The Potential Utilization of High-Fiber Agricultural By-Products as Monogastric Animal Feed and Feed Additives: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Potential Utilization of Feed Crop By-Products
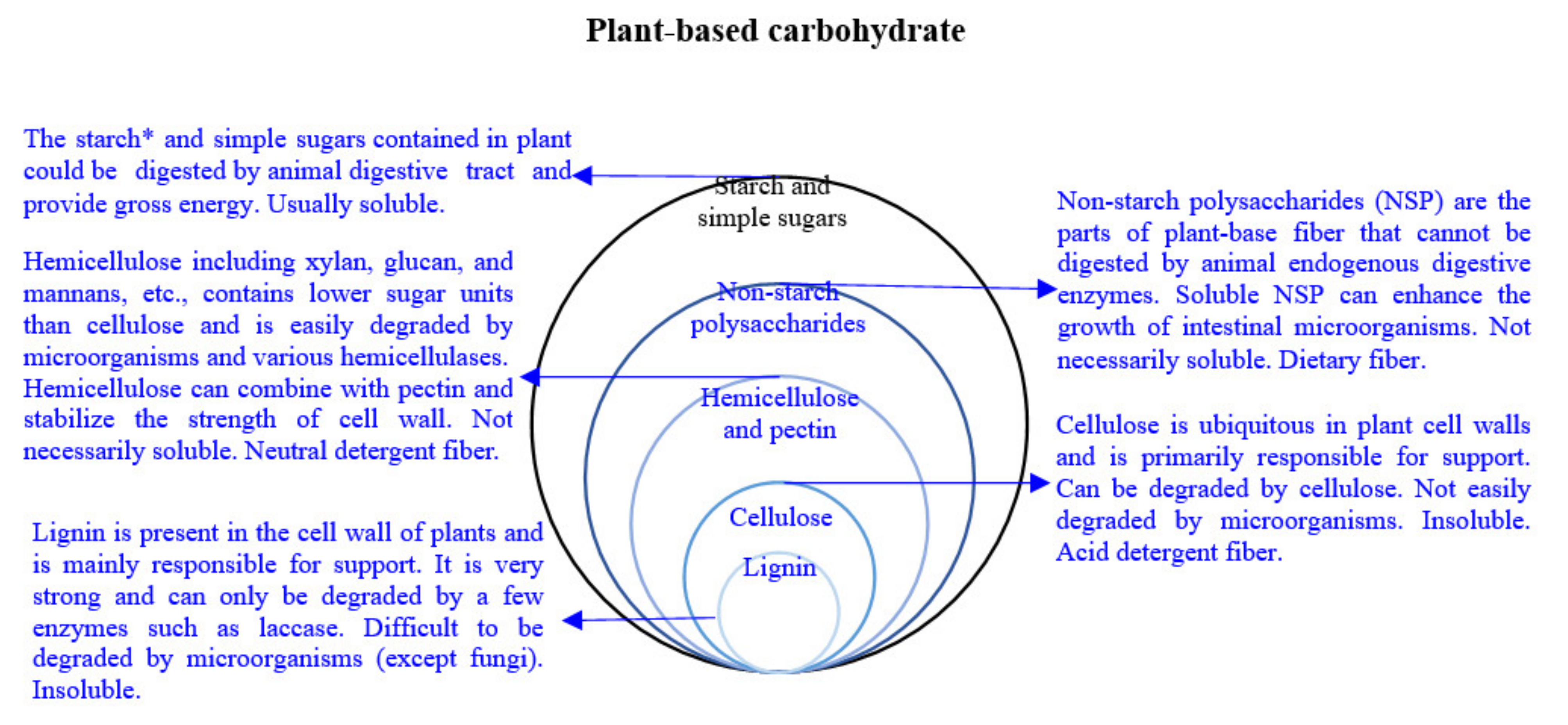
3. Composition of Fiber
4. Partial Plant Phytochemicals and Anti-Nutrition Factors in High-Fiber By-Products
4.1. Phenol
4.2. Flavonoids
4.3. Gossypol
4.4. Tannic Acid
4.5. Phytic Acids
4.6. Trypsin Inhibitor
4.7. Lectin
5. Effect of Fiber Addition on Animal Production Performance and Microbiota
5.1. Effects of Fiber on Broilers
5.2. Effects of Fiber on Swine
6. Effect of Functional Components of Fibers on Animal Physiology
6.1. Fibers Antioxidant and Anti-Inflammatory Responses in Animals
6.2. Fibers Satiety in Animals
7. Fibers Can Increase Animal Performances
7.1. Intestinal Health and Immune Regulation
7.2. Digestibility Adjustment in Animals
7.3. Fat Metabolism and Muscle Generation
8. Fermented Fiber
8.1. Solid-State and Liquid Fermentations
8.2. Stage Fermentation and Co-Fermentation
9. Evaluation of the Use of Agricultural By-Products
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemery, Y.; Rouau, X.; Lullien, V.; Barron, C.; Abécassis, J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J. Cereal Sci. 2007, 46, 327–347. [Google Scholar] [CrossRef]
- Wen, X.; Chen, W.; Chen, B.; Yang, C.; Tu, G.; Cheng, T. Does the prohibition on open burning of straw mitigate air pollution? An empirical study in Jilin Province of China in the post-harvest season. J. Environ. Manag. 2020, 264, 110451. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.Y.; Hsieh, Y.C.; Lee, T.T. The Effects of fungal feed additives in animals: A review. Animals 2020, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitote 2010, 81, 961–968. [Google Scholar] [CrossRef]
- Buxton, D.R.; Redfearn, D.D. Plant limitations to fiber digestion and utilization. J. Nutr. 1997, 127, 8145–8185. [Google Scholar] [CrossRef]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fiber-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Stappenbeck, T.S.; Martens, E.C. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2017, 4, 151–159. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Backhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Chuang, W.Y.; Liu, C.L.; Tsai, C.F.; Lin, W.C.; Chang, S.C.; Shih, H.D.; Shy, Y.M.; Lee, T.T. Evaluation of waste mushroom compost as a feed supplement and its effects on the fat metabolism and antioxidant capacity of broilers. Animals 2020, 10, 455. [Google Scholar] [CrossRef]
- Jørgensen, H.; Theil, P.K.; Knudsen, K.E.B. Satiating properties of diets rich in dietary fibre fed to sows as evaluated by physico-chemical properties, gastric emptying rate and physical activity. Livest. Sci. 2010, 134, 37–40. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondt, S.C. Dietary fibers and their fermented short-chain fatty acids in prevention of human diseases. Bioact. Carbohydr. Diet. Fibre 2019, 17, 100170. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2009, 23, 3838–3850. [Google Scholar] [CrossRef]
- Kermanshahi, H.; Shakouri, M.D.; Daneshmand, A. Effects of non-starch polysaccharides in semi-purified diets on performance, serum metabolites, gastrointestinal morphology, and microbial population of male broiler chickens. Livest. Sci. 2018, 214, 93–97. [Google Scholar] [CrossRef]
- Wang, C.C.; Lin, L.J.; Chao, Y.P.; Chiang, C.J.; Lee, M.T.; Chang, S.C.; Yu, B.; Lee, T.T. Anti-oxidant molecular targets of wheat bran fermented by white rot fungi and its potential modulation of antioxidative status in broiler chickens. Br. Poult. Sci. 2017, 58, 262–271. [Google Scholar] [CrossRef]
- Teng, P.Y.; Chang, C.L.; Huang, C.M.; Chang, S.C.; Lee, T.T. Effects of solid-state fermented wheat bran by Bacillus amyloliquefaciens and Saccharomyces cerevisiae on growth performance and intestinal microbiota in broiler chickens. Ital. J. Anim. Sci. 2017, 16, 552–562. [Google Scholar] [CrossRef]
- Chuang, W.Y.; Lin, W.C.; Hsieh, Y.C.; Huang, C.M.; Chang, S.C.; Lee, T.T. Evaluation of the combined use of Saccharomyces cerevisiae and Aspergillus oryzae with phytase fermentation products on growth, inflammatory, and intestinal morphology in broilers. Animals 2019, 9, 1051. [Google Scholar] [CrossRef]
- Pereira, J.L.A.R.; Pinho, R.G.V.; Filho, A.X.S.; Pereira, M.N.; Santos, Á.O.; Borges, I.D. Quantitative characterization of corn plant components according to planting time and grain maturity stage. R. Bras. Zootec. 2012, 41, 1110–1117. [Google Scholar] [CrossRef][Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 2019, 25, 803–814. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; D’Souza, D.; Biswas, D.; Hanning, I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015, 94, 1419–1430. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.; Wang, S.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Tech. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Beltranena, E.; Zijlstra, R.T. Effect of anti-nutritional factors of oilseed co-products on feed intake of pigs and poultry. Anim. Feed Sci. Tech. 2017, 233, 76–86. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the genus Vitis: Phenolic compounds, anticancer properties and clinical relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Lima, M.C.; de Sousa, C.P.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Bio. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In vitro colonic fermentation of dietary fibers: Fermentation rate, short-chain fatty acid production and changes in microbiota. Trends Food Sci. Tech. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Beyazit, N.; Çakran, H.S.; Cabir, A.; Akışcan, Y.; Demetgül, C. Synthesis, characterization and antioxidant activity of chitosan Schiff base derivatives bearing (−)-gossypol. Carbohydr. Polym. 2020, 240, 116–333. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, E.M. Gossypol: A contraceptive for men. Contraception 2002, 65, 259–263. [Google Scholar] [CrossRef]
- Moon, D.O.; Choi, Y.H.; Moon, S.K.; Kim, W.J.; Kim, G.Y. Gossypol decreases tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 expression via suppression of NF-kappa B activity. Food Chem. Toxicol. 2011, 49, 999–1005. [Google Scholar] [CrossRef]
- Keshmiri-Neghab, H.; Goliaei, B. Therapeutic potential of gossypol: An overview. Pharm. Biol. 2013, 52, 124–128. [Google Scholar] [CrossRef]
- Wedegaertner, T.; Rathore, K. Elimination of gossypol in cottonseed will improve its utilization. Procedia Environ. Sci. 2015, 29, 124–125. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Pan, Z.Y.; Qin, J.F.; Zhong, W.J.; Wang, W.C.; Yang, L. Relative toxicity of dietary free gossypol concentration in ducklings from 1 to 21 d of age. Anim. Feed Sci. Tech. 2017, 228, 32–38. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, A.; Mu, Y.; Zhang, N.; Sun, L.; Rajput, S.A.; Qi, D. Effects of dietary cottonseed oil and cottonseed meal supplementation on the structure, nutritional composition of egg yolk and gossypol residue in eggs. Poult. Sci. 2019, 98, 381–392. [Google Scholar] [CrossRef]
- Bullock, S.L.; Hewitt, D.G.; Stanko, R.L.; Dowd, M.K.; Rutledge, J.; Draeger, D.A. Plasma gossypol dynamics in white-tailed deer: Implications for whole cottonseed as a supplemental feed. Small Rumin. Res. 2010, 93, 165–170. [Google Scholar] [CrossRef]
- Goel, G.; Puniya, A.K.; Singh, K. Tannic acid resistance in ruminal streptococcal isolates. J. Basic Microbiol. 2005, 45, 243–245. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Kubena, L.F.; Byrd, J.A.; Young, C.R.; Corrier, D.E. Effects of tannic acid on cecal volatile fatty acids and susceptibility to Salmonella typhimurium colonization in broiler chicks. Poult. Sci. 2001, 80, 1293–1298. [Google Scholar] [CrossRef]
- Mansoori, B.; Acamovic, T. The effect of tannic acid on the excretion of endogenous methionine, histidine and lysine with broilers. Anim. Feed Sci. Tech. 2007, 134, 198–210. [Google Scholar] [CrossRef]
- Mansoori, B.; Nodeh, H.; Modirsanei, M.; Kiaei, M.M.; Farkhoy, M. Evaluating the influence of tannic acid alone or with polyethylene glycol on the intestinal absorption capacity of broiler chickens, using d-xylose absorption test. Anim. Feed Sci. Tech. 2007, 134, 252–260. [Google Scholar] [CrossRef]
- Lee, S.H.; Shinde, P.L.; Choi, J.Y.; Kwon, K.; Lee, J.K.; Pak, S.I.; Cho, W.T.; Chae, B.J. Effects of tannic acid supplementation on growth performance, blood hematology, iron status and faecal microflora in weanling pigs. Livest. Sci. 2010, 131, 281–286. [Google Scholar] [CrossRef]
- Mansoori, B.; Modirsanei, M. Effects of dietary tannic acid and vaccination on the course of coccidiosis in experimentally challenged broiler chicken. Vet. Parasitol. 2012, 187, 119–122. [Google Scholar] [CrossRef]
- Tonda, R.M.; Rubach, J.K.; Lumpkins, B.S.; Mathis, G.F.; Poss, M.J. Effects of tannic acid extract on performance and intestinal health of broiler chickens following coccidiosis vaccination and/or a mixed-species Eimeria challenge. Poult. Sci. 2018, 97, 3031–3042. [Google Scholar] [CrossRef]
- Cengiz, Ö.; Köksal, B.H.; Tatlı, O.; Sevim, Ö.; Ahsan, U.; Bilgili, S.F.; Önol, A.G. Effect of dietary tannic acid supplementation in corn- or barley-based diets on growth performance, intestinal viscosity, litter quality, and incidence and severity of footpad dermatitis in broiler chickens. Livest. Sci. 2017, 202, 52–57. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Awati, A.; Schulze, H.; Partridge, G. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015, 95, 878–896. [Google Scholar] [CrossRef]
- Chuang, W.Y.; Hsieh, Y.C.; Lin, L.J.; Chang, S.C.; Lee, T.T. Saccharomyces cerevisiae and phytase co-fermentation wheat bran on growth, antioxidation, immunity and intestinal morphology in broilers. Anim. Biosci. 2021, 34, 1157–1168. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Ruckebusch, J.P.; Sorbara, J.O.B.; Wilson, J.W.; Guggenbuhl, P.; Roos, F.F. A systematic view on the effect of phytase on ileal amino acid digestibility in broilers. Anim. Feed Sci. Tech. 2017, 225, 182–194. [Google Scholar] [CrossRef]
- Hamdi, M.; Perez, J.F.; L’etourneau-Montminy, M.P.; Franco-Rossell’o, R.; Aligue, R.; Solà-Oriol, D. The effects of microbisal phytase and dietary calcium and phosphorus levels on the productive performance and bone mineralization of broilers. Anim. Feed Sci. Tech. 2018, 243, 41–51. [Google Scholar] [CrossRef]
- Huang, C.M.; Chuang, W.Y.; Lin, W.C.; Lin, L.J.; Chang, S.C.; Lee, T.T. Production performances and antioxidant activities of laying hens fed Aspergillus oryzae and phytase co-fermented wheat bran. Anim. Biosci. 2021, 34, 371–384. [Google Scholar] [CrossRef]
- Santos, T.T.; Srinongkote, S.; Bedford, M.R.; Walk, C.L. Effect of high phytase inclusion rates on performance of broilers fed diets not severely limited in available phosphorus. Asian-Aust. J. Anim. Sci. 2013, 26, 227–232. [Google Scholar] [CrossRef][Green Version]
- Evans, C.E.; Garlich, J.D.; Barasch, I.B.; Stark, C.R.; Fahrenholz, A.C.; Grimes, J.L. The effects of Miscanthus grass as a bedding source and the dietary inclusion of unheated, low-trypsin inhibitor soybeans on the performance of commercial tom turkeys reared to market age. J. Appl. Poult. Res. 2019, 28, 982–996. [Google Scholar] [CrossRef]
- Dunaevsky, Y.E.; Khadeeva, N.V.; Vassilevski, A.A.; Domash, V.I.; Belozersky, M.A. Chapter 36—Proteinase Inhibitors from Buckwheat (Fagopyrum esculentum Moench) Seeds. Nuts Seeds Health Dis. Prev. 2020, 521–532. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Rocha-Pizaña, M.R.; García-Lara, S.; López-Castillo, L.M.; Serna-Saldívar, S.O. Effect of thermal processing and reducing agents on trypsin inhibitor activity and functional properties of soybean and chickpea protein concentrates. LWT Food Sci. Tech. 2018, 98, 629–634. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Xue, Z.; Gao, X.; Jia, Y.; Wang, Y.; Lua, Y.; Zhang, J.; Zhang, M.; Chen, H. Insight into the inactivation mechanism of soybean Bowman-Birk trypsin inhibitor (BBTI) induced by epigallocatechin gallate and epigallocatechin: Fluorescence, thermo dynamics and docking studies. Food Chem. 2020, 303, 125–380. [Google Scholar] [CrossRef]
- Vasconcelos, I.M.; Oliveira, J.T.A. Antinutritional properties of plant lectins. Toxicon 2004, 44, 385–403. [Google Scholar] [CrossRef]
- Cavada, B.S.; Osterne, V.J.S.; Oliveira, M.V.; Pinto-Junior, V.R.; Silva, M.T.L.; Bari, A.U.; Lima, L.D.; Lossio, C.F.; Nascimento, K.S. Reviewing Mimosoideae lectins: A group of under explored legume lectins. Int. J. Biol. Macromol. 2020, 154, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.T.; Lo, C.T.; Chang, S.C.; Lee, T.T. Effects of Trichoderma fermented wheat bran on growth performance, intestinal morphology and histological findings in broiler chickens. Ital. J. Anim. Sci. 2017, 16, 82–92. [Google Scholar] [CrossRef]
- Okrathok, S.; Khempaka, S. Modified-dietary fiber from cassava pulp reduces abdominal fat and meat cholesterol contents without affecting growth performance of broiler chickens. J. Appl. Poult. Res. 2019, 29, 229–239. [Google Scholar] [CrossRef]
- Lin, W.C.; Lee, T.T. Laetiporus sulphureus fermented wheat bran enhanced the broiler growth performance by improving the intestinal microflora and inflammation status. Poult. Sci. 2020, 99, 3606–3616. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Luo, J.; et al. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet. Anim. Feed Sci. Tech. 2020, 260, 114335. [Google Scholar] [CrossRef]
- Wu, X.; Chen, D.; Yu, B.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; He, J. Effect of different dietary non-starch fiber fractions on growth performance, nutrient digestibility, and intestinal development in weaned pigs. Nutrition 2018, 51–52, 20–28. [Google Scholar] [CrossRef]
- Mateos, G.G.; Jiménez-Moreno, E.; Serrano, M.P.; Lázaro, R.P. Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. J. Appl. Poult. Res. 2012, 21, 156–174. [Google Scholar] [CrossRef]
- Cheng, L.K.; Wang, L.X.; Xu, Q.S.; Huang, L.J.; Zhou, D.S.; Li, Z.; Li, S.G.; Du, Y.G.; Yin, H. Chitooligosaccharide supplementation improves the reproductive performance and milk composition of sows. Livest. Sci. 2015, 174, 74–81. [Google Scholar] [CrossRef]
- Naya, A.; Gertz, M.; Hasler, M.; Beilage, E.G.; Krieter, J. Does a higher content of fibre in the piglet diet have an influence on tail biting in growing pigs? Livest. Sci. 2019, 223, 133–137. [Google Scholar] [CrossRef]
- Huang, C.M.; Lee, T.T. Immunomodulatory effects of phytogenics in chickens and pigs—A review. Asian-Aust. J. Anim. Sci. 2018, 31, 617–627. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Aust. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Lee, T.T. Potential crosstalk of oxidative stress and immune response in poultry through phytochemicals—A review. Asian-Aust. J. Anim. Sci. 2019, 32, 309–319. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, Y.S.; Li, S.; Zhao, Y.; Deng, K.; Chao, D.; Jin, C.; Zhuo, Y.; Che, L.; Li, J.; et al. Effects of prebiotic inulin addition to low- or high-fat diet on maternal metabolic status and neonatal traits of offspring in a pregnant sow model. J. Funct. Foods. 2018, 48, 125–133. [Google Scholar] [CrossRef]
- Bench, C.J.; Rioja-Lang, F.C.; Hayne, S.M.; Gonyou, H.W. Group gestation housing with individual feeding—I: How feeding regime, resource allocation, and genetic factors affect sow welfare. Livest. Sci. 2013, 152, 208–217. [Google Scholar] [CrossRef]
- Oelke, C.A.; Bernardi, M.L.; Nunes, P.R.; Weber, N.C.; Veit, F.C.; Ribeiro, A.M.L. Physiological and behavioral response of sows fed with different levels of dietary fiber during gestation. J. Vet. Behav. 2018, 28, 54–57. [Google Scholar] [CrossRef]
- Meunier-Salaun, M.C.; Edwards, S.A.; Robert, S. Effect of dietary fibre on the behavior and health of the restricted fed sow. Anim. Feed Sci. Tech. 2001, 90, 53–69. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, N.; Xue, Y.; Liu, S.; Lei, H.; Tu, W.; Lu, Y.; Xia, D. Crude fiber modulates the fecal microbiome and steroid hormones in pregnant Meishan sows. Gen. Comp. Endocrinol. 2019, 277, 141–147. [Google Scholar] [CrossRef]
- Lai, L.P.; Lee, M.T.; Chen, C.S.; Yu, B.; Lee, T.T. Effects of co-fermented Pleurotus eryngii stalk residues and soybean hulls by Aureobasidium pullulans on performance and intestinal morphology in broiler chickens. Poult. Sci. 2015, 94, 2959–2969. [Google Scholar] [CrossRef]
- Wang, C.L.; Chiang, C.J.; Chao, Y.P.; Yu, B.; Lee, T.T. Effect of Cordyceps Militaris waster medium on production performance, egg traits and egg yolk cholesterol of laying hens. J. Poult. Sci. 2015, 52, 188–196. [Google Scholar] [CrossRef]
- Chang, S.C.; Lin, M.J.; Chao, Y.P.; Chiang, C.J.; Jea, Y.S.; Lee, T.T. Effects of spent mushroom compost meal on growth performance and meat characteristics of grower geese. R. Bras. Zootec. 2016, 45, 281–287. [Google Scholar] [CrossRef]
- Gao, L.; Chen, L.; Huang, Q.; Meng, L.; Zhong, R.; Liu, C.; Tang, X.; Zhang, H. Effect of dietary fiber type on intestinal nutrient digestibility and hindgut fermentation of diets fed to finishing pigs. Livest. Sci. 2015, 174, 53–58. [Google Scholar] [CrossRef]
- Choi, H.; Kim, B.G. A low-fiber diet requires a longer adaptation period before collecting feces of pigs compared with a high-fiber diet in digestibility experiments using the inert marker method. Anim. Feed Sci. Tech. 2019, 256, 114–254. [Google Scholar] [CrossRef]
- Jim’enez-Moreno, E.; Gonz’alez-Alvarado, J.M.; de-Coca-Sinova, A.; L’azaro, R.P.; C’amara, L.; Mateos, G.G. Insoluble fiber sources in mash or pellets diets for young broilers. 2. Effects on gastrointestinal tract development and nutrient digestibility. Poult. Sci. 2019, 98, 2531–2547. [Google Scholar] [CrossRef]
- Pereira, T.C.J.; Ribeiro, L.S.O.; Pires, A.J.V.; Pereira, M.L.A.; Santos, A.B.; Silva, H.G.O.; de Carvalho, G.G.P. Growth performance and apparent digestibility by goats fed diets with peach palm meal replacing maize. J. Appl. Anim. Sci. 2019, 35, 563–569. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sdadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrance expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Wiele, T.V.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Zeng, M.Y.; Inohara, N.; Nunez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Stahlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Beackhed, F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2017, 23, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Edwin, T.; Moran, J. Gastric digestion of protein through pancreozyme action optimizes intestinal forms for absorption, mucin formation and villus integrity. Anim. Feed Sci. Tech. 2016, 221, 284–303. [Google Scholar]
- Edwin, T.; Moran, J. Intestinal events and nutritional dynamics predispose clostridium perfringens virulence in broilers. Poult. Sci. 2014, 93, 3028–3036. [Google Scholar]
- Navarro, D.M.D.L.; Bruininx, E.M.A.M.; de-Jong, L.; Stein, H.H. Effects of inclusion rate of high fiber dietary ingredients on apparent ileal, hindgut, and total tract digestibility of dry matter and nutrients in ingredients fed to growing pigs. Anim. Feed Sci. Tech. 2019, 248, 1–9. [Google Scholar] [CrossRef]
- Nanclares, M.P.; Trudeau, M.P.; Hansen, J.Ø.; Mydland, L.T.; Urriola, P.E.; Shurson, G.C.; Åkesson, C.P.; Kjos, N.P.; Arntzen, M.Ø.; Øverland, M. High-fiber rapeseed co-product diet for Norwegian Landrace pigs: Effect on digestibility. Livest. Sci. 2017, 203, 1–9. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.M.; Zhang, Z.J.; Zhang, Y.J.; Zhang, Y.W.; Chen, L.; Zhuang, S. Effect of fiber source and enzyme addition on the apparent digestibility of nutrients and physicochemical properties of digesta in cannulated growing pigs. Anim. Feed Sci. Tech. 2016, 216, 262–272. [Google Scholar] [CrossRef]
- Jim’enez-Moreno, E.; de-Coca-Sinova, A.; Gonz’alez-Alvarado, J.M.; Mateos, G.G. Inclusion of insoluble fiber sources in mash or pellet diets for young broilers. 1. Effects on growth performance and water intake. Poult. Sci. 2016, 95, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, R.; Gao, H.; Yang, J.; Zhang, W.; Qin, L. Oat fiber inhibits atherosclerotic progression through improving lipid metabolism in ApoE−/− mice. J. Funct. Food. 2019, 56, 14–20. [Google Scholar] [CrossRef]
- Soccol, C.R.; da-Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.S. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Pan, Y.G.; Lin, W.C.; Lo, C.T.; Chang, S.C.; Yu, B.; Lee, T.T. Effects of substitution of Bermuda grass hay with Trichoderma fermented rice straw on growth, blood and rumen fluid parameters in Barbados sheep. J. Appl. Anim. Res. 2018, 46, 1162–1168. [Google Scholar] [CrossRef]
- Soumeh, E.A.; Mohebodini, H.; Toghyani, M.; Shabani, A.; Ashayerizadeh, A.; Jazi, V. Synergistic effects of fermented soybean meal and mannan-oligosaccharide on growth performance, digestive functions, and hepatic gene expression in broiler chickens. Poult. Sci. 2019, 98, 6797–6807. [Google Scholar] [CrossRef]
- Leathers, T.D.; Dien, B.S. Xylitol production from corn fibre hydrolysates by a two-stage fermentation process. Process Biochem. 2000, 35, 765–769. [Google Scholar] [CrossRef]
- Shin, H.S.; Kim, D.H. A Two-stage fermentation process converting waste and wastewater to hydrogen and methane. Environment. Anaerob. Tech. 2010, 345–363. [Google Scholar] [CrossRef]
- Khongkliang, P.; Jehlee, A.; Kongjan, P.; Reungsang, A.; O-Thong, S. High efficient biohydrogen production from palm oil mill effluent by two-stage dark fermentation and microbial electrolysis under thermophilic condition. Int. J. Hydrog. Energ. 2019, 44, 31841–31852. [Google Scholar] [CrossRef]
- Mukherjee, M.; Nandi, A.; Chandra, K.; Saikia, S.K.; Jana, C.K.; Das, N. Protein extraction from Saccharomyces cerevisiae at different growth phases. J. Microbiol. Methods 2020, 172, 105906. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Tech. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Menconi, A.; Kuttappan, V.A.; Hernandez-Velasco, X.; Urbano, T.; Matté, F.; Layton, S.; Kallapura, G.; Latorre, J.; Morales, B.E.; Prado, O.; et al. Evaluation of a commercially available organic acid product on body weight loss, carcass yield, and meat quality during preslaughter feed withdrawal in broiler chickens: A poultry welfare and economic perspective. Poult. Sci. 2014, 93, 448–455. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Mycotoxins in corn: Occurrence, impacts, and management. In Corn: Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Washington, DC, USA, 2019; pp. 235–287. ISBN 9780128119716. [Google Scholar]
- Maciorowski, K.G.; Herrera, P.; Jones, F.T.; Pillai, S.D.; Ricke, S.C. Effects on poultry and livestock of feed contamination with bacteria and fungi. Anim. Feed Sci. Tech. 2007, 133, 109–136. [Google Scholar] [CrossRef]
- Peng, W.X.; Marchal, J.L.M.; van der Poel, A.F.B. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim. Feed Sci. Tech. 2018, 237, 129–153. [Google Scholar] [CrossRef]
- Chen, L.W.; Chuang, W.Y.; Hsieh, Y.C.; Lin, H.H.; Lin, W.C.; Lin, L.J.; Chang, S.C.; Lee, T.T. Effects of dietary supplementation with Taiwanese tea byproducts and probiotics on growth performance, lipid metabolism, and the immune response in red feather native chickens. Anim. Biosci. 2021, 34, 393–404. [Google Scholar] [CrossRef]
- De-Sordi, L.; Lourenco, M.; Debarbieux, L. The battle within, interactions of bacteriophages and bacteria in the gastrointestinal tract. Cell Host Microbe 2019, 25, 210–218. [Google Scholar] [CrossRef]
| Animal Type | Ingredient | Age | Effect 1 | References | ||
|---|---|---|---|---|---|---|
| ADG | FCR | Ileum Microbes (log CFU) | ||||
| Ross 308 broilers | 10% wheat bran replace | 1–35 days | −4% | +1% | Coliform: −1.53 Clostridium perfringens: +0.08 | [61] |
| 10% Trichoderma fermented wheat bran replace | −1% | −6% | Coliform: −0.6 C. perfringens: −0.26 | |||
| Ross 308 broilers | 3% pectin | 1–14 days | −14% | +21% | E. coli: +0.97 * Lactobacillus spp.: −1.57 * | [14] |
| 3% carboxymethyl cellulose | −14% | +21% | E. coli: +1.54 * Lactobacillus spp.: −1.72 * | |||
| 3% cellulose | −3% | +5% | E. coli: +0.15 Lactobacillus spp.: +0.1 | |||
| Ross 308 broilers | 0.5% cassava pulp addition | 1–42 days | +0% | −1% | - | [62] |
| 1% cassava pulp addition | +0% | −2% | - | |||
| 1.5% cassava pulp addition | +1% | −1% | - | |||
| Ross 308 broilers | 5% wheat bran replace | 1–35 days | +2% | −1% | Coliform: −0.12 Lactic acid bacteria: +0.16 | [63] |
| 10% wheat bran replace | −3% | +1% | Coliform: +0.16 Lactic acid bacteria: +0.2 | |||
| 5% Laetiporus sulphureus fermented wheat bran replace | +2% | −3% * | Coliform: −0.68 Lactic acid bacteria: +0.37 | |||
| 10% Laetiporus sulphureus fermented wheat bran replace | +1% | −3% * | Coliform: −0.29 Lactic acid bacteria: +0.47 | |||
| Weaning piglets | 1% insoluble fiber | 24–52 days | +3% | −3% | E. coli: +0.38 Lactobacillus spp.: +0.9 * | [64] |
| 1% soluble fiber | −3% | −1% | E. coli: +0.22 Lactobacillus spp.: +0.57 | |||
| CRMDF 2 | +11% | −8% * | E. coli: −0.36 Lactobacillus spp.: +0.53 | |||
| 0.5% insoluble fiber and 0.5% soluble fiber | +6% | −7% * | E. coli: +0.61 Lactobacillus spp.: +0.95 | |||
| Weaning piglets | 5% cellulose | 21–46 days | −19% | +9% | - | [65] |
| 5% xylan | −15% | +0% | - | |||
| 5% glucan | −22% * | +3% | - | |||
| Animal Type | Ingredient | Periods | Effects | References |
|---|---|---|---|---|
| Goat | 10, 40, 60, and 85% palm meal replace corn | 90–188 day | Reduce the feed intake, apparent digestibility, and palatability on higher replacement | [84] |
| Weaning pig | 1% inulin or lignocellulose addition | 24–52 day | Increase apparent digestibility of ileum and tight junction expression | [64] |
| Growing pig | 5% inulin | 21.3 ± 1.0 kg | Decrease the dry matter digestibility of ileum, neutral and carbohydrates; increase the ether extract digestibility and total short-chain fatty acids in feces | [81] |
| 5% carboxymethyl cellulose sodium | Increase the digestibility of ileum, detergent fiber, and ether extract | |||
| Sow | 40 ppm chitooligosaccharide | Production and lactation | Increase litter number, litter weight, and survival rate | [67] |
| Barrows | High-fiber treatment 1 | 81.5 kg | Increase total tract digestibility of gross energy, dry matter, organic matter, and crude protein | [82] |
| Weaning piglets | 5% cellulose | 21–46 day | Decrease fecal digestibility of dry matter, calcium, phosphorus, energy, and crude fiber | [65] |
| 5% xylan | Decrease fecal digestibility of calcium | |||
| 5% glucan | Decrease fecal digestibility of calcium, phosphorus, and crude fiber; enhance the gut barrier function | |||
| Cobb-500 broilers | 2.5 or 5% oat hulls supplement | 1–21 day | Enhance the gizzard weight and decrease the pH value in the intestine, increase nutrient digestibility | [83] |
| Ross 308 broilers | 0.5, 1, and 1.5% cassava pulp addition | 1–42 day | Decrease the cholestenone concentration in liver, serum, and muscle, increase nutrition digestibility and gizzard weight | [62] |
| Ross 308 broilers | 0.5, 1, and 2% PWMC 2 | 1–35 day | Increase antioxidant capacities, tight junction expression, and enhance fat metabolism | [3] |
| Ross 308 broilers | 5% wheat bran supplement | 1–35 day | Increase IL-6 and IL-1β mRNA expression | [63] |
| 5% Laetiporus sulphureus fermented wheat bran supplement | Increase IgA secretion in serum and ileum; decrease IL-1β and TNF-α concentration in serum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, W.-Y.; Lin, L.-J.; Shih, H.-D.; Shy, Y.-M.; Chang, S.-C.; Lee, T.-T. The Potential Utilization of High-Fiber Agricultural By-Products as Monogastric Animal Feed and Feed Additives: A Review. Animals 2021, 11, 2098. https://doi.org/10.3390/ani11072098
Chuang W-Y, Lin L-J, Shih H-D, Shy Y-M, Chang S-C, Lee T-T. The Potential Utilization of High-Fiber Agricultural By-Products as Monogastric Animal Feed and Feed Additives: A Review. Animals. 2021; 11(7):2098. https://doi.org/10.3390/ani11072098
Chicago/Turabian StyleChuang, Wen-Yang, Li-Jen Lin, Hsin-Der Shih, Yih-Min Shy, Shang-Chang Chang, and Tzu-Tai Lee. 2021. "The Potential Utilization of High-Fiber Agricultural By-Products as Monogastric Animal Feed and Feed Additives: A Review" Animals 11, no. 7: 2098. https://doi.org/10.3390/ani11072098
APA StyleChuang, W.-Y., Lin, L.-J., Shih, H.-D., Shy, Y.-M., Chang, S.-C., & Lee, T.-T. (2021). The Potential Utilization of High-Fiber Agricultural By-Products as Monogastric Animal Feed and Feed Additives: A Review. Animals, 11(7), 2098. https://doi.org/10.3390/ani11072098






