Experimental Tests for Measuring Individual Attentional Characteristics in Songbirds
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Method
2.1. Subjects
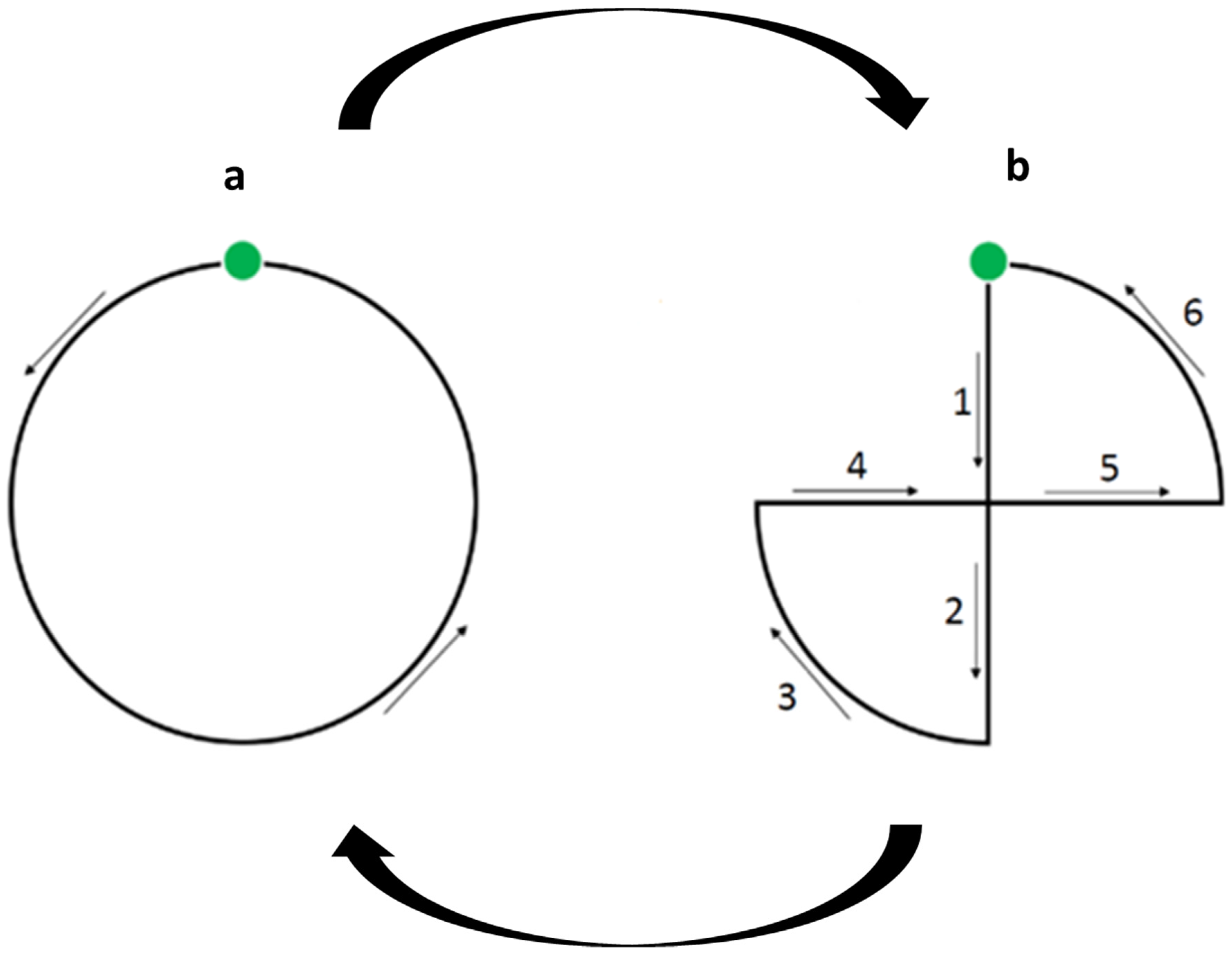
2.2. Experimental TESTS
2.3. The Non-Social Visual Attention Test (VAT)
2.4. The Social Visual Attention Test (SVAT)
2.5. The Auditory Attention Test (AAT)
2.6. Data and Statistical Analyses
- Glances: monocular or binocular looks towards the stimulus lasting less than one second;
- Gazes: a monocular or binocular look towards the stimulus lasting more than one second;
- Total gaze duration: total time during which a subject gazed at the stimulus during a test session.
3. Results
3.1. Visual Attention Tests
Non-SOCIAL (VAT)
3.2. Social Visual Attention (sVAT)
3.3. Auditory Test (AT)
3.4. Individual Consistency between Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Posner, M.I.; Snyder, C.R.; Davidson, B.J. Attention and the detection of signals. J. Exp. Psychol. Gen. 1980, 109, 160–174. [Google Scholar] [CrossRef]
- Bushnell, P. Behavioral approaches to the asessment of attention in animals. Psychopharmacology 1998, 138, 231–259. [Google Scholar] [CrossRef] [PubMed]
- Kruschke, J.K. Attention in learning. Curr. Dir. Psychol. Sci. 2003, 12, 171. [Google Scholar] [CrossRef]
- Cowan, N. Activation, attention, and short-term memory. Mem. Cogn. 1993, 21, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Hanggi, E.B.; Ingersoll, J.F. Long-term memory for categories and concepts in horses (Equus caballus). Anim. Cog. 2009, 12, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. Attention and Memory; University Press: Oxford, UK, 1997. [Google Scholar]
- Emery, N. The eye have it: The neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 2000, 24, 581–604. [Google Scholar] [CrossRef]
- Shepherd, S.V. Following Gaze: Gaze-Following Behavior as a Window into Social Cognition. Front. Integr. Neurosci. 2010, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Dalmaso, M.; Castelli, L.; Galfano, G. Social Modulators of Gaze-Mediated Orienting of Attention: A Review. Psychon. Bull. Rev. 2020, 27, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W. The 5-choice serial reaction time task: Behavioural pharmacology and functional neurochemistry. Psychopharmacology 2002, 163, 362–380. [Google Scholar] [CrossRef]
- Posner, M.I. Cognitive Neuroscience of Attention; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Carli, M.; Robbins, T.W.; Evenden, J.L.; Everitt, B.J. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav. Bran Res. 1983, 9, 361–380. [Google Scholar] [CrossRef]
- Rochais, C.; Sébilleau, M.; Houdebine, M.; Bec, P.; Hausberger, M.; Henry, S. A novel test for evaluating horses’ spontaneous visual attention is predictive of attention in operant learning tasks. Naturwissenschaften 2017, 104, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Steckler, T.; Sauvage, M.; Holsboer, F. Glucocorticoid receptor impairment enhances impulsive responding in transgenic mice performing on a simultaneous visual discrimination task. Eur. J. Neurosci. 2000, 12, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Range, F.; Huber, L. Attention in common marmosets: Implications for social-learning experiments. Anim. Behav. 2007, 73, 1033–1041. [Google Scholar] [CrossRef]
- Horn, L.; Range, F.; Huber, L. Dogs’ attention towards humans depends on their relationship, not only on social familiarity. Anim. Cogn. 2013, 16, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Rochais, C.; Henry, S.; Sankey, C.; Nassur, F.; Góracka-Bruzda, A.; Hausberger, M. Visual attention, an indicator of human-animal relationships? A study of domestic horses (Equus caballus). Front. Psychol. 2014, 5, 108. [Google Scholar] [CrossRef]
- Blois-Heulin, C. Visual gaze in Red-Capped Mangabey (Cercocebus torquatus torquatus) and the Grey-Cheeked Mangabey (Cercocebus albigena albigena). Folia Primatol. 1999, 70, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Range, F.; Horn, L.; Bugnyar, T.; Gajdon, G.K.; Huber, L. Social attention in Keas, dogs, and human children. Anim. Cogn. 2009, 12, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Grandgeorge, M.; Gautier, Y.; Bourreau, Y.; Mossu, H.; Hausberger, M. Visual Attention Patterns Differ in Dog vs. Cat Interactions with Children with Typical Development or Autism Spectrum Disorders. Front. Psychol. 2020, 11, 2047. [Google Scholar] [CrossRef]
- Scheid, C.; Range, F.; Bugnyar, T. When, What, and Whom to Watch? Quantifying Attention in Ravens (Corvus corax) and Jackdaws (Corvus monedula). J. Comp. Psychol. 2007, 121, 380–386. [Google Scholar] [CrossRef]
- Kuhl, P.K. Human speech and birdsong: Communication and the social brain. Proc. Natl. Acad. Sci. USA 2003, 100, 9645–9646. [Google Scholar] [CrossRef]
- Ten Cate, C. Listening behaviour and song learning in zebra finches. Anim. Behav. 1986, 34, 1267–1268. [Google Scholar] [CrossRef]
- Poirier, C.; Henry, L.; Mathelier, M.; Lumineau, S.; Cousillas, H.; Hausberger, M. Direct Social Contacts Override Auditory Information in the Song-Learning Process in Starlings (Sturnus vulgaris). J. Comp. Psychol. 2004, 118, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Bertin, A.; Hausberger, M.; Henry, L.; Richard-Yris, M.-A. Adult and peer influences on starling song development. Dev. Psychobiol. 2007, 49, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Bertin, A.; Hausberger, M.; Henry, L.; Richard-Yris, M.-A. Adult:young ratio influences song acquisition in female European starlings (Sturnus vulgaris). J. Comp. Psychol. 2009, 123, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Cousillas, H.; George, I.; Henry, L.; Richard, J.-P.; Hausberger, M. Linking Social and Vocal Brains: Could Social Segregation Prevent a Proper Development of a Central Auditory Area in a Female Songbird? PLoS ONE 2008, 3, e2194. [Google Scholar] [CrossRef] [PubMed]
- Rochais, C.; Henry, S.; Hausberger, M. Spontaneous attention-capture by auditory distractors as predictor of distractibility: A study of domestic horses (Equus caballus). Sci. Rep. 2017, 7, 15283. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Dalton, P. Ear-catching? Real-world distractibility scores predict susceptibility to auditory attentional capture. Psychol. Bull. Rev. 2014, 21, 1209–1213. [Google Scholar] [CrossRef]
- Hausberger, M. Social influences on song acquisition and sharing in the European starling (Sturnus vulgaris). In Social Influences on Vocal Development; Snowdon, C.T., Hausberger, M., Eds.; Cambridge University Press: Cambridge, MA, USA, 1997; pp. 128–156. [Google Scholar]
- Cousillas, H.; Henry, L.; George, I.; Marchesseau, S.; Hausberger, M. Lateralization of social signal brain processing correlates with the degree of social integration in a songbird. Sci. Rep. 2020, 10, 14093. [Google Scholar] [CrossRef]
- Hausberger, M.; Forasté-Mathelier, M.; Richard-Yris, M.A.; Nygren, C. Differential response of female starlings to shared and nonshared song types. Etología 1997, 5, 31–38. [Google Scholar]
- Cohen, L.B. Attention-Getting and Attention-Holding Processes of Infant Visual Preferences. Child Dev. 1972, 43, 869–879. [Google Scholar] [CrossRef] [PubMed]
- SanMiguel, I.; Linden, D.; Escera, C. Attention Capture by Novel Sounds: Distraction versus Facilitation. Eur. J. Cogn. Psychol. 2010, 22, 481–515. [Google Scholar] [CrossRef]
- Hausberger, M.; Henry, L.; Rethoré, B.; Pougnault, L.; Kremers, D.; Rössler, C.; Aubry, C.; Cousillas, H.; Boye, M.; Lemasson, A. When Perceptual Laterality Vanishes with Curiosity: A Study in Dolphins and Starlings. Laterality 2021, 26, 238–259. [Google Scholar] [CrossRef] [PubMed]
- Suthers, H.B. Analysis of a flock of starlings. Bird Band. 1978, 49, 35–46. [Google Scholar] [CrossRef]
- Feare, C. The Starling; Oxford University Press: Oxford, UK, 1984. [Google Scholar]
- Hart, N.S.; Partridge, J.C.; Cuthill, I.C. Visual pigments, oil droplets and cone photoreceptor dictribution in the European starling. J. Exp. Biol. 1998, 201, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- George, I.; Richard, J.P.; Cousillas, H.; Hausberger, M. No need to talk, I know you: Familiarity influences early multisensory integration in a songbird’s brain. Front. Behav. Neurosci. 2011, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Perret, A.; Henry, L.; Coulon, M.; Caudal, J.-P.; Richard, J.-P.; Cousillas, H.; Hausberger, M.; George, I. Social visual contact, a primary “drive” for social animals? Anim. Cogn. 2015, 18, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Belin, L.; Formanek, L.; Heyraud, C.; Hausberger, M.; Henry, L. Influence of early experience on processing 2D threatening pictures by European starlings (Sturnus vulgaris). Anim. Cog. 2018, 21, 749–758. [Google Scholar] [CrossRef]
- Davidson, G.L.; Butler, S.; Fernández-Juricic, E.; Thornton, A.; Clayton, N.S. Gaze Sensitivity: Function and Mechanisms from Sensory and Cognitive Perspectives. Anim. Behav. 2014, 87, 3–15. [Google Scholar] [CrossRef]
- Sridharan, D.; Schwarz, J.S.; Knudsen, E.I. Selective attention in birds. Curr. Biol. 2014, 24, 510–513. [Google Scholar] [CrossRef][Green Version]
- Boogert, N.J.; Reader, S.M.; Hoppitt, W.; Laland, K.N. The Origin and Spread of Innovations in Starlings. Anim. Behav. 2008, 75, 1509–1518. [Google Scholar] [CrossRef]
- Dalmaso, M.; Pavan, G.; Castelli, L.; Galfano, G. Social Status Gates Social Attention in Humans. Biol. Lett. 2012, 8, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.-P.; Leppelsack, H.-J.; Hausberger, M. A Rapid Correlation Method for the Analysis of Spectrotemporal Receptive-Fields of Auditory Neurons. J. Neurosci. Methods 1995, 61, 99–103. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 276–282. [Google Scholar] [CrossRef]
- Blois-Heulin, C.; Girona, B. Patterns of Social Visual Attention in the Red-Capped Mangabey (Cercocebus Torquatus Torquatus) in the Context of Food Competition. Folia Primatol. 1999, 70, 180–184. [Google Scholar] [CrossRef]
- Siegel, S.; Castellan, N.J. Nonparametric Statistics for the Behavioral Sciences, 2nd ed.; McGraw-Hill Humanities: New York, NY, USA, 1988. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 22 July 2021).
- Barlow, G.W. Ethological units of behavior. In Central Nervous System and Fish Behavior; Ingle, D., Ed.; University of Chicago Press: Chicago, IL, USA, 1968; pp. 217–232. [Google Scholar]
- Chelazzi, L.; Perlato, A.; Santandrea, E.; Della Libera, C. Rewards teach visual selective attention. Vis. Res. 2013, 85, 58–72. [Google Scholar] [CrossRef]
- Bari, A.; Dalley, J.W.; Robbins, T.W. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat. Protocol. 2008, 3, 759–767. [Google Scholar] [CrossRef]
- Alkam, T.; Hiramatsu, M.; Mamiya, T.; Aoyama, Y.; Nitta, A.; Yamada, K.; Kim, H.C.; Nabeshima, T. Evaluation of object-based attention in mice. Behav. Brain Res. 2011, 220, 185–193. [Google Scholar] [CrossRef]
- Waring, G.H. Horse Behavior (Park Ridge); Noyes Publications/William Andrew Pub: New York, NY, USA, 2003. [Google Scholar]
- Dawkins, M. Shifts of “attention” in chicks during feeding. Anim. Behav. 1971, 19, 575–582. [Google Scholar] [CrossRef]
- Pietrewicz, A.T.; Kamil, A.C. Search image formation in the blue jay (Cyanocitta cristata). Science 1979, 204, 1332–1333. [Google Scholar] [CrossRef] [PubMed]
- Tinbergen, L. The Natural Control of Insects in Pinewoods. Arch. Nerlandaises Zool. 1960, 13, 265–343. [Google Scholar] [CrossRef]
- Goto, K.; Bond, A.B.; Burks, M.; Kamil, A.C. Visual search and attention in blue jays (Cyanocitta cristata): Associative cuing and sequential priming. J. Exp. Psychol. Anim. Learn. Cogn. 2014, 40, 185–194. [Google Scholar] [CrossRef] [PubMed]
- George, I.; Cousillas, H.; Richard, J.-P.; Hausberger, M. Experience with Adults Shapes Multisensory Representation of Social Familiarity in the Brain of a Songbird. PLoS ONE 2012, 7, e38764. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, A.; Boutin, A.; Boivin, S.; Blois-Heulin, C.; Hausberger, M. Horse (Equus Caballus) Whinnies: A Source of Social Information. Anim. Cogn. 2009, 12, 693–704. [Google Scholar] [CrossRef]
- Shepherd, S.V.; Deaner, R.O.; Platt, M.L. Social status gates social attention in monkeys. Curr. Biol. 2006, 16, R119–R120. [Google Scholar] [CrossRef]
- Lemasson, A.; Blois-Heulin, C.; Jubin, R.; Hausberger, M. Female Social Relationships in a Captive Group of Campbell’s Monkeys (Cercopithecus campbelli campbelli). Am. J. Primatol. 2006, 68, 1161–1170. [Google Scholar] [CrossRef]
- Hausberger, M.; Richard-Yris, M.-A.; Henry, L.; Lepage, L.; Schmidt, I. Song Sharing Reflects the Social-Organization in a Captive Group of European Starlings (Sturnus vulgaris). J. Comp. Psychol. 1995, 109, 222–241. [Google Scholar] [CrossRef]
- Henry, L.; Bourguet, C.; Coulon, M.; Aubry, C.; Hausberger, M. Sharing Mates and Nest Boxes Is Associated with Female “Friendship” in European Starlings, Sturnus vulgaris. J. Comp. Psychol. 2013, 127, 1–13. [Google Scholar] [CrossRef]
- Cadieu, J.C.; Cadieu, N. Influence of Some Interactions between Fledglings and Adults on the Food Choice in Young Canaries (Serinus Canarius). J. Ethol. 1996, 14, 99–109. [Google Scholar] [CrossRef]
- Clergeau, P. Attractivité et utilisation du milieu chez les étourneaux en alimentation. Acta Oecologica 1982, 3, 307–320. [Google Scholar]
- Fernández-Juricic, E.; Smith, R.; Kacelnik, A. Increasing the costs of conspecific scanning in socially foraging starlings affects vigilance and foraging behaviour. Anim. Behav. 2005, 69, 73–81. [Google Scholar] [CrossRef]
- Rodriguez, A.; Hausberger, M.; Clergeau, P. Flexibility in European starlings’ use of social information: Experiments with decoys in different populations. Anim. Behav. 2010, 80, 965–973. [Google Scholar] [CrossRef]
- Martin, G.R. The eye of a passeriform bird, the European starling (Sturnus vulgaris): Eye movement amplitude, visual fields and schematic optics. J. Comp. Physiol. A 1986, 159, 545–557. [Google Scholar] [CrossRef]
- Rochais, C.; Henry, S.; Fureix, C.; Hausberger, M. Investigating Attentional Processes in Depressive-like Domestic Horses (Equus Caballus). Behav. Process. 2016, 124, 93–96. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pougnault, L.; Cousillas, H.; Heyraud, C.; Huber, L.; Hausberger, M.; Henry, L. Experimental Tests for Measuring Individual Attentional Characteristics in Songbirds. Animals 2021, 11, 2233. https://doi.org/10.3390/ani11082233
Pougnault L, Cousillas H, Heyraud C, Huber L, Hausberger M, Henry L. Experimental Tests for Measuring Individual Attentional Characteristics in Songbirds. Animals. 2021; 11(8):2233. https://doi.org/10.3390/ani11082233
Chicago/Turabian StylePougnault, Loïc, Hugo Cousillas, Christine Heyraud, Ludwig Huber, Martine Hausberger, and Laurence Henry. 2021. "Experimental Tests for Measuring Individual Attentional Characteristics in Songbirds" Animals 11, no. 8: 2233. https://doi.org/10.3390/ani11082233
APA StylePougnault, L., Cousillas, H., Heyraud, C., Huber, L., Hausberger, M., & Henry, L. (2021). Experimental Tests for Measuring Individual Attentional Characteristics in Songbirds. Animals, 11(8), 2233. https://doi.org/10.3390/ani11082233




