Seaweed Supplementation Failed to Affect Fecal Microbiota and Metabolome as Well as Fecal IgA and Apparent Nutrient Digestibility in Adult Dogs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Experimental Design and Sample Collection
2.3. Chemical Analyses and ATTD Assessment
2.4. Microbial Analyses
2.5. Fecal IgA Determination
2.6. Statistical Analyses
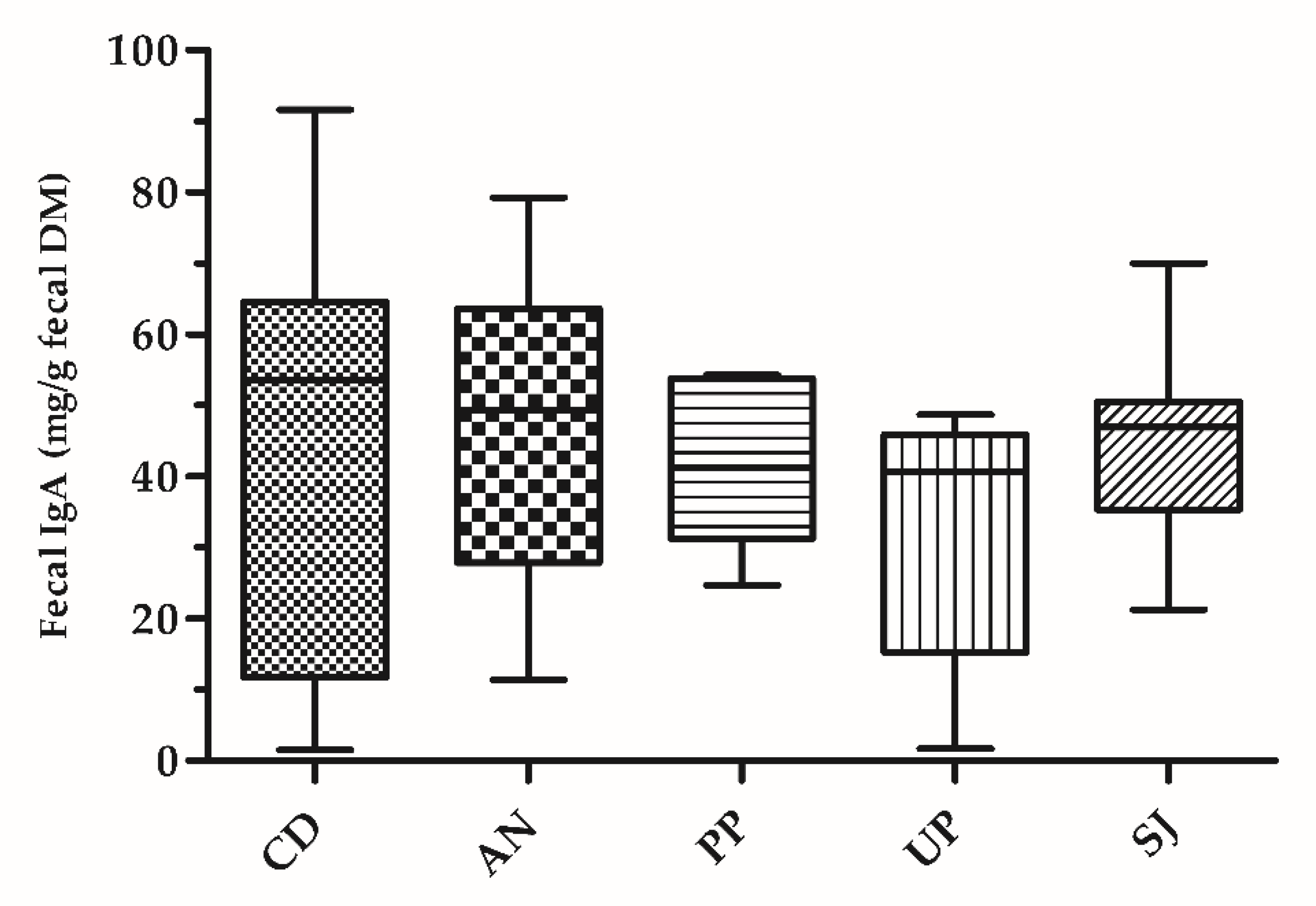
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuddenham, S.; Sears, C.L. The intestinal microbiome and health. Curr. Opin. Infect. Dis. 2015, 28, 464–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Ju, Z.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrition 2018, 51, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J. Modulation of the Gastrointestinal Microbiome with Nondigestible Fermentable Carbohydrates to Improve Human Health. Microbiol. Spectr. 2017, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Pinna, C.; Biagi, G. The Utilisation of Prebiotics and Synbiotics in Dogs. It. J. Anim. Sci. 2014, 13, 169–178. [Google Scholar] [CrossRef]
- Redfern, A.; Suchodolski, J.; Jergens, A. Role of the gastrointestinal microbiota in small animal health and disease. Vet. Rec. 2017, 181, 370. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Cabrita, A.R.J.; Maia, M.R.G.; Oliveira, H.M.; Sousa-Pinto, I.; Almeida, A.A.; Pinto, E.; Fonseca, A.J.M. Tracing seaweeds as mineral sources for farm-animals. J. Appl. Phycol. 2016, 28, 3135–3150. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardari, R.R.R.; Nordberg Karlsson, E. Marine Poly- and Oligosaccharides as Prebiotics. J. Agric. Food Chem. 2018, 66, 11544–11549. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from Seaweeds: An Ocean of Opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef] [Green Version]
- Gotteland, M.; Riveros, K.; Gasaly, N.; Carcamo, C.; Magne, F.; Liabeuf, G.; Beattie, A.; Rosenfeld, S. The Pros and Cons of Using Algal Polysaccharides as Prebiotics. Front. Nutr. 2020, 7, 163. [Google Scholar] [CrossRef]
- Okolie, C.L.; Rajendran, S.R.C.K.; Udenigwe, C.C.; Aryee, A.N.A.; Mason, B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J. Food Biochem. 2017, 41, e12392. [Google Scholar] [CrossRef]
- Corino, C.; Modina, S.C.; Di Giancamillo, A.; Chiapparini, S.; Rossi, R. Seaweeds in Pig Nutrition. Animals 2019, 9, 1126. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.M.; Patil, A.R.; Fahey, G.C., Jr.; Merchen, N.R.; Wolf, B.W.; Lai, C.S.; Garleb, K.A. Apparent digestibility and glycaemic responses to an experimental induced viscosity dietary fibre incorporated into an enteral formula fed to dogs cannulated in the ileum. Food Chem. Toxicol. 1999, 37, 47–56. [Google Scholar] [CrossRef]
- Zentek, J.; Kaufmann, D.; Pietrzak, T. Digestibility and effects on fecal quality of mixed diets with various hydrocolloid and water contents in three breeds of dogs. J. Nutr. 2002, 132, 1679S–1681S. [Google Scholar] [CrossRef] [Green Version]
- Gawor, J.; Jodkowska, K.; Jank, M. Effects of an Ascophyllum nodosum formulation on oral health index in dogs and cats. Weter. Prakt. 2013, 10, 74–79. [Google Scholar]
- Gawor, J.; Jank, M.; Jodkowska, K.; Klim, E.; Svensonn, U.K. Effects of edible treats containing Ascophyllum nodosum on the oral health of dogs: A double-blind, randomized, placebo-controlled single-center study. Front. Vet. Sci. 2018, 5, 168. [Google Scholar] [CrossRef]
- Isidori, M.; Rueca, F.; Trabalza-Marinucci, M. Palatability of extruded dog diets supplemented with Ascophyllum nodosum L. (Fucaceae, Phaeophyceae). J. Appl. Phycol. 2019, 31, 3275–3281. [Google Scholar] [CrossRef]
- Laflamme, D. Development and validation of a body condition score system for dogs. Can. Pract. 1997, 22, 10–15. [Google Scholar]
- FEDIAF (European Pet Food Industry Federation). Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs. September 2020. Available online: https://fediaf.org/images/FEDIAF_Nutritional_Guidelines_2020_20200917.pdf (accessed on 7 January 2021).
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Vogtmann, H.P.; Frirter, P.; Prabuck, A.L. A new method of determining metabolizability of energy and digestibility of fatty acids in broiler diets. Br. Poult. Sci. 1975, 16, 531–534. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. EN ISO 6869:2000. In Animal Feeding Stuffs. Determination of the Contents of Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc—Method Using Atomic Absorption Spectrometry; ISO: Geneva, Switzerland, 2020; Available online: https://www.iso.org/obp/ui/#iso:std:iso:6869:ed-1:v1:en (accessed on 4 December 2020).
- Carpenè, E.; Andreani, G.; Ferlizza, E.; Menotta, S.; Fedrizzi, G.; Isani, G. Trace Elements in Home-Processed Food Obtained from Unconventional Animals. Life 2020, 10, 75. [Google Scholar] [CrossRef]
- Stefanelli, C.; Carati, D.; Rossoni, C. Separation of N1- and N8-acetylspermidine isomers by reversed-phase column liquid chromatography after derivatization with dansyl chloride. J. Chromatogr. 1986, 375, 49–55. [Google Scholar] [CrossRef]
- Muyzer, G.; de Waal, E.C.; Uitterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Mühling, M.; Woolven-Allen, J.; Murrell, J.C.; Joint, I. Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. ISME J. 2008, 2, 379–392. [Google Scholar] [CrossRef]
- Rinttila, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNAtargeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Malinen, E.; Rinttila, T.; Kajander, K.; Matto, J.; Kassinen, A.; Krogius, L.; Saarela, M.; Korpela, R.; Palva, A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 2005, 100, 373–382. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.; Finegold, S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004, 70, 6459–6465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinen, E. Comparison of real-time PCR with SYBR Green I or 5′-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology 2003, 149, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Zannoni, A.; Pietra, M.; Gaspardo, A.; Accorsi, P.A.; Barone, M.; Turroni, S.; Laghi, L.; Zhu, C.; Brigidi, P.; Forni, M. Non-invasive Assessment of Fecal Stress Biomarkers in Hunting Dogs during Exercise and at Rest. Front. Vet. Sci. 2020, 7, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattigan, R.; Sweeney, T.; Maher, S.; Thornton, K.; Rajauria, G.; O’Doherty, J.V. Laminarin-rich extract improves growth performance, small intestinal morphology, gene expression of nutrient transporters and the large intestinal microbial composition of piglets during the critical post-weaning period. Br. J. Nutr. 2020, 123, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish Brown Seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Patra, J.K.; Lee, S.; Park, J.G.; Baek, K. Antioxidant and antibacterial properties of essential oil extracted from an edible seaweed Undaria pinnatifida. J. Food Biochem. 2017, 41, e12278. [Google Scholar] [CrossRef]
- Cian, R.E.; Drago, S.R.; De Medina, F.S.; Martínez-Augustin, O. Proteins and Carbohydrates from Red Seaweeds: Evidence for Beneficial Effects on Gut Function and Microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef] [Green Version]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahaye, M.; Michel, C.; Barry, J.L. Chemical, physicochemical and in-vitro fermentation characteristics of dietary fibres from Palmaria palmata (L.) Kuntze. Food Chem. 1993, 47, 29–36. [Google Scholar] [CrossRef]
- Karimi, S.H. Effects of Red Seaweed (Palmaria palmata) Supplemented Diets Fed to Broiler Chickens Raised under Normal or Stressed Conditions. Master’s Thesis, Dalhousie University, Halifax, NS, Canada, September 2015. [Google Scholar]
- Kulshreshtha, G.; Rathgeber, B.; Stratton, G.; Thomas, N.; Evans, F.; Critchley, A.; Hafting, J.; Prithiviraj, B. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult. Sci. 2014, 93, 2991–3001. [Google Scholar] [CrossRef]
- Heim, G.; Walsh, A.M.; Sweeney, T.; Doyle, D.N.; O’Shea, C.J.; Ryan, M.T.; O’Doherty, J.V. Effect of Seaweed-Derived Laminarin and Fucoidan and Zinc Oxide on Gut Morphology, Nutrient Transporters, Nutrient Digestibility, Growth Performance and Selected Microbial Populations in Weaned Pigs. Br. J. Nutr. 2014, 111, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.; Skrivanova, E.; Missotten, J.; Ovyn, A.; Mrazek, J.; De Smet, S.; Dierick, N. Intact brown seaweed (Ascophyllum nodosum) in diets of weaned piglets: Effects on performance, gut bacteria and morphology and plasma oxidative status. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1101–1111. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [Green Version]
- Sartor, R.B. Gut microbiota: Optimal sampling of the intestinal microbiota for research. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 253–254. [Google Scholar] [CrossRef]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Healy, H.P.; Dawson, K.A.; Merchen, N.R.; Fahey, G.C., Jr. Effects of Supplemental Fructooligosaccharides Plus Mannanoligosaccharides on Immune Function and Ileal and Fecal Microbial Populations in Adult Dogs. Arch. Tierernahr. 2002, 56, 309–318. [Google Scholar] [CrossRef]
- Kraehenbuhl, J.P.; Neutra, M.R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol. Rev. 1992, 72, 853–879. [Google Scholar] [CrossRef]
- Fagarasan, S. Evolution, development, mechanism and function of IgA in the gut. Curr. Opin. Immunol. 2008, 20, 170–177. [Google Scholar] [CrossRef]
- Sutherland, D.B.; Suzuki, K.; Fagarasan, S. Fostering of advanced mutualism with gut microbiota by Immunoglobulin A. Immunol. Rev. 2016, 270, 20–31. [Google Scholar] [CrossRef]
- Frei, R.; Akdis, M.; O’Mahony, L. Prebiotics, probiotics, synbiotics, and the immune system: Experimental data and clinical evidence. Curr. Opin. Gastroenterol. 2015, 31, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Mogno, I.; Contijoch, E.J.; Borgerding, J.N.; Aggarwala, V.; Li, Z.; Siu, S.; Grasset, E.K.; Helmus, D.S.; Dubinsky, M.C.; et al. Fecal IgA Levels Are Determined by Strain-Level Differences in Bacteroides ovatus and Are Modifiable by Gut Microbiota Manipulation. Cell Host Microbe 2020, 27, 467–475.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomax, A.R.; Calder, P.C. Prebiotics, immune function, infection and inflammation: A review of the evidence. Br. J. Nutr. 2009, 101, 633–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Healy, H.P.; Dawson, K.A.; Merchen, N.R.; Fahey, G.C., Jr. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 2002, 132, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Benyacoub, J.; Czarnecki-Maulden, G.L.; Cavadini, C.; Sauthier, T.; Anderson, R.E.; Schiffrin, E.J.; von der Weid, T. Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. J. Nutr. 2003, 133, 1158–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlinden, A.; Hesta, M.; Hermans, J.M.; Janssens, G.P. The effects of inulin supplementation of diets with or without hydrolysed protein sources on digestibility, faecal characteristics, haematology and immunoglobulins in dogs. Br. J. Nutr. 2006, 96, 936–944. [Google Scholar] [CrossRef] [Green Version]
- Theodoro, S.S.; Putarov, T.C.; Tiemi, C.; Volpe, L.M.; de Oliveira, C.A.F.; Glória, M.B.A.; Carciofi, A.C. Effects of the solubility of yeast cell wall preparations on their potential prebiotic properties in dogs. PLoS ONE 2019, 14, e0225659. [Google Scholar] [CrossRef]
- Gaspardo, A.; Zannoni, A.; Turroni, S.; Barone, M.; Sabetti, M.C.; Zanoni, R.G.; Forni, M.; Brigidi, P.; Pietra, M. Influence of Lactobacillus kefiri on Intestinal Microbiota and Fecal IgA Content of Healthy Dogs. Front. Vet. Sci. 2020, 7, 146. [Google Scholar] [CrossRef]
- Ndeh, D.; Gilbert, H.J. Biochemistry of complex glycan depolymerisation by the human gut microbiota. FEMS Microbiol. Rev. 2018, 42, 146–164. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, S.; Touvrey-Loiodice, M.; Poulet, L.; Drouillard, S.; Vincentelli, R.; Henrissat, B.; Skjåk-Bræk, G.; Helbert, W. Ancient acquisition of “alginate utilization loci” by human gut microbiota. Sci. Rep. 2018, 8, 8075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, T.; O’Doherty, J.V. Marine macroalgal extracts to maintain gut homeostasis in the weaning piglet. Domest. Anim. Endocrinol. 2016, 56, S84–S89. [Google Scholar] [CrossRef]
- Dierick, N.; Ovyn, A.; De Smet, S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J. Sci. Food Agric. 2009, 89, 584–594. [Google Scholar] [CrossRef]
- Gardiner, G.E.; Campbell, A.J.; O’Doherty, J.V.; Pierce, E.; Lynch, P.B.; Leonard, F.C.; Stanton, C.; Ross, R.P.; Lawlor, P.G. Effect of Ascophyllum nodosum extract on growth performance, digestibility, carcass characteristics and selected intestinal microflora populations of grower–finisher pigs. Anim. Feed Sci. Technol. 2008, 141, 259–273. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Ghosh, T.S.; Mande, S.S. Global Profiling of Carbohydrate Active Enzymes in Human Gut Microbiome. PLoS ONE 2015, 10, e0142038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| CD | Ascophyllum Nodosum | Palmaria Palmata | Undaria Pinnatifida | Saccharina Japonica | |
|---|---|---|---|---|---|
| Dry matter (g/kg) | 939 | 910 | 876 | 795 | 831 |
| On a dry matter basis (g/kg) | |||||
| Crude protein | 271 | 51.4 | 142 | 126 | 117 |
| Ether extract | 164 | 33.5 | 0.77 | 2.65 | 2.83 |
| Crude fiber | 16.2 | 34.6 | 25.6 | 42.5 | 74.7 |
| Insoluble dietary fiber | 59.2 | 392.7 | 180.4 | 275.1 | 435.5 |
| Soluble dietary fiber | 18.2 | 156.4 | 167.5 | 53.8 | 115.7 |
| Total dietary fiber | 77.4 | 549.1 | 347.9 | 328.9 | 551.2 |
| Crude ash | 70.2 | 212 | 291 | 385 | 217 |
| Acid-insoluble ash | 5.37 | 2.19 | 1.09 | 0.49 | 0.30 |
| Macrominerals (g/kg) | |||||
| Ca | 0.175 | 0.215 | 0.058 | 0.104 | 0.169 |
| P | 0.085 | 0.005 | 0.019 | 0.032 | 0.010 |
| Mg | 0.011 | 0.080 | 0.039 | 0.115 | 0.088 |
| Na | 0.050 | 0.384 | 0.292 | 0.760 | 0.278 |
| K | 0.030 | 0.088 | 0.470 | 0.386 | 0.224 |
| Trace minerals (mg/kg) | |||||
| Zn | 166.3 | 46.3 | 18.1 | 25.7 | 57.5 |
| Mn | 42.6 | 12.3 | 58.6 | 5.77 | 4.68 |
| Fe | 158.5 | 161.1 | 249.9 | 133.7 | 54.4 |
| Cu | 12.9 | n.q. | n.q. | n.q. | n.q. |
| Metals and other trace elements (mg/kg) | |||||
| Pb | 0.078 | 1.98 | 0.47 | 0.23 | |
| Cd | 0.30 | 0.05 | 0.22 | 0.20 | |
| Cr | 0.61 | 1.16 | 0.24 | 0.29 | |
| Hg | 0.02 | 0.02 | 0.02 | 0.03 | |
| As | 33.8 | 3.84 | 36.1 | 24.2 | |
| Al | 27.0 | 322 | 33.1 | 23.8 | |
| Co | 0.43 | 0.30 | 0.18 | 0.08 | |
| Ni | 1.08 | 1.96 | 2.03 | 0.21 | |
| Se | 0.06 | 0.18 | 0.21 | 0.08 | |
| Mo | 0.84 | 0.64 | 0.29 | 0.17 | |
| Ag | 0.05 | 0.20 | 0.01 | 0.01 | |
| Ti | n.q. | 0.01 | n.q. | n.q. | |
| U | 0.62 | 0.10 | 0.51 | 0.45 | |
| Sb | 0.08 | 0.03 | 0.03 | 0.03 | |
| V | 1.25 | 12.7 | 0.41 | 2.43 |
| Target Bacterial Populations | Primers | Sequence (5′–3′) | Reference |
|---|---|---|---|
| Total bacteria | UniF | CCTACGGGAGGCAGCAG | [32] |
| UniR | ATTACCGCGGCTGCTGG | ||
| Firmicutes | Firm350f | GGCAGCAGTRGGGAATCTTC | [33] |
| Firm814r | ACACYTAGYACTCATCGTTT | ||
| Bifidobacterium spp. | Bif_F | TCGCGTCYGGTGTGAAAG | [34] |
| Bif_R | CCACATCCAGCRTCCAC | ||
| Enterococcus spp. | Ent_F | CCCTTATTGTTAGTTGCCATCATT | [34] |
| Ent_R | ACTCGTTGTACTTCCCATTGT | ||
| Lactobacillus spp. | Lac_F | AGCAGTAGGGAATCTTCCA | [35] |
| Lac_R | CACCGCTACACATGGAG | ||
| Clostridium cluster I | CloI-F | TACCHRAGGAGGAAGCCAC | [36] |
| CloI-R | GTTCTTCCTAATCTCTACGCAT | ||
| Escherichia coli | Coli_F | GTTAATACCTTTGCTCATTGA | [37] |
| Coli_R | ACCAGGGTATCTAATCCTGTT | ||
| Faecalibacterium prausnitzii | Fprau 07 | CCATGAATTGCCTTCAAAACTGTT | [38] |
| Fprau 02 | GAGCCTCAGCGTCAGTTGGT |
| Chemical Parameters | CD | AN | PP | UP | SJ | p-Value | Pooled SEM |
|---|---|---|---|---|---|---|---|
| DM | 40.6 | 39.6 | 40.2 | 41.2 | 40.1 | 0.984 | 1.5 |
| Ammonia | 71.0 | 71.7 | 76.6 | 72.7 | 79.6 | 0.887 | 6.5 |
| Acetic acid | 125 | 131 | 144 | 129 | 149 | 0.562 | 11.6 |
| Propionic acid ° | 85.9 | 99.3 | 88.1 | 78.4 | 91.3 | 0.738 | 9.5 |
| n-Butyric acid ° | 20.7 | 25.1 | 24.6 | 25.1 | 30.5 | 0.271 | 2.9 |
| Valeric acid ° | 0.47 | 0.16 | 1.21 | 0.67 | 0.45 | 0.414 | 0.36 |
| iso-Butyric acid ° | 4.21 | 4.27 | 4.62 | 4.66 | 4.75 | 0.958 | 0.45 |
| iso-Valeric acid ° | 5.70 | 6.33 | 6.22 | 6.41 | 6.54 | 0.968 | 0.69 |
| Total SCFA | 231 | 255 | 257 | 233 | 271 | 0.676 | 21.6 |
| Total BCFA ° | 9.91 | 10.6 | 10.8 | 11.1 | 11.3 | 0.974 | 1.13 |
| SCFA + BCFA | 242 | 266 | 269 | 244 | 282 | 0.668 | 21.8 |
| Putrescine ° | 3155 | 4398 | 2751 | 3269 | 3554 | 0.634 | 736 |
| Cadaverine ° | 1215 | 1423 | 1233 | 1772 | 1481 | 0.971 | 454 |
| Spermidine ° | 1249 | 1049 | 1206 | 1221 | 1256 | 0.832 | 136 |
| Spermine | 700 | 613 | 653 | 592 | 705 | 0.840 | 79.8 |
| Bacterial Populations Item | CD | AN | PP | UP | SJ | p-Value | Pooled SEM |
|---|---|---|---|---|---|---|---|
| Total bacteria | 5.92 | 5.91 | 6.16 | 6.29 | 6.18 | 0.614 | 0.17 |
| Firmicutes | 2.87 | 2.62 | 2.79 | 2.68 | 3.00 | 0.807 | 0.24 |
| Bifidobacterium spp. | 0.30 | 0.26 | 0.19 | 0.44 | 0.46 | 0.852 | 0.18 |
| Lactobacillus spp. | n.q. | n.q. | 0.08 | 0.02 | 0.20 | 0.190 | 0.07 |
| Faecalibacterium prausnitzii | 1.20 | 0.75 | 0.96 | 0.65 | 0.98 | 0.178 | 0.16 |
| Enterococcus spp. | 4.01 | 3.91 | 4.13 | 4.60 | 4.44 | 0.308 | 0.23 |
| Clostridium cluster I | 2.49 | 2.72 | 2.85 | 2.79 | 2.67 | 0.587 | 0.14 |
| E. coli | 1.38 | 1.09 | 1.15 | 0.95 | 0.99 | 0.898 | 0.34 |
| Nutrients Item | CD | AN | PP | UP | SJ | p-Value | Pooled SEM |
|---|---|---|---|---|---|---|---|
| Dry matter | 87.7 | 90.2 | 89.9 | 90.5 | 89.7 | 0.568 | 1.2 |
| Crude protein | 88.6 | 91.2 | 91.0 | 91.2 | 90.7 | 0.523 | 1.2 |
| Crude ash | 50.1 | 65.2 | 61.5 | 63.9 | 60.5 | 0.218 | 4.6 |
| Macrominerals | |||||||
| Ca | 40.1 | 61.8 * | 58.3 | 50.3 | 57.1 | 0.073 | 5.32 |
| P | 39.5 | 52.5 | 55.8 | 50.0 | 51.7 | 0.512 | 6.36 |
| Mg ° | 41.0 | 42.6 | 30.0 | 31.7 | 38.8 | 0.916 | 10.5 |
| Na | 96.4 | 97.3 | 98.1 | 97.9 | 97.4 | 0.108 | 0.41 |
| K | 95.7 | 95.4 | 94.8 | 94.7 | 95.5 | 0.749 | 0.63 |
| Trace minerals | |||||||
| Zn | 16.7 | 39.6 | 35.4 | 32.8 | 35.6 | 0.406 | 8.42 |
| Mn | 12.8 | 34.0 | 33.9 | 25.2 | 30.1 | 0.420 | 8.33 |
| Fe | −2.41 | 16.6 | 0.67 | 7.49 | 14.7 | 0.276 | 7.20 |
| Cu | 53.2 | 59.2 | 57.0 | 54.8 | 61.4 | 0.840 | 5.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinna, C.; Vecchiato, C.G.; Grandi, M.; Stefanelli, C.; Zannoni, A.; Biagi, G. Seaweed Supplementation Failed to Affect Fecal Microbiota and Metabolome as Well as Fecal IgA and Apparent Nutrient Digestibility in Adult Dogs. Animals 2021, 11, 2234. https://doi.org/10.3390/ani11082234
Pinna C, Vecchiato CG, Grandi M, Stefanelli C, Zannoni A, Biagi G. Seaweed Supplementation Failed to Affect Fecal Microbiota and Metabolome as Well as Fecal IgA and Apparent Nutrient Digestibility in Adult Dogs. Animals. 2021; 11(8):2234. https://doi.org/10.3390/ani11082234
Chicago/Turabian StylePinna, Carlo, Carla Giuditta Vecchiato, Monica Grandi, Claudio Stefanelli, Augusta Zannoni, and Giacomo Biagi. 2021. "Seaweed Supplementation Failed to Affect Fecal Microbiota and Metabolome as Well as Fecal IgA and Apparent Nutrient Digestibility in Adult Dogs" Animals 11, no. 8: 2234. https://doi.org/10.3390/ani11082234
APA StylePinna, C., Vecchiato, C. G., Grandi, M., Stefanelli, C., Zannoni, A., & Biagi, G. (2021). Seaweed Supplementation Failed to Affect Fecal Microbiota and Metabolome as Well as Fecal IgA and Apparent Nutrient Digestibility in Adult Dogs. Animals, 11(8), 2234. https://doi.org/10.3390/ani11082234







