, D.S.; Chenge, J.; Louters, L.L. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 2011, 93, 1187–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardie, D.G. AMP-activated protein kinase: A master switch in glucose and lipid metabolism. Rev. Endocr. Metab. Disord. 2004, 5, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.S.; Choi, S.B.; Park, S.K.; Jang, J.S.; Kim, Y.E.; Park, S. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol. Pharm. Bull. 2005, 28, 1431–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Weng, J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE 2011, 6, e16556. [Google Scholar] [CrossRef] [Green Version]
- Leng, S.H.; Lu, F.E.; Xu, L.J. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol. Sin. 2004, 25, 496–502. [Google Scholar]
- Iranian Council of Animal Care. Guide to the Care and Use of Experimental Animals; Isfahan University of Technology: Isfahan, Iran, 1995. [Google Scholar]
- Durairajan, S.S.K.; Liu, L.F.; Lu, J.H.; Chen, L.L.; Yuan, Q.; Chung, S.K.; Li, M. Berberine ameliorates β-amyloid pathology, gliosis, and cognitive impairment in an Alzheimer’s disease transgenic mouse model. Neurobiol. Aging. 2012, 33, 2903–2919. [Google Scholar] [CrossRef]
- He, W.; Wang, C.; Chen, Y.; He, Y.; Cai, Z. Berberine attenuates cognitive impairment and ameliorates tau hyperphosphorylation by limiting the self-perpetuating pathogenic cycle between NF-κB signaling, oxidative stress and neuroinflammation. Pharmacol. Rep. 2017, 69, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y.I. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [Green Version]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Osorio, J.S.; Trevisi, E.; Ji, P.; Drackley, J.K.; Luchini, D.; Bertoni, G.; Loor, J.J. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J. Dairy Sci. 2014, 97, 7437–7450. [Google Scholar] [CrossRef] [Green Version]
- Villaquiran, M.; Gipson, T.A.; Merkel, R.C.; Goetsch, A.L.; Sahlu, T. Body Condition Scores in Goats; American Institute for Goat Research, Langston University: Lanston, OK, USA, 2004. [Google Scholar]
- Gaines, W.L. An efficiency formula for dairy cows. Science 1928, 67, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Reist, M.; Erdin, D.K.; von Euw, D.; Tschümperlin, K.M.; Leuenberger, H.; Hammon, H.M.; Blum, J.W. Postpartum reproductive function: Association with energy, metabolic and endocrine status in high yielding dairy cows. Theriogenology 2003, 59, 1707–1723. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. A comparative study of the metabolic profile, insulin sensitivity and inflammatory response between organically and conventionally managed dairy cattle during the periparturient period. Animal 2014, 8, 1516–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisi, E.; Amadori, M.; Archetti, I.; Lacetera, N.; Bertoni, G. Inflammatory response and acute phase proteins in the transition period of high-yielding dairy cows. Acute Phase Proteins Early Non-Specif. Biomark. Hum. Vet. Dis. 2011, 15, 355–379. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.F.; Cao, Y.C.; Cai, C.J.; Chao, Y.U.; Li, S.X.; Yao, J.H. Temporal dynamics of nutrient balance, plasma biochemical and immune traits, and liver function in transition dairy cows. J. Integr. Agric. 2020, 19, 820–837. [Google Scholar] [CrossRef]
- Albay, M.K.; Karakurum, M.C.; Şahinduran, S.; Sezer, K.; Yıldız, R.; Büyükoğlu, T. Selected serum biochemical parameters and acute phase protein levels in a herd of Saanen goats showing signs of pregnancy toxaemia. Vet. Med. 2014, 59, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Cabiddu, A.; Dattena, M.; Decandia, M.; Molle, G.; Lopreiato, V.; Minuti, A.; Trevisi, E. The effect of parity number on the metabolism, inflammation, and oxidative status of dairy sheep during the transition period. J. Dairy Sci. 2020, 103, 8564–8575. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Zhang, J.; Sun, C.; Lopez, A. Berberine improves glucose homeostasis in streptozotocin-induced diabetic rats in association with multiple factors of insulin resistance. Int. Sch. Res. Not. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tillhon, M.; Ortiz, L.M.G.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef]
- Sadjadian, R.; Seifi, H.A.; Mohri, M.; Naserian, A.A.; Farzaneh, N. Effects of monensin on metabolism and production in dairy Saanen goats in periparturient period. Asian-Australas J. Anim. Sci. 2013, 26, 82. [Google Scholar] [CrossRef]
- Zhang, F.; Nan, X.; Wang, H.; Zhao, Y.; Guo, Y.; Xiong, B. Effects of propylene glycol on negative energy balance of postpartum dairy cows. Animals 2020, 10, 1526. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, H.K.; Baluchnejadmojarad, T.; Roghani, M.; Goshadrou, F.; Ronaghi, A. Berberine chloride improved synaptic plasticity in STZ induced diabetic rats. Metab. Brain Dis. 2013, 28, 421–428. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Piao, X.S.; Zhang, Q.; Li, P.; Yi, J.Q.; Liu, J.D.; Wang, G.Q. The effects of Forsythia suspensa extract and berberine on growth performance, immunity, antioxidant activities, and intestinal microbiota in broilers under high stocking density. Poult. Sci. J. 2013, 92, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Pang, T.; Gu, M.; Gao, A.H.; Xie, C.M.; Li, J.Y.; Li, J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim. Biophys. Acta 2006, 1760, 1682–1689. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Kim, J.B. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.; Li, J.Y.; Gosby, A.; To, S.W.; Cheng, Z.; Miyoshi, H.; Ye, J.M. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: A mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 2008, 57, 1414–1418. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Ye, J.; Jia, W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.W.; Williams, E.J.; Evans, A.C.O. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef]
- Tosto, M.S.L.; Santos, S.A.; da Costa Pinto Filho, R.; de Carvalho Rodrigues, T.C.G.; Nicory, I.M.C.; de Carvalho, G.G.P.; de Jesus Pereira, T.C. Metabolic and behavior changings during the transition period as predictors of calving proximity and welfare of dairy goats. Vet. Anim. Sci. 2021, 11, 100–108. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.J.; Veerkamp, R.F. Energy balance of dairy cattle in relation to milk production variables and fertility. J. Dairy Sci. 2000, 83, 62–69. [Google Scholar] [CrossRef]
- Staples, C.R.; Burke, J.M.; Thatcher, W.W. Influence of supplemental fats on reproductive tissues and performance of lactating cows. J. Dairy Sci. 1998, 81, 856–871. [Google Scholar] [CrossRef]
- Ballou, M.A.; Gomes, R.C.; Juchem, S.O.; De Peters, E.J. Effects of dietary supplemental fish oil during the peripartum period on blood metabolites and hepatic fatty acid compositions and total triacylglycerol concentrations of multiparous Holstein cows. J. Dairy Sci. 2009, 92, 657–669. [Google Scholar] [CrossRef] [Green Version]
- Salin, S.; Vanhatalo, A.; Jaakkola, S.; Elo, K.; Taponen, J.; Boston, R.C.; Kokkonen, T. Effects of dry period energy intake on insulin resistance, metabolic adaptation, and production responses in transition dairy cows on grass silage–based diets. J. Dairy Sci. 2018, 101, 11364–11383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drong, C.; Meyer, U.; Von Soosten, D.; Frahm, J.; Rehage, J.; Breves, G.; Dänicke, S. Effect of monensin and essential oils on performance and energy metabolism of transition dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Liang, T.; Muckey, M.B.; Mendonça, L.G.D.; Hulbert, L.E.; Elrod, C.C.; Bradford, B.J. Yeast product supplementation modulated feeding behavior and metabolism in transition dairy cows. J. Dairy Sci. 2015, 98, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Koster, J.D.; Opsomer, G. Insulin resistance in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef]
- Chilliard, Y.; Sauvant, D.; Hervieu, J.; Dorleans, M.; Morand-Fehr, P. Lipoprotein lipase activity and composition of omental adipose tissue as related to lipid metabolism of the goat in late pregnancy and early lactation. Ann. Sci. Nat. B 1977, 17, 1021–1033. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Liu, Q.; Li, Q.; Liu, B.; Yang, Y.; Zhang, N. Berberine reduces pyruvate-driven hepatic glucose production by limiting mitochondrial import of pyruvate through mitochondrial pyruvate carrier1. EBioMedicine 2018, 34, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Li, X.B.; Peng, Z.C.; Fu, S.P.; Zhao, C.X.; Du, X.L.; Li, X.W. Berberine protects against NEFA-induced impairment of mitochondrial respiratory chain function and insulin signaling in bovine hepatocytes. Int. J. Mol. Sci. 2018, 19, 1691. [Google Scholar] [CrossRef] [Green Version]
- Berlinguer, F.; Porcu, C.; Molle, G.; Cabiddu, A.; Dattena, M.; Gallus, M.; Zinellu, A. Circulating concentrations of key regulators of nitric oxide production in undernourished sheep carrying single and multiple fetuses. Animals 2020, 10, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruderman, N.B.; Cacicedo, J.M.; Itani, S.; Yagihashi, N.; Saha, A.K.; Ye, J.M.; Ido, Y. Malonyl-CoA and AMP-activated protein kinase (AMPK): Possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem. Soc. Trans. 2003, 31, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Shinjyo, N.; Parkinson, J.; Bell, J.; Katsuno, T.; Bligh, A. Berberine for prevention of dementia associated with diabetes and its comorbidities: A systematic review. J. Integr. Med. 2020, 18, 125–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othman, M.S.; Safwat, G.; Aboulkhair, M.; Moneim, A.E.A. The potential effect of berberine in mercury-induced hepatorenal toxicity in albino rats. Food Chem. Toxicol. 2014, 69, 175–181. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Hu, H.; Lu, Y.; Chen, J.; Yasuda, K.; Wang, H. Berberine inhibits hepatic stellate cell proliferation and prevents experimental liver fibrosis. Biol. Pharm. Bull. 2009, 32, 1533–1537. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
| Item | Diets | |
|---|---|---|
| Pre-Partum a | Post-Partum b | |
| Ingredient (% of DM) | ||
| Alfalfa hay | 4.00 | 29.5 |
| Corn silage | 34.3 | 10.8 |
| Wheat straw | 17.9 | 5.00 |
| Barley grain, ground | 7.70 | 10.8 |
| Corn grain, ground | 31.5 | 22.2 |
| Soybean meal | 1.00 | 17.0 |
| Wheat bran | 1.80 | 2.20 |
| Calcium carbonate | 0.90 | 1.00 |
| Minerals and vitamins premix c | 0.90 | 0.50 |
| Salt | 0.00 | 1.00 |
| Chemical composition | ||
| ME (Mcal/kg of DM) | 2.60 | 2.90 |
| NEL (Mcal/kg of DM) | 1.60 | 1.82 |
| CP (% DM) | 18.5 | 15.5 |
| Ether extract (% DM) | 2.50 | 2.50 |
| Ash (% DM) | 7.60 | 8.00 |
| NDF (% DM) | 43.0 | 37.3 |
| NFC (% DM) d | 38.0 | 36.7 |
| Item 1 | Treat 2 | SEM | p-Values | |||
|---|---|---|---|---|---|---|
| Control | BBR1 | BBR2 | BBR4 | |||
| Post-partum DMI, kg/d | 2.21 b | 2.28 b | 2.47 a | 2.42 a | 0.058 | 0.021 |
| Milk yield, kg/d | 1.66 b | 1.96 b | 2.48 a | 2.10 ab | 0.143 | 0.007 |
| ECM, kg/d | 1.69 b | 2.15 a | 2.77 a | 2.21 ab | 0.131 | 0.002 |
| 4% FCM, kg/d | 1.62 b | 2.12 ab | 2.68 a | 2.11 ab | 0.152 | 0.003 |
| Milk Fat | ||||||
| Yield, kg/d | 0.08 | 0.09 | 0.11 | 0.09 | 0.012 | 0.219 |
| Content, % | 4.68 | 4.74 | 4.55 | 4.11 | 0.582 | 0.697 |
| Milk protein | ||||||
| Yield, kg/d | 0.07 | 0.08 | 0.09 | 0.08 | 0.008 | 0.451 |
| Content, % | 4.56 | 3.92 | 3.76 | 3.72 | 0.363 | 0.314 |
| Milk lactose | ||||||
| Yield, kg/d | 0.06 b | 0.09 ab | 0.11 a | 0.10 ab | 0.012 | 0.042 |
| Content, % | 3.94 | 4.47 | 4.52 | 4.70 | 0.252 | 0.224 |
| Milk total solids | ||||||
| Yield, kg/d | 0.23 | 0.28 | 0.31 | 0.27 | 0.028 | 0.287 |
| Content, % | 14.02 | 13.89 | 12.16 | 12.86 | 0.596 | 0.144 |
| Milk total solids non-fat | ||||||
| Yield, kg/d | 0.15 | 0.19 | 0.22 | 0.20 | 0.018 | 0.147 |
| Content, % | 9.28 | 9.27 | 8.61 | 9.31 | 0.196 | 0.078 |
| Feed efficiency | ||||||
| Milk yield: DMI, kg/d | 0.80 b | 0.94 ab | 1.09 a | 0.99 a | 0.061 | 0.050 |
| ECM: DMI, kg/d | 0.97 b | 1.17 ab | 1.31 a | 1.13 ab | 0.085 | 0.050 |
| FCM: DMI, kg/d | 0.82 b | 1.01 ab | 1.18 a | 1.00 ab | 0.070 | 0.019 |
| Item | Time 1 | Treat 2 | SEM | p-Values | |||
|---|---|---|---|---|---|---|---|
| Control | BBR1 | BBR2 | BBR4 | ||||
| AST (U/L) | −21 | 73.50 | 75.34 | 75.60 | 73.67 | 4.173 | 0.839 |
| −14 | 74.16 | 78.30 | 74.66 | 69.00 | 4.320 | 0.532 | |
| −7 | 86.16 | 83.66 | 73.34 | 71.00 | 5.590 | 0.225 | |
| 0 | 93.17 a | 87.70 ab | 70.34 b | 70.84 b | 5.625 | 0.044 | |
| 7 | 94.15 a | 87.00 a | 75.84 b | 79.50 ab | 3.804 | 0.037 | |
| 14 | 93.17 | 90.00 | 78.17 | 80.83 | 4.281 | 0.107 | |
| 21 | 89.50 | 85.34 | 77.50 | 75.50 | 5.525 | 0.307 | |
| ALT (U/L) | −21 | 23.00 | 22.00 | 22.67 | 23.8 | 1.35 | 0.837 |
| −14 | 24.00 | 22.34 | 23.00 | 19.00 | 1.715 | 0.265 | |
| −7 | 25.34 | 24.33 | 20.65 | 18.67 | 1.83 | 0.101 | |
| 0 | 29.33 a | 25.00 ab | 19.00 b | 20.65 b | 1.433 | 0.003 | |
| 7 | 29.00 a | 23.67 ab | 19.66 b | 20.66 ab | 2.041 | 0.045 | |
| 14 | 28.33 | 23.60 | 21.66 | 22.00 | 1.802 | 0.101 | |
| 21 | 28.00 | 23.00 | 20.34 | 21.00 | 2.944 | 0.316 | |
| ALP (U/L) | −21 | 129.00 | 135.66 | 131.33 | 131.00 | 11.904 | 0.881 |
| −14 | 141.67 | 140.33 | 131.00 | 127.00 | 11.880 | 0.783 | |
| −7 | 168.00 a | 153.33 ab | 122.34 c | 134.33 bc | 5.300 | 0.001 | |
| 0 | 201.67 a | 176.66 ab | 146.00 c | 151.67 bc | 6.258 | 0.001 | |
| 7 | 178.00 a | 160.34 ab | 137.00 bc | 125.00 c | 6.888 | 0.026 | |
| 14 | 173.00 | 160.33 | 136.67 | 141.00 | 15.91 | 0.116 | |
| 21 | 171.67 | 170.00 | 129.00 | 142.00 | 17.91 | 0.287 | |
| Albumin (g/dL) | −21 | 4.47 | 4.73 | 4.60 | 4.46 | 0.230 | 0.820 |
| −14 | 4.47 | 4.43 | 4.90 | 5.03 | 0.182 | 0.111 | |
| −7 | 4.33 c | 4.47 bc | 5.23 a | 4.97 ab | 0.151 | 0.010 | |
| 0 | 3.73 c | 3.80 bc | 4.36 ab | 4.50 a | 0.140 | 0.009 | |
| 7 | 3.87 c | 4.13 bc | 5.03 a | 4.67 ab | 0.171 | 0.005 | |
| 14 | 3.97 b | 3.86 b | 5.24 a | 4.74 a | 0.212 | 0.005 | |
| 21 | 3.50 c | 4.13 bc | 5.26 a | 4.96 ab | 0.200 | 0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghavipanje, N.; Fathi Nasri, M.H.; Farhangfar, S.H.; Ghiasi, S.E.; Vargas-Bello-Pérez, E. Regulation of Nutritional Metabolism in Transition Dairy Goats: Energy Balance, Liver Activity, and Insulin Resistance in Response to Berberine Supplementation. Animals 2021, 11, 2236. https://doi.org/10.3390/ani11082236
Ghavipanje N, Fathi Nasri MH, Farhangfar SH, Ghiasi SE, Vargas-Bello-Pérez E. Regulation of Nutritional Metabolism in Transition Dairy Goats: Energy Balance, Liver Activity, and Insulin Resistance in Response to Berberine Supplementation. Animals. 2021; 11(8):2236. https://doi.org/10.3390/ani11082236
Chicago/Turabian StyleGhavipanje, Navid, Mohammad Hasan Fathi Nasri, Seyyed Homayoun Farhangfar, Seyyed Ehsan Ghiasi, and Einar Vargas-Bello-Pérez. 2021. "Regulation of Nutritional Metabolism in Transition Dairy Goats: Energy Balance, Liver Activity, and Insulin Resistance in Response to Berberine Supplementation" Animals 11, no. 8: 2236. https://doi.org/10.3390/ani11082236
APA StyleGhavipanje, N., Fathi Nasri, M. H., Farhangfar, S. H., Ghiasi, S. E., & Vargas-Bello-Pérez, E. (2021). Regulation of Nutritional Metabolism in Transition Dairy Goats: Energy Balance, Liver Activity, and Insulin Resistance in Response to Berberine Supplementation. Animals, 11(8), 2236. https://doi.org/10.3390/ani11082236







