A Comparison of the Behavior, Physiology, and Offspring Resilience of Gestating Sows When Raised in a Group Housing System and Individual Stalls
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethistall Statements
2.2. Animals and Management
2.3. Housing Systems
2.4. Behavioral Observations
2.5. Sample Collection and Physiological Analysis
2.6. Disease Resilience Test of Piglets
2.7. Statistical Analysis
3. Results
3.1. The Behavioral Response of Gestating Sows Housed in GS or IS
3.2. Effects of IS or GS Housing Systems on the Physiological Responses of Gestating Sows
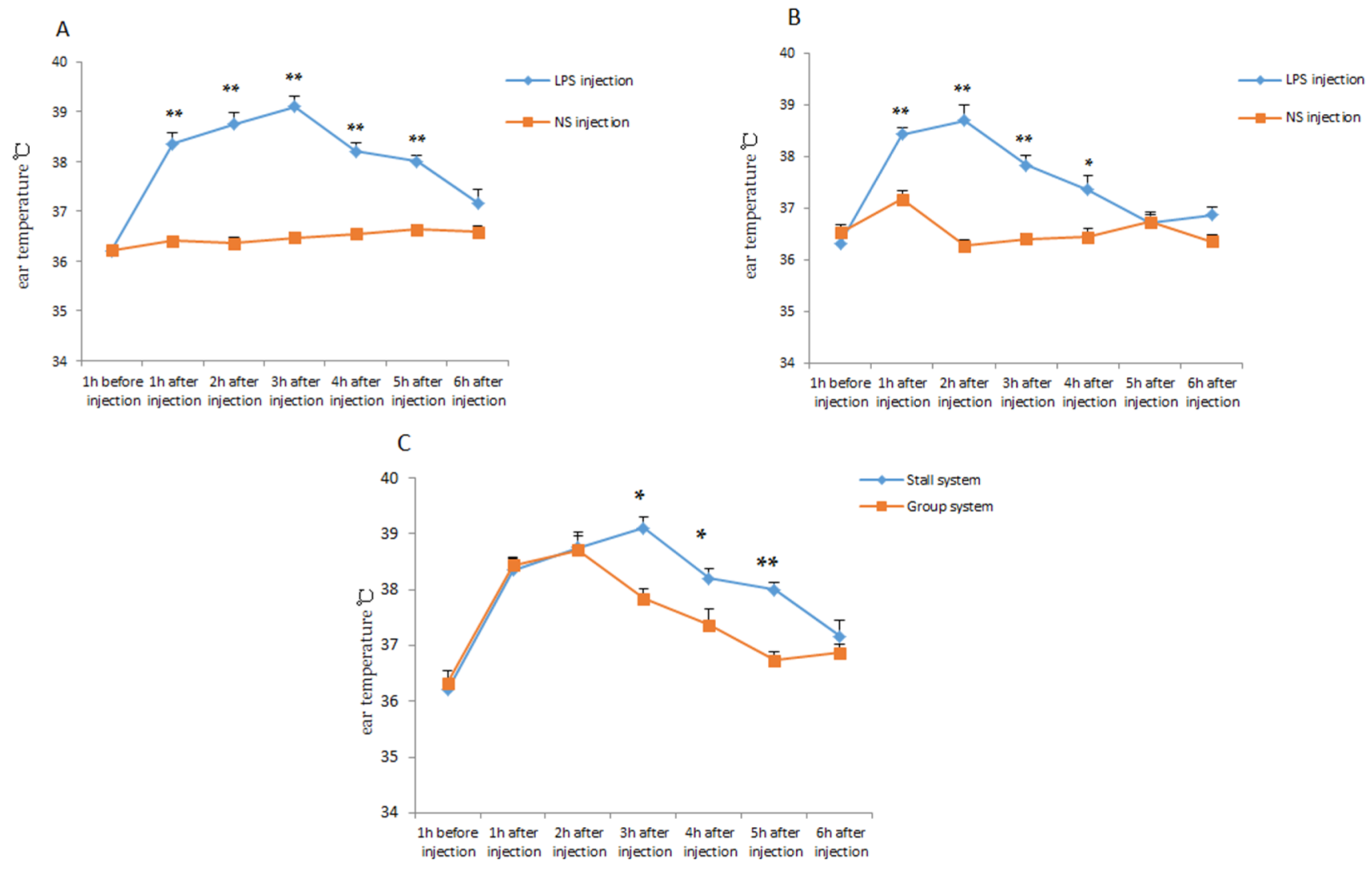
3.3. Comparison of Resistance and Resilience of Offspring Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Marco-Ramell, A.; Arroyo, L.; Peña, R.; Pato, R.; Saco, Y.; Fraile, L.; Bendixen, E.; Bassols, A. Biochemical and proteomic analyses of the physiological response induced by individual housing in gilts provide new potential stress markers. BMC Vet. Res. 2016, 12, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salak-Johnson, J.L.; McGlone, J.J. Making sense of apparently conflicting data: Stress and immunity in swine and cattle. J. Anim. Sci. 2007, 85 (Suppl. 13), E81–E88. [Google Scholar] [CrossRef]
- Van der Beek, E.M.; Wiegant, V.M.; Schouten, W.G.; van Eerdenburg, F.J.; Loijens, L.W.; van der Plas, C.; Benning, M.A.; de Vries, H.; de Kloet, E.R.; Lucassen, P.J. Neuronal number, volume, and apoptosis of the left dentate gyrus of chronically stressed pigs correlate negatively with basal saliva cortisol levels. Hippocampus 2004, 14, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Poole, T.B. The Nature and Evolution of Behavioural Needs in Mammals. Anim. Welf. 1992, 3, 203–220. [Google Scholar]
- Marchant, J.N.; Broom, D.M. Factors affecting posture-changing in loose-housed and confined gestating sows. Anim. Sci. 1996, 63, 477–485. [Google Scholar] [CrossRef]
- Jang, J.C.; Jung, S.W.; Jin, S.S.; Ohh, S.J.; Kim, J.E.; Kim, Y.Y. The Effects of Gilts Housed Either in Group with the Electronic Sow Feeding System or Conventional Stall. Asian Australas J. Anim. Sci. 2015, 28, 1512–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bench, C.J.; Rioja-Lang, F.C.; Hayne, S.M.; Gonyou, H.W. Group gestation sow housing with individual feeding—II: How space allowance, group size and composition, and flooring affect sow welfare. Livest. Sci. 2013, 152, 218–227. [Google Scholar] [CrossRef]
- Chapinal, N.; de la Torre, J.L.R.; Cerisuelo, A.; Gasa, J.; Baucells, M.D.; Coma, J.; Vidal, A.; Manteca, X. Evaluation of welfare and productivity in pregnant sows kept in stalls or in 2 different group housing systems. J. Vet. Behav. 2010, 5, 82–93. [Google Scholar] [CrossRef]
- Min, Y.; Choi, Y.; Kim, J.; Kim, D.; Jeong, Y.; Kim, Y.; Song, M.; Jung, H. Comparison of the Productivity of Primiparous Sows Housed in Individual Stalls and Group Housing Systems. Animal 2020, 10, 1940. [Google Scholar] [CrossRef]
- Morgan, L.; Klement, E.; Novak, S.; Eliahoo, E.; Younis, A.; Sutton, G.A.; Abu-Ahmad, W.; Raz, T. Effects of group housing on reproductive performance, lameness, injuries and saliva cortisol in gestating sows. Prev. Vet. Med. 2018, 160, 10–17. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, Q.; Wang, G.; Zhou, B.; Lu, M.; Marchant-Forde, J.N.; Yang, X.; Zhao, R. Group housing during gestation affects the behaviour of sows and the physiological indices of offspring at weaning. Animal 2014, 8, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Colpoys, J.D.; Johnson, A.K.; Gabler, N.K. Daily feeding regimen impacts pig growth and behavior. Physiol. Behav. 2016, 159, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, E.C.; Plush, K.J.; van Wettere, W.H.E.J.; Hughes, P.E. Group and individual sow behavior is altered in early gestation by space allowance in the days immediately following grouping. J. Anim. Sci. 2016, 94, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmid, S.M.; Büscher, W.; Steinhoff-Wagner, J. Suitability of Different Thermometers for Measuring Body Core and Skin Temperatures in Suckling Piglets. Animal 2021, 11, 1004. [Google Scholar] [CrossRef] [PubMed]
- Rooney, H.B.; O’driscoll, K.; O’doherty, J.V.; Lawlor, P.G. Effect of increasing dietary energy density during late gestation and lactation on sow performance, piglet vitality, and lifetime growth of offspring. J. Anim. Sci. 2020, 98, 379. [Google Scholar] [CrossRef] [PubMed]
- Verdon, M.; Hansen, C.F.; Rault, J.L.; Jongman, E.; Hansen, L.U.; Plush, K.; Hemsworth, P.H. Effects of group housing on sow welfare: A review. J. Anim. Sci. 2015, 93, 1999–2017. [Google Scholar] [CrossRef] [Green Version]
- Koketsu, Y.; Iida, R. Sow housing associated with reproductive performance in breeding herds. Mol. Reprod. Dev. 2017, 84, 979–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damgaard, B.M.; Malmkvist, J.; Pedersen, L.J.; Jensen, K.H.; Thodberg, K.; Jørgensen, E.; Juul-Madsen, H.R. The effects of floor heating on body temperature, water consumption, stress response and immune competence around parturition in loose-housed sows. Res. Vet. Sci. 2009, 86, 136–145. [Google Scholar] [CrossRef]
- Vieuille-Thomas, C.; Pape, G.L.; Signoret, J.P. Stereotypies in pregnant sows: Indications of influence of the housing system on the patterns expressed by the animals. Appl. Anim. Behav. Sci. 1995, 44, 19–27. [Google Scholar] [CrossRef]
- Braastad, B.O. Effects of prenatal stress on behaviour of offspring of laboratory and farmed mammals. Appl. Anim. Behav. Sci. 1998, 61, 159–180. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Li, X.; Zhang, X.H.; Liu, H.G.; Li, J.H.; Bao, J. Effects of confinement duration and parity on stereotypic behavioral and physiological responses of pregnant sows. Physiol. Behav. 2017, 179, 369–376. [Google Scholar] [CrossRef]
- Haley, D.B.; de Passillé, A.M.; Rushen, J. Assessing cow comfort: Effects of two floor types and two tie stall designs on the behaviour of lactating dairy cows. Appl. Anim. Behav. Sci. 2001, 71, 105–117. [Google Scholar] [CrossRef]
- Janssens, C.J.; Helmond, F.A.; Wiegant, V.M. Increased cortisol response to exogenous adrenocorticotropic hormone in chronically stressed pigs: Influence of housing conditions. J. Anim. Sci. 1994, 72, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Mcglone, J.J.; von Borell, E.H.; Deen, J.; Johnson, A.K.; Levis, D.G.; Meunier-Salaon, M.; Morrow, J.; Reeves, D.; Salak-Johnson, J.L.; Sundberg, P.L. Compilation of the Scientific Literature Comparing Housing Systems for Gestating Sows and Gilts Using Measures of Physiology, Behavior, Performance, and Health. Prof. Anim. Sci. 2004, 20, 105–117. [Google Scholar] [CrossRef]
- Otten, W.; Puppe, B.; Kanitz, E.; Schön, P.C.; Stabenow, B. Physiological and behavioral effects of different success during social confrontation in pigs with prior dominance experience. Physiol. Behav. 2002, 75, 127–133. [Google Scholar] [CrossRef]
- Henry, J.P. Biological basis of the stress response. Intgr. Physiol. Behav. Sci. 1992, 27, 66–83. [Google Scholar] [CrossRef]
- Merlot, E.; Pastorelli, H.; Prunier, A.; Père, M.C.; Louveau, I.; Lefaucheur, L.; Perruchot, M.H.; Meunier-Salaün, M.C.; Gardan-Salmon, D.; Gondret, F.; et al. Sow environment during gestation: Part I. Influence on maternal physiology and lacteal secretions in relation with neonatal survival. Animal 2019, 13, 1432–1439. [Google Scholar] [CrossRef]
- Quesnel, H.; Père, M.C.; Louveau, I.; Lefaucheur, L.; Perruchot, M.H.; Prunier, A.; Pastorelli, H.; Meunier-Salaün, M.C.; Gardan-Salmon, D.; Merlot, E.; et al. Sow environment during gestation: Part II. Influence on piglet physiology and tissue maturity at birth. Animal 2019, 13, 1440–1447. [Google Scholar] [CrossRef]
- Merlot, E.; Calvar, C.; Prunier, A. Influence of the housing environment during sow gestation on maternal health, and offspring immunity and survival. Anim. Prod. Sci. 2017, 57, 1751. [Google Scholar] [CrossRef]
- Seck, J.R. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 2004, 151 (Suppl. 3), U49–U62. [Google Scholar] [CrossRef] [Green Version]
- Couret, D.; Jamin, A.; Kuntz-Simon, G.; Prunier, A.; Merlot, E. Maternal stress during late gestation has moderate but long-lasting effects on the immune system of the piglets. Vet. Immunol. Immunopathol. 2009, 131, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Merlot, E.; Quesnel, H.; Prunier, A. Prenatal stress, immunity and neonatal health in farm animal species. Animal 2013, 7, 2016–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raetz, C.R. Biochemistry of endotoxins. Annu. Rev. Biochem. 1990, 59, 129–170. [Google Scholar] [CrossRef]
- Wyns, H.; Plessers, E.; De Backer, P.; Meyer, E.; Croubels, S. In vivo porcine lipopolysaccharide inflammation models to study immunomodulation of drugs. Vet. Immunol. Immunopathol. 2015, 166, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Nordgreen, J.; Munsterhjelm, C.; Aae, F.; Popova, A.; Boysen, P.; Ranheim, B.; Heinonen, M.; Raszplewicz, J.; Piepponen, P.; Lervik, A.; et al. The effect of lipopolysaccharide (LPS) on inflammatory markers in blood and brain and on behavior in individually-housed pigs. Physiol. Behav. 2018, 195, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Harlizius, B.; Mathur, P.; Knol, E.F. Breeding for resilience: New opportunities in a modern pig breeding program. J. Anim. Sci. 2020, 98 (Suppl. 1), S150–S154. [Google Scholar] [CrossRef]
- Mulder, H.A.; Rashidi, H. Selection on resilience improves disease resistance and tolerance to infections. J. Anim. Sci. 2017, 95, 3346–3358. [Google Scholar] [CrossRef]
- Bacou, E.; Haurogné, K.; Mignot, G.; Allard, M.; De Beaurepaire, L.; Marchand, J.; Terenina, E.; Billon, Y.; Jacques, J.; Bach, J.M.; et al. Acute social stress-induced immunomodulation in pigs high and low responders to ACTH. Physiol. Behav. 2017, 169, 1–8. [Google Scholar] [CrossRef] [PubMed]


| Behavior Categories | Definitions |
|---|---|
| Standing behavior | All four hooves are on the pen floor with limbs extended or the pig is walking with limbs in both extension and flexion and moving throughout the pen [12] |
| Dog sitting behavior | The front limbs are extended and bearing weight the rear limbs and body are in contact with the pen floor [12] |
| Lying down behavior | The pig’s body and limbs are in contact with the pen floor [12] |
| Vacuum chewing behavior | Continuous chewing while no feed is present in the mouth [8] |
| Exploratory behavior | Actively manipulating and exploring the surrounding environment [13] |
| Piglet | Hormone | NS Injection | LPS Injection | p-Value |
|---|---|---|---|---|
| PS | COR (ng/mL) | 72.30 ± 14.27 A | 158.34 ± 13.50 a,C | 0.0003 |
| PG | COR (ng/mL) | 32.79 ± 10.77 B | 112.74 ± 21.08 b,c | 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Song, P.; Yan, H.; Zhang, L.; Wang, L.; Zhao, F.; Gao, H.; Hou, X.; Shi, L.; Li, B.; et al. A Comparison of the Behavior, Physiology, and Offspring Resilience of Gestating Sows When Raised in a Group Housing System and Individual Stalls. Animals 2021, 11, 2076. https://doi.org/10.3390/ani11072076
Liu X, Song P, Yan H, Zhang L, Wang L, Zhao F, Gao H, Hou X, Shi L, Li B, et al. A Comparison of the Behavior, Physiology, and Offspring Resilience of Gestating Sows When Raised in a Group Housing System and Individual Stalls. Animals. 2021; 11(7):2076. https://doi.org/10.3390/ani11072076
Chicago/Turabian StyleLiu, Xin, Pengkang Song, Hua Yan, Longchao Zhang, Ligang Wang, Fuping Zhao, Hongmei Gao, Xinhua Hou, Lijun Shi, Bugao Li, and et al. 2021. "A Comparison of the Behavior, Physiology, and Offspring Resilience of Gestating Sows When Raised in a Group Housing System and Individual Stalls" Animals 11, no. 7: 2076. https://doi.org/10.3390/ani11072076
APA StyleLiu, X., Song, P., Yan, H., Zhang, L., Wang, L., Zhao, F., Gao, H., Hou, X., Shi, L., Li, B., & Wang, L. (2021). A Comparison of the Behavior, Physiology, and Offspring Resilience of Gestating Sows When Raised in a Group Housing System and Individual Stalls. Animals, 11(7), 2076. https://doi.org/10.3390/ani11072076







