Anthropogenic and Environmental Factors Determining Local Favourable Conditions for Wolves during the Cold Season
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Wolf Monitoring and Sampling Procedure
2.3. Antropogenic and Enviromental Predictors
2.4. Statistical Analysis
3. Results
3.1. Groups Comparisons (WRS vs. Availability)—Multivariate Test
3.2. Model Development—Logistic LASSO Regression
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Harrington, F.H.; Mech, L.D. Wolf howling and its role in territory maintenance. Behaviour 1979, 68, 207–249. [Google Scholar] [CrossRef] [Green Version]
- Mech, L.D.; Boitani, L. Wolves. Behaviour, Ecology, and Conservation; Mech, L.D., Boitani, L., Eds.; University of Chicago Press: Chicago, IL, USA; London, UK, 2003; ISBN 0-226-51696-2. [Google Scholar]
- Gazzola, A.; Avanzinelli, E.; Mauri, L.; Scandura, M.; Apollonio, M. Temporal changes of howling in south European wolf packs. Ital. J. Zool. 2002, 69, 157–161. [Google Scholar] [CrossRef]
- Nowak, S.; Jędrzejewski, W.; Schmidt, K.; Theuerkauf, J.; Mysłajek, R.W.; Jędrzejewska, B. Howling activity of free-ranging wolves (Canis lupus) in the Białowieża Primeval Forest and the Western Beskidy Mountains (Poland). J. Ethol. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Cassidy, K.A.; MacNulty, D.R.; Stahler, D.R.; Smith, D.W.; Mech, L.D. Group composition effects on aggressive interpack interactions of gray wolves in Yellowstone National Park. Behav. Ecol. 2015, 26, 1352–1360. [Google Scholar] [CrossRef] [Green Version]
- Massolo, A.; Meriggi, A. Factors affecting habitat occupancy by wolves in northern Apennines (northern Italy): A model of habitat suitability. Ecography 1998, 21, 97–107. [Google Scholar] [CrossRef]
- Glenz, C.; Massolo, A.; Kuonen, D.; Schlaepfer, R. A wolf habitat suitability prediction study in Valais (Switzerland). Landsc. Urban Plan. 2001, 55, 55–65. [Google Scholar] [CrossRef]
- Jȩdrzejewski, W.; Niedziałkowska, M.; Nowak, S.; Jȩdrzejewska, B. Habitat variables associated with wolf (Canis lupus) distribution and abundance in northern Poland. Divers. Distrib. 2004, 10, 225–233. [Google Scholar] [CrossRef]
- Jedrzejewski, W.; Jedrzejewska, B.; Zawadzka, B.; Borowik, T.; Nowak, S.; Mysłajek, R.W. Habitat suitability model for Polish wolves based on long-term national census. Anim. Conserv. 2008, 11, 377–390. [Google Scholar] [CrossRef]
- Huck, M.; Jędrzejewski, W.; Borowik, T.; Jędrzejewska, B.; Nowak, S.; Mysłajek, R.W. Analyses of least cost paths for determining effects of habitat types on landscape permeability: Wolves in Poland. Acta Theriol. 2011, 56, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capitani, C.; Mattioli, L.; Avanzinelli, E.; Gazzola, A.; Lamberti, P.; Mauri, L.; Scandura, M.; Viviani, A.; Apollonio, M. Selection of rendezvous sites and reuse of pup raising areas among wolvesCanis lupus of north-eastern Apennines, Italy. Acta Theriol. 2006, 51, 395–404. [Google Scholar] [CrossRef]
- Iliopoulos, Y.; Youlatos, D.; Sgardelis, S. Wolf pack rendezvous site selection in Greece is mainly affected by anthropogenic landscape features. Eur. J. Wildl. Res. 2014, 60, 23–34. [Google Scholar] [CrossRef]
- Bassi, E.; Willis, S.G.; Passilongo, D.; Mattioli, L.; Apollonio, M. Predicting the Spatial Distribution of Wolf (Canis lupus) Breeding Areas in a Mountainous Region of Central Italy. PLoS ONE 2015, 10, e0124698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPhee, H.M.; Webb, N.F.; Merrill, E.H. Hierarchical predation: Wolf (Canis lupus) selection along hunt paths and at kill sites. Can. J. Zool. 2012, 90, 555–563. [Google Scholar] [CrossRef]
- Hebblewhite, M.; Merrill, E.H.; Mcdonald, T.L. Spatial decomposition of predation risk using resource selection functions: An example in a w olf-elk predator-prey system. OIKOS 2005, 111, 101–111. [Google Scholar] [CrossRef]
- Gervasi, V.; Sand, H.; Zimmermann, B.; Mattisson, J.; Wabakken, P.; Linnell, J.D.C. Decomposing risk: Landscape structure and wolf behavior generate different predation patterns in two sympatric ungulates. Ecol. Appl. 2013, 23, 1722–1734. [Google Scholar] [CrossRef] [Green Version]
- Bojarska, K.; Kwiatkowska, M.; Skórka, P.; Gula, R.; Theuerkauf, J. Forest Ecology and Management Anthropogenic environmental traps: Where do wolves kill their prey in a commercial forest ? For. Ecol. Manag. 2017, 397, 117–125. [Google Scholar] [CrossRef]
- Blasi, C. Fitoclimatologia del Lazio; Regione Lazio: Rome, Italy, 1994.
- Boscagli, G.; Adriani, S.; Tribuzi, S.; Incandela, M.; Calò, C.M. Stima del popolamento di Lupo (Canis lupus L.) e del randagismo canino nel Cicolano (RI) durante l’inverno 2006/2007. In Ricerca Scientifica e Strategie per la Conservazione del Lupo in Italia; Caniglia, R., Fabbri, E., Greco, C.R.E., Eds.; Min. Ambiente-ISPRA: Bologna, Italy, 2010; pp. 255–268. [Google Scholar]
- Apollonio, M. Wolves in the Casentinesi Forests: Insights for wolf conservation in Italy from a protected area with a rich wild prey community. Biol. Conserv. 2004, 120, 249–260. [Google Scholar] [CrossRef]
- Freegard, C. Standard Operating Procedure Ground-Based Radio-Tracking. Available online: https://www.dpaw.wa.gov.au/images/documents/conservation-management/off-road-conservation/urban-nature/sop/sop13.4_groundradiotrack_v1.0_20090826.pdf (accessed on 25 October 2010).
- De Clercq, E.M.; De Wulf, R.; Van Herzele, A. Relating spatial pattern of forest cover to accessibility. Landsc. Urban Plan. 2007, 80, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Petroelje, T.R.; Belant, J.L.; Beyer, D.E. Factors affecting the elicitation of vocal responses from coyotes Canis latrans. Wildlife Biol. 2013, 19, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Borowik, T.; Cornulier, T.; Jędrzejewska, B. Environmental factors shaping ungulate abundances in Poland. Acta Theriol. 2013, 58, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Torretta, E.; Serafini, M.; Imbert, C.; Milanesi, P.; Meriggi, A. Wolves and wild ungulates in the Ligurian Alps (Western Italy): Prey selection and spatial-temporal interactions. Mammalia 2016, 81. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Wierzchowski, J.; Kučas, A.; Balčiauskienė, L. Habitat Suitability Based Models for Ungulate Roadkill Prognosis. Animals 2020, 10, 1345. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lozano, C.; Varga, D.; Pintó, J.; Roig-Munar, F.X. Landscape Connectivity and Suitable Habitat Analysis for Wolves (Canis lupus L.) in the Eastern Pyrenees. Sustainability 2020, 12, 5762. [Google Scholar] [CrossRef]
- Santilli, F.; Varuzza, P. Factors affecting wild boar (Sus scrofa) abundance in southern Tuscany. Hystrix 2014, 24, 169–173. [Google Scholar] [CrossRef]
- Tellería, J.L.; Virgós, E. Distribution of an Increasing Roe Deer Population in a Fragmented Mediterranean Landscape. Ecography 1997, 20, 247–252. [Google Scholar] [CrossRef]
- Evcin, O.; Kucuk, O.; Akturk, E. Habitat suitability model with maximum entropy approach for European roe deer (Capreolus capreolus) in the Black Sea Region. Environ. Monit. Assess. 2019, 191, 669. [Google Scholar] [CrossRef]
- Llaneza, L.; López-Bao, J.V.; Sazatornil, V. Insights into wolf presence in human-dominated landscapes: The relative role of food availability, humans and landscape attributes. Divers. Distrib. 2012, 18, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Neumann, W.; Ericsson, G.; Dettki, H.; Bunnefeld, N.; Keuler, N.S.; Helmers, D.P.; Radeloff, V.C. Difference in spatiotemporal patterns of wildlife road-crossings and wildlife-vehicle collisions. Biol. Conserv. 2012, 145, 70–78. [Google Scholar] [CrossRef]
- Mancinelli, S.; Falco, M.; Boitani, L.; Ciucci, P. Social, behavioural and temporal components of wolf (Canis lupus) responses to anthropogenic landscape features in the central Apennines, Italy. J. Zool. 2019, 309, 114–124. [Google Scholar] [CrossRef]
- Zini, V.; Wäber, K.; Dolman, P.M. Habitat quality, configuration and context effects on roe deer fecundity across a forested landscape mosaic. PLoS ONE 2019, 14, e0226666. [Google Scholar] [CrossRef] [Green Version]
- Belda, A.; Zaragozí, B. Can spatial distribution of ungulates be predicted by modeling camera trap data related to landscape indices ? A case study in a fragmented mediterranean landscape. Caldasia 2020, 42, 96–104. [Google Scholar] [CrossRef]
- Zimmermann, B.; Nelson, L.; Wabakken, P.; Sand, H.; Liberg, O. Behavioral responses of wolves to roads: Scale-dependent ambivalence. Behav. Ecol. 2014, 25, 1353–1364. [Google Scholar] [CrossRef]
- Keuling, O.; Stier, N.; Roth, M. How does hunting influence activity and spatial usage in wild boar Sus scrofa L.? Eur. J. Wildl. Res. 2008, 54, 729–737. [Google Scholar] [CrossRef]
- Tolon, V.; Dray, S.; Loison, A.; Zeileis, A.; Fischer, C.; Baubet, E. Responding to spatial and temporal variations in predation risk: Space use of a game species in a changing landscape of fear. Can. J. Zool. 2009, 87, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Grignolio, S.; Merli, E.; Bongi, P.; Ciuti, S.; Apollonio, M. Effects of hunting with hounds on a non-target species living on the edge of a protected area. Biol. Conserv. 2011, 144, 641–649. [Google Scholar] [CrossRef]
- Valente, A.M.; Marques, T.A.; Fonseca, C.; Torres, R.T. A new insight for monitoring ungulates: Density surface modelling of roe deer in a Mediterranean habitat. Eur. J. Wildl. Res. 2016, 62, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Brogi, R.; Grignolio, S.; Brivio, F.; Apollonio, M. Protected areas as refuges for pest species? The case of wild boar. Glob. Ecol. Conserv. 2020, 22. [Google Scholar] [CrossRef]
- McCune, B.; Keon, D. Equations for potential annual direct incident radiation and heat load. J. Veg. Sci. 2002, 13, 603. [Google Scholar] [CrossRef]
- Riley, S.J.; DeGloria, S.D.; Elliot, R. A Terrain_Ruggedness_Index.pdf. Int. J. Sci. 1999, 5, 23–27. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Hotelling, H. The generalization of Student’s ratio. Ann. Math. Stat. 1931, 2, 360–378. [Google Scholar] [CrossRef]
- Mardia, K.V.; Kent, J.T.; Bibby, J.M. Multivariate Analysis; Academic Press: New York, NY, USA, 1979; ISBN 9780124712522. [Google Scholar]
- Curran, J. Hotelling: Hotelling’s T2 Test and Variants. R Package Version 1.0-5. 2018. Available online: https://CRAN.R-project.org/package=Hotelling (accessed on 15 March 2021).
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning. Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009; ISBN 978-0-387-84857-0. [Google Scholar]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Torretta, E.; Caviglia, L.; Serafini, M.; Meriggi, A. Wolf predation on wild ungulates: How slope and habitat cover influence the localization of kill sites. Curr. Zool. 2018, 64, 271–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopcraft, J.G.C.; Sinclair, A.R.E.; Packer, C. Planning for success: Serengeti lions seek prey accessibility rather than abundance. J. Anim. Ecol. 2005, 74, 559–566. [Google Scholar] [CrossRef]
- Woodruff, S.P.; Jimenez, M.D.; Johnson, T.R. Characteristics of Winter Wolf Kill Sites in the Southern Yellowstone Ecosystem. J. Fish Wildl. Manag. 2018, 9, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Kunkel, K.; Pletscher, D.H. Winter Hunting Patterns of Wolves in and Near Glacier National Park, Montana. J. Wildl. Manag. 2001, 65, 520–530. [Google Scholar] [CrossRef]
- Balme, G.; Hunter, L.; Slotow, R. Feeding habitat selection by hunting leopards Panthera pardus in a woodland savanna: Prey catchability versus abundance. Anim. Behav. 2007, 74, 589–598. [Google Scholar] [CrossRef]
- Mattioli, L.; Capitani, C.; Avanzinelli, E.; Bertelli, I.; Gazzola, A.; Apollonio, M. Predation by wolves (Canis lupus) on roe deer (Capreolus capreolus) in north-eastern Apennine, Italy. J. Zool. 2004, 264, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Sławski, M. Collembolan Assemblages Response to Wild Boars (Sus scrofa L.) Rooting in Pine Forest Soil. Forests 2020, 11, 1123. [Google Scholar] [CrossRef]
- Mysterud, A. Cover as a habitat element for temperate ungulates: Effects on habita selection and demography. Wildl. Soc. Bull. 2006, 27, 385–394. [Google Scholar]
- Safford, R.K. Modelling critical winter habitat of four ungulate species in the Robson Valley, British Columbia. J. Ecosyst. Manag. 2004, 4, 1–13. [Google Scholar]
- Borkowski, J.; Ukalska, J. Winter habitat use by red and roe deer in pine-dominated forest. For. Ecol. Manag. 2008, 255, 468–475. [Google Scholar] [CrossRef]
- Roads, A. Balkan Chamois (Rupicapra rupicapra balcanica) Avoids Roads, Settlements, and Hunting Grounds: An Ecological Overview from Timfi Mountain, Greece. Diversity 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Eggermann, J.; da Costa, G.F.; Guerra, A.M.; Kirchner, W.H.; Petrucci-Fonseca, F. Presence of Iberian wolf (Canis lupus signatus) in relation to land cover, livestock and human influence in Portugal. Mamm. Biol. 2011, 76, 217–221. [Google Scholar] [CrossRef]
- Ciucci, P.; Mancinelli, S.; Boitani, L.; Gallo, O.; Grottoli, L. Anthropogenic food subsidies hinder the ecological role of wolves: Insights for conservation of apex predators in human-modi fi ed landscapes. Glob. Ecol. Conserv. 2020, 21, e00841. [Google Scholar] [CrossRef]
- Theuerkauf, J.; Rouys, S. Habitat selection by ungulates in relation to predation risk by wolves and humans in the Białowieza Forest, Poland. For. Ecol. Manag. J. 2008, 256, 1325–1332. [Google Scholar] [CrossRef]
- Brown, J.S.; Laundré, J.W.; Gurung, M. The ecology of fear: Optimal foraging, game theory, and trophic interactions. J. Mammal. 1999, 80, 385–399. [Google Scholar] [CrossRef]
- Bonnot, N.; Morellet, N.; Verheyden, H. Habitat use under predation risk: Hunting, roads and human dwellings influence the spatial behaviour of roe deer. Eur. J. Wildl. Res. 2013, 59, 185–193. [Google Scholar] [CrossRef]
- Mancinelli, S. Being in the Right Place at the Right Time: Wolf Spatio-Temporal Niche in a Human-Modified Environment. Ph.D. Thesis, Università La Sapienza, Roma, Italy, 2017. [Google Scholar]
- Cagnacci, F.; Focardi, S.; Heurich, M.; Stache, A.; Hewison, A.J.M.; Morellet, N.; Kjellander, P.; Linnell, J.D.C.; Mysterud, A.; Neteler, M.; et al. Partial migration in roe deer: Migratory and resident tactics are end points of a behavioural gradient determined by ecological factors. Oikos 2011, 120, 1790–1802. [Google Scholar] [CrossRef]
- Wilmers, C.C.; Stahler, D.R.; Crabtree, R.L.; Smith, D.W.; Getz, W.M. Resource dispersion and consumer dominance: Scavenging at wolf- and hunter-killed carcasses in Greater Yellowstone, USA. Ecol. Lett. 2003, 6, 996–1003. [Google Scholar] [CrossRef] [Green Version]
- Saïd, S.; Servanty, S. The influence of landscape structure on female roe deer home-range size. Landsc. Ecol. 2005, 20, 1003–1012. [Google Scholar] [CrossRef]
- Mysterud, A. Seasonal migration pattern and home range of roe deer (Capreolus capreolus) in an altitudinal gradient in southern Norway. J. Zool. Lond. 1999, 247, 479–486. [Google Scholar] [CrossRef]
- Mysterud, A.; Loe, L.E.; Zimmermann, B.; Bischof, R. Partial migration in expanding red deer populations at northern latitudes–A role for density dependence? Oikos 2011. [Google Scholar] [CrossRef]
- Ramanzin, M.; Sturaro, E.; Zanon, D. Seasonal migration and home range of roe deer (Capreolus capreolus) in the Italian eastern Alps. Can. J. Zool. 2007, 85, 280–289. [Google Scholar] [CrossRef]
- Gula, R. Influence of snow cover on wolf Canis lupus predation patterns in Bieszczady Mountains, Poland. Wildl. Biol. 2004, 10, 17–23. [Google Scholar] [CrossRef]
- Pagon, N.; Grignolio, S.; Pipia, A.; Bongi, P.; Bertolucci, C.; Apollonio, M. Seasonal variation of activity patterns in roe deer in a temperate forested area. Chronobiol. Int. 2013, 30, 772–785. [Google Scholar] [CrossRef]
- Theuerkauf, J.; Jedrzejewski, W.; Schmidt, K.; Gula, R. Spatiotemporal Segregation of Wolves from Humans in the Bialowieza Forest. J. Wildl. Manag. 2003, 67, 706–716. [Google Scholar] [CrossRef]
- Molnar, B.; Fattebert, J.; Palme, R.; Ciucci, P.; Betschart, B.; Smith, D.W.; Diehl, P.-A. Environmental and Intrinsic Correlates of Stress in Free-Ranging Wolves. PLoS ONE 2015, 10, e0137378. [Google Scholar] [CrossRef]
- Zapata-Ríos, G.; Branch, L.C. Mammalian carnivore occupancy is inversely related to presence of domestic dogs in the high Andes of Ecuador. PLoS ONE 2018, 13, e0192346. [Google Scholar] [CrossRef]
- Schenone, L.A.; Aristarchi, C.L.; Meriggi, A.L.; Animale, B.; Pavia, U.; Botta, P. Ecologia del lupo in provincia di Genova: Distribuzione, consistenza della popolazione, alimentazione, impatto sulla zootecnia. Hystrix Ital. J. Mammal. 2003, 14, 13–30. [Google Scholar] [CrossRef]

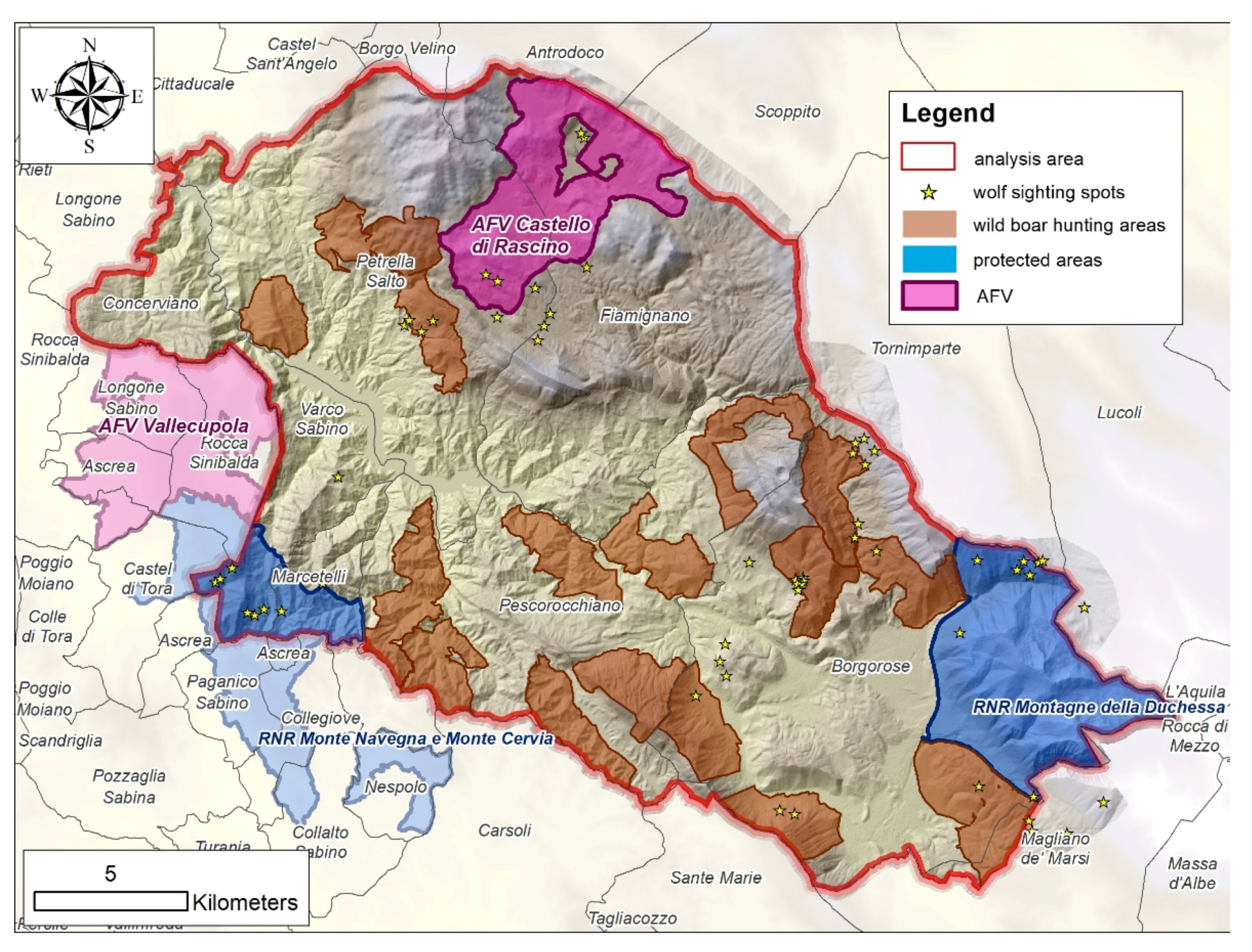

| Name | Description | Area (km2) | % |
|---|---|---|---|
| Urban areas | Human settlements | 6.94 | 1.39 |
| Principal roads | Main paved roads | 6.29 | 1.26 |
| Secondary roads | Gravel roads | 2.44 | 0.49 |
| Cultivated lands | Arable lands and permanent crops | 50.16 | 10.02 |
| Open areas | Pastures and natural grassland | 74.58 | 14.90 |
| Broad-leaved forests | Oak, chestnut, beech, and other mixed coppice woods | 299.61 | 59.87 |
| Coniferous forest | Black pine | 6.72 | 1.34 |
| Scrubland | Bushes and shrubs | 39.56 | 7.91 |
| Bare grounds | Rocks and sparsely vegetated areas | 2.49 | 0.50 |
| Water bodies | Lake and rivers | 7.45 | 1.49 |
| Fruit chestnuts | Cultivated woods for fruits production | 4.16 | 0.83 |
| Total | 500.40 | 100.00 |
| Predictor Categories | Name | Description | Unit |
|---|---|---|---|
| Land cover (LC) | Cultivated lands | Arable lands and permanent crops | % |
| Open areas | Pastures and natural grassland | % | |
| Broad-leaved forests | % | ||
| Coniferous forest | % | ||
| Scrubland | Bushes and shrubs | % | |
| Bare grounds | Rocks and sparsely vegetated areas | % | |
| Water bodies | Lake and rivers | % | |
| Fruit chestnuts | % | ||
| Topography (TPG) | Average slope | % | |
| HLI index | Heat load index—aspect rescaling equation 1 | ||
| Average altitude | m a.s.l. | ||
| Roughness | Index of topographic heterogeneity 2 | ||
| Human disturbance (HD) | Urban areas Main roads | Villages, transport, industrial/commercial | % |
| Density of paved roads | km km-2 | ||
| Secondary roads | Density of gravel roads | km km-2 | |
| Dogs | Dogs responding to simulated howls | yes/not | |
| Territorial planning and wildlife management (TPWM) | Protected areas | Hunting ban regional protected areas | % |
| Drive hunting areas | Specifically assigned for wild boar drive hunting | % | |
| Private hunting areas | Privately managed for hunting purpose | % | |
| Time since the last hunt | Time since the last hunt occurred within wild boar drive hunting areas | hours 3 | |
| Landscape features (LF) | Patches number | Number of fragmented patches | n° |
| Patch richness Edge density | Number of land cover types | n° | |
| Ecotone between closed 4 and open habitats | km km−2 |
| Predictor Categories | Variables | WRS | Availability | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| LC | Cultivated lands | 8.81 | 15.14 | 12.65 | 19.34 |
| Open areas | 8.49 | 13.22 | 8.42 | 10.56 | |
| Broad-leaved forests | 38.12 | 28.66 | 56.64 | 27.48 | |
| Coniferous forest | 2.32 | 6.49 | 0.44 | 2.18 | |
| Scrubland | 8.29 | 10.63 | 8.23 | 9.72 | |
| Bare grounds | 6.11 | 12.56 | 6.68 | 13.80 | |
| Water bodies | 0.09 | 0.49 | 2.25 | 11.48 | |
| Fruit chestnuts | 0.00 | 0.00 | 1.08 | 5.30 | |
| TPG | Average slope | 39.18 | 13.22 | 34.16 | 13.52 |
| HLI index | 0.79 | 0.04 | 0.76 | 0.04 | |
| Average altitude | 1175.64 | 226.81 | 973.02 | 296.01 | |
| Roughness | 69,487.98 | 48,141.72 | 58,968.91 | 45,288.13 | |
| HD | Urban areas Principal roads | 0.48 | 1.35 | 1.70 | 4.30 |
| 0.72 | 1.54 | 1.61 | 2.07 | ||
| Secondary roads | 0.91 | 0.91 | 0.85 | 0.99 | |
| TPWM | Protected areas | 27.44 | 41.80 | 7.77 | 25.00 |
| Drive hunting areas | 17.22 | 30.15 | 17.56 | 31.87 | |
| Private hunting areas | 4.70 | 18.83 | 7.77 | 24.88 | |
| LF | Patches number | 24.97 | 11.73 | 31.14 | 16.12 |
| Patch richness Edge density | 6.90 | 1.85 | 7.15 | 2.24 | |
| 5.93 | 3.53 | 6.35 | 4.07 | ||
| Predictor Category | Statistic | p |
|---|---|---|
| All variables | 12.428 | 0.000 |
| LC | 19.580 | 0.000 |
| TPG | 6.447 | 0.000 |
| HD | 7.506 | 0.000 |
| TPWM | 6.504 | 0.000 |
| LF | 3.365 | 0.005 |
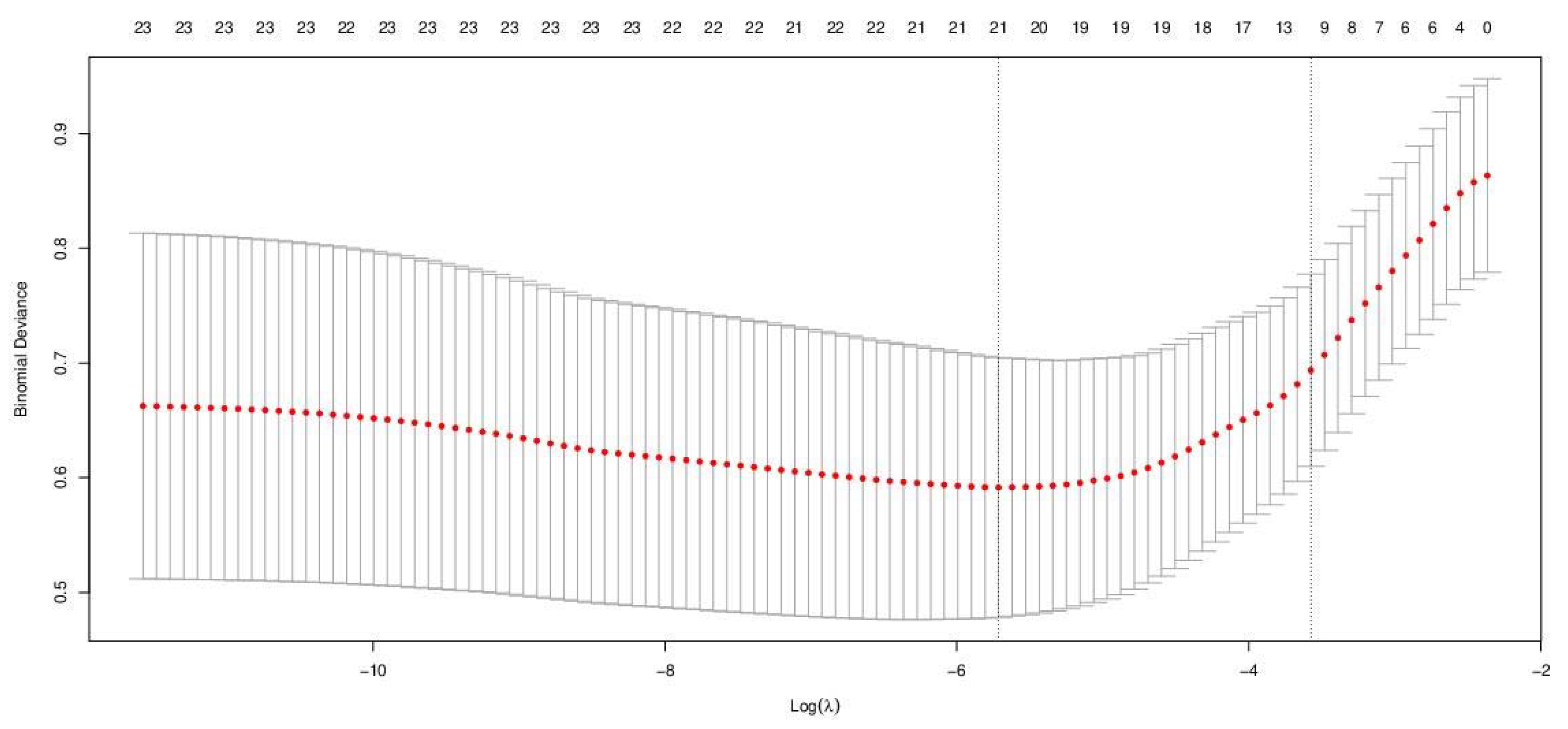
| Lambda | Binomial Deviance | Standard Error | No. of Non-Null Parameters | |
|---|---|---|---|---|
| Lambda.min | 0.0033 | 0.5915 | 0.1133 | 21 |
| Lambda.1se | 0.0280 | 0.6936 | 0.0838 | 12 |
| Lambda | %DEV | R2 | No. of Non-Null Parameters |
|---|---|---|---|
| 0.0025 | 0.5022 | 0.5291 | 21 |
| Predictor Category | Name | Coefficient | ODDS | Increase or Decrease in the ODDS 1 |
|---|---|---|---|---|
| Intercept | 1.274 | 3.577 | ||
| LC | Cultivated lands | −0.05532 | 0.946 | −5.382% |
| Open areas | −0.09742 | 0.907 | −9.282% | |
| Broad-leaved forests | −0.09941 | 0.905 | −9.462% | |
| Coniferous forest | 0.02733 | 1.028 | +2.771% | |
| Scrubland | −0.06320 | 0.939 | −6.125% | |
| Bare grounds | −0.08875 | 0.915 | −8.493% | |
| Water bodies | −0.09636 | 0.908 | −9.187% | |
| Fruit chestnuts | −0.16660 | 0.846 | −15.349% | |
| TPG | Average slope | 0.01412 | 1.014 | +1.422% |
| HLI index | 4.74000 | 114.485 | +11,348.540% | |
| Average altitude | 0.08186 | 1.001 | +0.082% | |
| Roughness | −0.08278 | 0.999 | −0.000% | |
| HD | Urban areas Principal roads | −0.13550 | 0.873 | −12.674% |
| - | - | - | ||
| Secondary roads | - | - | - | |
| Dogs | −1.57700 | 0.207 | −79.250% | |
| TPWM | Protected areas | 0.01367 | 1.014 | +1.376% |
| Drive hunting areas | 0.01511 | 1.015 | +1.522% | |
| Private hunting areas | −0.01376 | 0.986 | −1.367% | |
| Time since the last hunt | 0.01883 | 1.019 | +1.901% | |
| LF | Patches number | −0.03198 | 0.968 | −3.147% |
| Patch richness Edge density | 0.14270 | 1.153 | +15.339% | |
| 0.01819 | 1.018 | +1.828% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viola, P.; Adriani, S.; Rossi, C.M.; Franceschini, C.; Primi, R.; Apollonio, M.; Amici, A. Anthropogenic and Environmental Factors Determining Local Favourable Conditions for Wolves during the Cold Season. Animals 2021, 11, 1895. https://doi.org/10.3390/ani11071895
Viola P, Adriani S, Rossi CM, Franceschini C, Primi R, Apollonio M, Amici A. Anthropogenic and Environmental Factors Determining Local Favourable Conditions for Wolves during the Cold Season. Animals. 2021; 11(7):1895. https://doi.org/10.3390/ani11071895
Chicago/Turabian StyleViola, Paolo, Settimio Adriani, Carlo Maria Rossi, Cinzia Franceschini, Riccardo Primi, Marco Apollonio, and Andrea Amici. 2021. "Anthropogenic and Environmental Factors Determining Local Favourable Conditions for Wolves during the Cold Season" Animals 11, no. 7: 1895. https://doi.org/10.3390/ani11071895
APA StyleViola, P., Adriani, S., Rossi, C. M., Franceschini, C., Primi, R., Apollonio, M., & Amici, A. (2021). Anthropogenic and Environmental Factors Determining Local Favourable Conditions for Wolves during the Cold Season. Animals, 11(7), 1895. https://doi.org/10.3390/ani11071895







