Simple Summary
Two Boleodorus species were detected in cultivated areas of southern Alberta. The aim of the present work was to characterize the discovered populations of Boleodorus using morphological and molecular methods. Boleodorus is the least studied genus in family Tylenchidae, with very few species reported after formal descriptions and outside their type locality. To date, Boleodorus species are not considered nematode pest species, rather they can serve as environmental indicators. Therefore, it is important to quantify and monitor the population densities of these species for soil health management studies. The current study encompasses the distribution and host association of all described Boleodorus species. In addition, morphometrical characters of all valid species are listed for their prompt identification.
Abstract
The present study provides the morphological and molecular characterization of Boleodorus thylactus and B. volutus populations, recovered from agricultural fields of southern Alberta. Despite a significant abundance of this group of nematodes, none of the Boleodorus species were previously reported in Canada. Therefore, representative adult specimens of each population were photographed and examined morphometrically. Phylogenetic trees were reconstructed using partial D2–D3 expansion segments of the 28S and 18S rDNA sequences to understand the relationships of Boleodorus species with Tylenchidae-related genera. Boleodorus species are relevant to soil ecological studies and therefore we summarized the important morphological and morphometric characters in tabular form for easy and efficient species identification. Moreover, we discuss the associated hosts and the distribution of all described Boleodorus species. This study will serve as a guide and basic framework for species diagnostics in the genus Boleodorus and will aid in filling the gaps in our knowledge of the species present in our cultivated lands.
1. Introduction
In southern Alberta, sustainable crop production is achieved not only through advanced agronomic practices but also through constant surveying and surveillance of the crop fields. The latter includes the collection of soil samples from cultivated areas and examination for the presence of plant-parasitic nematode species [1,2]. Various nematode species are associated with cultivated plants but only a select few of them are attributed importance in terms of crop yield reduction [3].
In addition to root-lesion nematodes, stunt and pin nematodes were recently reported in cultivated areas of southern Alberta [1,2,4]. During our recent survey, we isolated several populations of Boleodorus spp. that exhibited four lateral lines, curved to hooked tails, and delicate stylets. Since no Boleodorus species from Canada was previously reported, we elaborated on these findings by carrying out a detailed morphological and molecular analyses of these populations. By comparing morphometrical and morphological characters and molecular data we identified these species as B. thylactus Thorne [5] and B. volutus Lima and Siddiqi [6].
The genus Boleodorus was established by Thorne in 1941 [5] with the type species B. thylactus. Since then, several new members of this genus have been isolated and described from different crops and agricultural regions. Currently, this genus ranks as the second largest genus of subfamily Boleodorinae [7]. Although the members in this subfamily frequently occur in agricultural soils, they receive little attention compared with other plant-parasitic species [8]. The feeding habits of the majority of nematodes in this group are unknown, but members of the genus Boleodorus are considered epidermal cell or root hair feeders and are characterized either 1e or 2 on the colonizer-persister (cp) scale [9,10]. The nematodes in this category have short generations, high reproduction rates, and tolerance to ecological disturbances, thus serving as indicators of soil health [10]. Boleodorus species are also considered to be herbivores which rely on the roots of higher plants as their food source [11,12]. This highlights the fact that, even though Boleodorus species have short and delicate stylets, their feeding elicits a sort of mechanical injury to the roots. The genus Boleodorus is not recognized as being within the important soil pests that affect plants; however, having a non-parasitic status does not exclude Boleodorus species from disease management programs.
Literature studies indicated that B. thylactus was originally described in the USA, but also reported in several Asian and European countries [5,7,13]. The present study is the first report of B. thylactus and B. volutus found in the cultivated areas of southern Alberta, Canada. Despite its frequent occurrence and abundance, previous studies did not characterize the qualitative/quantitative characters and distribution pattern of Boleodorus species in a way that could stimulate more advanced research. Here, we carried out a detailed study of the morphological and morphometrical characters of the valid species of Boleodorus. In addition, we examined the phylogenetic relationships and distribution of the genus. The present study will serve as a guide and basic framework for species identification in the Boleodorus genus and will fill gaps in our knowledge of the species present in our cultivated areas.
2. Materials and Methods
2.1. Isolation and Morphological Studies
Soil samples were collected from different cultivated areas of southern Alberta. Nematodes were extracted from soil samples using the modified Cobb sieving and flotation-centrifugation method [14]. Mixed populations of Boleodorus species were detected in the samples from four different fields. Boleodorus members were collected individually from the mixture of soil nematodes and assigned the population numbers 40, 50, 61, and 62. For preliminary examinations, fresh Boleodorus adults were transferred to a drop of distilled water, heat relaxed at 60 °C and observed under a Zeiss Axioskope 40 microscope. Nematodes were fixed and permanent slides were prepared according to the methods of Seinhorst [15] and De Grisse [16]. Photo documentation of each specimen was carried out using a Zeiss Axioskope 40 microscope equipped with a Zeiss Axiocam 208 camera (Carl Zeiss Microscopy, Jena, Germany). Measurements on images were performed using ZEN blue 3.1 imaging software (Carl Zeiss Microscopy).
2.2. DNA Extraction, PCR, and Sequencing
Nematode DNA was prepared according to Maria et al. [17]. Three sets of DNA primers (Integrated DNA Technologies, Coralville, IA, USA) were used in the PCR analyses to amplify the nucleotide sequences of the partial 18S, 28S (LSU), and ITS of ribosomal RNA genes (rDNA). The partial 18S rRNA region was amplified with 1813F and 2646R primers [18]. The LSU rDNA regions were amplified using 28–81F and 28–1006rev primers [19] and the ITS gene was amplified using F194 [20] and AB28-R primers [21]. The PCR conditions were as described in Holterman et al. [18,19] and in Ferris et al. [20]. Amplified PCR products were resolved by electrophoresis in 1% agarose gels and visualized by staining with GelRed (Biotium, Fremont, CA, USA). Amplified DNA fragments were purified using an E.Z.N.A. Gel Extraction Kit (Omega Biotek, Norcross, GA, USA) following the manufacturer’s instructions, ligated into the pJET1.2 vector (Thermo Fisher Scientific, Mississauga, ON, Canada), and introduced into Escherichia coli DH5α competent cells (Thermo Fisher Scientific). The presence of the PCR-derived inserts in the plasmids from transformed E. coli cells was confirmed by PCR. Plasmid DNA was isolated and purified using an E.Z.N.A. Plasmid DNA Mini Kit I (Omega Biotek), according to the manufacturer’s instructions, and sent to Genewiz, Inc. for DNA sequencing (South Plainfield, NJ, USA). DNA sequences were aligned using the BioEdit sequence alignment tool and compared for similarities with all known nematode species sequences in the GenBank database.
2.3. Phylogenetic Analyses
In the present study, D2–D3 expansion segments of 28S rRNA, 18S rRNA, and ITS rRNA sequences of the Boleodorus populations were obtained. These sequences and other sequences from species of Tylenchidae from GenBank were used for phylogenetic analysis. Selection of outgroup taxa for each dataset were based on previously published studies [22]. Multiple sequence alignments of the different genes were completed using the FFT-NS-2 algorithm of MAFFT v.7.450 [23]. The BioEdit program v7.2.5 [24] was used for sequence alignment visualization and edited using Gblocks v0.91b [25] on the Castresana Laboratory server (available online: http://molevol.cmima.csic.es/castresana/Gblocks_server.html (accessed on 21 April 2021)) using options for a less stringent selection (minimum number of sequences for a conserved or a flanking position: 50% of the number of sequences + 1; maximum number of contiguous non-conserved positions: 8; minimum length of a block: 5; allowed gap positions: with half). Phylogenetic analyses of the sequence datasets were based on Bayesian inference (BI) using MrBayes v3.1.2 [26]. The best-fit model of DNA evolution was achieved using JModelTest v2.1.7 [27] with the Akaike Information Criterion (AIC). The best-fit model, the base frequency, the proportion of invariable sites, and the gamma distribution shape parameters and substitution rates in the AIC were then used in MrBayes for the phylogenetic analyses. The transversion model with invariable sites and a gamma-shaped distribution (TVM + I + G) for the D2–D3 segments of the 28S rRNA and the transition model with a gamma-shaped distribution (TIM1 + G) for the 18S rRNA gene were run with four chains for 4 and 4 × 106 generations, respectively. A combined analysis of the two ribosomal genes was not undertaken due to several sequences not being available for all species. The sampling for Markov chains was carried out at intervals of 100 generations. For each analysis, two runs were conducted. After discarding burn-in samples of 30% and evaluating convergence, the remaining samples were retained for more in-depth analyses. The topologies were used to generate a 50% majority-rule consensus tree. On each appropriate clade, posterior probabilities (PP) were given. FigTree software v1.42 [28] was used for visualization of trees from all analyses.
3. Results
3.1. Systematics
3.1.1. Morphological and Morphometrical Observations on Boleodorus Species
This section briefly addresses the key diagnostic characters of Boleodorus species that can be used in species identification or discrimination. The majority of these species were described decades ago, with some original descriptions in languages other than English and often difficult to access. In this study, all original descriptions were collected through web search queries and personal communications with authors and journals’ editorial staff. By examining all the original descriptions, we found that important morphological and morphometrical characters—such as the presence/absence of males, the number of lateral lines, the presence/absence of crenations on the lip area or lateral field, lip and tail morphology, body and stylet length, and de Man ratios a, b, c, c′, and V—were discussed in almost all reports on Boleodorus species. Therefore, we summarize all these characters here and present them in Table 1 and Table 2; we anticipate that these efforts will aid in species identification of genus Boleodorus. Moreover, because the measurements presented in Table 1 and Table 2 were collected from the original descriptions, this information may help to identify intraspecific variations. Male body habitus and the morphology of the anterior and tail region were found to be similar to those of females; hence, only the important body ratios and measurements of spicule and stylet were included in Table 2.
In general, the females of Boleodorus occur much more abundantly than males [29], therefore priority was given to morphological characters and morphometrical values of females (Table 1). Apparently, males have not been described for nearly half of the Boleodorus species; very few reports acquired sufficient numbers of males for analysis. In most studies, fewer than 10 males were morphometrically characterized (Table 2). Interestingly, female spermathecae were reported to contain sperm in species described without males, which indicates that males are required for reproduction despite their notably lower numbers. The number of lateral lines has a diagnostic value in most nematode genera—all the described Boleodorus species have four lateral lines except B. typicus Hussain and Khan [30] and B. zaini Maqbool [31], both of which are reported to have six lateral lines. Moreover, the lateral field is generally smooth in this genus, however a few species (B. constrictus Rahman and Ahmad [32], B. filiformis Hussain and Khan [33], B. impar Khan and Basir [34], and B. mirus Khan [35]) were reported to have crenations at the outer lines of the lateral field.
The overall shape of the lip region in all the species ranges from conical to rounded and high to low, with or without depressions at the oral aperture. The lip region in Boleodorus species is smooth and without striations or annulation. Almost all the species are described as exhibiting a lip region continuous with the body contour, however some species—such as B. cylindricus Dhanachand, Renubala, and Anandi [36], B. filiformis, B. innuptus Andrassy [37], B. modicus Lal and Khan [38], B. punici Gambhir and Dhanachand [39], B. solomonensis Ye and Geraert [40], B. spiralis Egunjobi [41], B. tenuis Lal and Khan [38], B. thylactus, and B. volutus—are reported to have an offset lip region, though this offset is not by constriction or strong depression at the lip region.
The species of Boleodorus are not large worms; their body length ranges from 400 to 700 µm. The shortest species is B. citri Edward and Rai [42] (280–310 µm) and the longest is B. minustylus Mohilal, Anandi, and Dhanachand [43] (830–960 µm). The general body habitus is open C-shaped to spiral, however, B. acurvus Jairajpuri [44], B. spinnocaudatus Bina, Mohilal, Pramodini, and Majur-Shah [45], and B. solomonensis are outliers in this category and are reported to have almost straight body habitus. The stylets of Boleodorus species are delicate and short, ranging from 7 to 14 µm. The shortest stylet was reported for B. minustylus (4 µm) and the longest for B. solomonensis (12–14.5 µm). The excretory pore position is quite variable; however, it is always located in the region of the pharyngeal bulb or anterior to it. Only one species was exceptional, namely B. zaini, with the excretory pore located at the junction of the pharynx and intestine [31]. All the species of the genus have a monoprodelphic reproductive system and a post-uterine branch which is likely half a body width long. Boleodorus species can be regarded as dioecious—none are reported as hermaphroditic.
The tail is the most variable character of this genus, with a length ranging from 46 to 101 µm, the longest of which was reported for B. cylindricus (88–101 µm). The general morphology of the tail is slender and ventrally curved. Only in some species (B. azadkashmirensis Maqbool, Shahina, and Firoza [46], B. citri, B. constrictus, B. cynodeni Fotedar and Mahajan [47], B. innuptus, B. modicus, B. similis Khan and Basir [48], B. tenuis, B. thylactus, and B. volutus) the terminal region of the tail is curved and hooked in shape. Three types of tail tips were described for Boleodorus species, i.e., rounded, pointed, and clavate. Almost all the species are reported to exhibit rounded tail tips except B. citri, B. filiformis, B. flexuosus Eroshenko [49], B. longicaudatus Bina, Mohilal, Pramodini, and Majur-Shah [45], and B. spinnocaudatus, which all have pointed tips, whereas B. acurvus and B. clavicaudatus Thorne [5] are known to have clavate tips.
The de Man ratios a, b, c, c′, and V are widely used and provided for all described species. The most significant ratio is the V-value, which can be used to differentiate species together with the other indices. Amphids, deirids, and phasmids were observed for very few species—in our opinion their absence or presence in a broader context does not seem to provide enough evidence for species separation or diagnostics.
Overall, Boleodorus species are small nematodes with short stylets, a valveless median bulb, a monodelphic gonad, small PUS, and slender, ventrally curved to hooked tails. There are not any morphological peculiarities to classify each Boleodorus species, therefore one should combine all morphological and morphometrical characters in order to distinguish species.

Table 1.
Main morphological characters and morphometrics of female Boleodorus species. All measurements are in µm.
Table 1.
Main morphological characters and morphometrics of female Boleodorus species. All measurements are in µm.
| Species Name | Body Habitus | Lip Shape | Tail Shape | Tail Length | L | a | b | c | c′ | V | Stylet Length | DGO | 2 SE Pore Dist. | 3 LL | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 B. acurvus | Almost straight | Continuous, narrow conical | Filiform, terminus clavate | 75–90 | 470–540 | 27–37 | 4.2–4.7 | 6–9 | 7–9 | 60–63 | 10–11 | 4–6 | 80–90 | 4 | Jairajpuri [44] |
| Almost straight | Continuous, conical, depression at OA 1 | Filiform, terminus rounded | 77–93 | 475–605 | 27–39 | 4.1–5.1 | 5.7–6.9 | 7.2–8.5 | 62–64 | 10.5–11.5 | – | 79–95 | 4 | Zeidan and Geraert [50] | |
| 2 B. acutus | Arcuate to open C-shape | Continuous, conical, elevated at perioral region | Uniformly conoid | 63 | 500 | 22 | – | 8.0 | – | 67 | 13 | – | – | 4 | Thorne and Malek [51] |
| 3 B. azadkashmirensis | Open C-shape | Continuous, elevated, anteriorly truncated | Elongate-conoid, terminus hooked | 60 | 400–500 | 23–33 | 4.0–5.0 | 7.0–8.0 | 4.6–5.8 | 63.5–67.6 | 10.4–12 | 1.5–2.0 | 67–80 | 4 | Maqbool et al. [46] |
| 4 B. bambosus | Arcuate | Slightly offset, raised, cupolate | Elongate-conoid, terminus rounded and curved | 46–59 | 410–450 | 30–35 | 4.4–4.8 | 7.5–9.4 | 6.0–12 | 65.3–68.1 | 9.6–12.8 | 3.2 | 62.4–76.8 | 4 | Mohilal et al. [43] |
| 5 B. citri | Spiral | Continuous, cupolate, concave | Hooked, terminus subacute | 46 | 280–310 | 21–23 | – | 7.3–12 | – | 64–68 | 9–10.5 | – | 55–61 | 4 | Edward and Rai [42] |
| 6 B. clavicaudatus | – | Continuous, conical | Conoid, terminus clavate | – | 700 | 31 | 5.7 | 8.5 | – | 60 | 13 | – | – | 4 | Thorne [5] |
| – | Conical, anteriorly flattened | – | 61–74 | 625–740 | 37–49 | 5.2–6.1 | 8.5–12 | 5.1–7.2 | 54.4–62.6 | 11–12.3 | – | – | 4 | Sturhan and Hohberg [52] | |
| 7 B. constrictus | Spiral | Continuous, conical, depression at OA | Elongate-conoid, terminus hooked | 68–85 | 490–550 | 32–37 | 4.6–5.4 | 6.4–8 | 6.4–9.0 | 63.3–67.2 | 11 | 2.5 | – | 4 | Rahman and Ahmad [32] |
| 8 B. cylindricus | – | Offset, elevated | Filiform, terminus rounded | 88–101 | 480–610 | 37–47 | – | 6–7 | 9–13 | 63–64 | 6–8 | 7 | 4 | Dhanachand et al. [36] | |
| 9 B. cynodoni | Close C-shape | Continuous, low, rounded | Elongate, terminus hooked | 54 | 420–490 | 26–30 | 4.5–6.0 | 7–9 | – | 62–65 | 8 | – | 64 | 4 | Fotedar and Mahajan [47] |
| 10 B. filiformis | Arcuate | Slightly offset, cupolate, anteriorly flattened | Elongate, terminus acute | 62 | 460–550 | 24–31 | 5.0–5.6 | 7–9 | – | 63–69 | 9 | 1.5 | 75 | 4 | Husain and Khan [33] |
| 11 B. flexuosus | Spiral | Slightly offset, high, not annulated | Elongate-conoid, curved, terminus pointed | 91–96 | 550–570 | 25–28 | – | 5.7–6.1 | – | 61–65 | 10.5 | 4 | – | 4 | Eroshenko [49] |
| 12 B. hyderi | C-shape | Continuous, cupolate, flat | Elongate-conoid, terminus rounded | – | 440–500 | 23–27 | 4.3–5.0 | 6.8–7.8 | – | 63.6–68.5 | 10–11 | – | 75–80 | 4 | Husain and Khan [53] |
| 13 B. impar | Close C-shape | Continuous, conoid, elevated | Elongate, ventrally arcuate | 96 | 504–600 | 25–32 | 4.8–6.2 | 5–7 | – | 63–66 | 13–14 | 3 | 95 | 4 | Khan and Basir [34] |
| 14 B. innuptus | – | Offset, conical, not annulated | Ventrally arcuate, terminus hooked | 59 | 470–490 | 25–29 | – | 7.3–8.3 | – | 64–66 | 11.5–12.5 | – | – | 4 | Andrassy [37] |
| 15 B. longicaudatus | Slightly ventrally arcuate | Continuous, conical, depression at OA | Elongate, terminus pointed | 88–92 | 430–720 | 25–28 | 7.5–9.2 | 4.8–6.2 | 8.6–12 | 65–74 | 8.5 | 3.4 | 71–74 | 4 | Bina et al. [45] |
| 16 B. minustylus | Slightly curved ventrally | Continuous, rounded | Short, terminus rounded | 59–72 | 960–830 | 48–52 | 5.6–7.4 | 10–14 | 5.3–7.5 | 79–81 | 4.0–4.8 | 3–5 | 99–109 | 4 | Mohilal et al. [43] |
| 17 B. mirus | Ventrally arcuate | Continuous, elevated | Elongate- filiform, terminus rounded | – | 540–572 | 25–32 | 4.8–5.3 | 6.2–7.8 | – | 63–66 | 11–12 | 2 | 100 | 4 | Khan [35] |
| 18 B. modicus | Spiral | Slightly offset, raised, anteriorly-flattened, depression at OA | Hooked, terminus striated and rounded | 51 | 429–487 | 24–31 | 4.2–5.0 | 7.7–9.8 | 4.0–5.5 | 66.5–71 | 9.8–10.2 | 3 | 77–78 | 4 | Lal and Khan [38] |
| 19 B. neosimilis | – | Continuous, narrow, truncate | Uniform, terminus blunt | 51 | 460 | 23 | – | 9 | – | 68 | 10 | – | – | 4 | Geraert [54] |
| 20 B. pakistanensis | C-shape | Slightly offset, elevated, conoid, anteriorly truncated | Elongate-conoid, terminus rounded | 73 | 540–580 | 31–34 | 5.0–5.2 | 7.5–8.0 | – | 67–68 | 11–12 | 4 | – | 4 | Siddiqi [55] |
| 21 B. punici | Slightly ventrally arcuate | Setoff, elevated, wide | Long, filiform, terminus rounded | 73–82 | 450–510 | 37–43 | 4.9–6.6 | 5–7 | 9.6–12 | 58–64 | 7–8 | 7 | – | 4 | Gambhir and Dhanachand [39] |
| 22 B. rafiqi | C-shape | Continuous, cupolate, flat | Elongate, arcuate, terminus rounded | – | 500–600 | 22–35 | 4.7–5.0 | 7.0–10 | – | 65–68 | 8–11 | – | 80–90 | 4 | Husain and Khan [53] |
| 23 B. seshadrii | Open C-shape | Continuous, conoid | Dorsally curved, terminus rounded | – | 410–510 | 28–29 | 4.2–4.9 | 6.0–8.1 | – | 67–68 | 12–13 | 3 | – | 4 | Handoo et al. [56] |
| 24 B. similis | C-shape | Continuous, elevated | Elongate, terminus hooked | 63 | 390–440 | 19–24 | 3.0–4.7 | 5–7 | – | 65–68 | 10–11 | 2 | 75 | 4 | Khan and Basir [48] |
| 25 B. spinocaudatus | Almost straight | Continuous, high and wide | Elongate, terminus rounded or pointed | 49–80 | 600–690 | 25–36 | 6.6–11 | 7.5–13 | 4.6–6.7 | 76–83 | 8.5–10.2 | 5.1 | 62–88 | 4 | Bina et al. [45] |
| 26 B. solomonensis | Almost straight | Slightly offset, narrow, conical, depression at OA | Elongate, terminus rounded or clavate | 80–93 | 550–650 | 24–39 | 4.5–6.0 | 6.3–7.3 | 6.1–9.3 | 53.4–58 | 12–14.5 | – | 85–98 | 4 | Ye and Geraert [40] |
| 27 B. spiralis | Spiral | Slightly offset, truncate | Short, terminus rounded | 45 | 400–490 | 22–27 | 3.4–4.4 | 8.1–10 | – | 62.4–73.2 | 10–12 | – | – | 4 | Egunjobi [41] |
| 28 B. thylactus | Ventrally arcuate | Offset, convex conoid | Hooked, terminus acute | – | 600 | 31 | 5.5 | 8.0 | – | 12.0 | – | – | 4 | Thorne [5] | |
| 29 B. tenuis | Open C-shape | Slightly offset, raised, depression at OA | Hooked, terminus unstriated and rounded | 71 | 480–550 | 25–30 | 4.6–5.2 | 6.3–7.6 | 6.0–7.7 | 65–70 | 11.3–12.5 | 2.5 | 75–84.6 | 4 | Lal and Khan [38] |
| 30 B. teres | Ventrally arcuate | Continuous, conoid-truncate | Ventrally curved, terminus rounded | 71 | 420–560 | 23–35 | – | 6.0–8.5 | 6.5–10 | 54–63 | 10–12 | 5 | 90 | 4 | Nanjappa and Khan [57] |
| 31 B. typicus | Open C-shape | Continuous, truncated, cupolate | Elongate-conoid, terminus rounded | – | 440–580 | 23–27 | 3.9–5.3 | 6–9 | – | 62–68 | 12–14 | – | 90–100 | 6 | Husain and Khan [30] |
| 32 B. volutus | Spiral | Offset, raised, cupolate, anteriorly flattened | Hooked, terminus unstriated and rounded | 35–60 | 450–510 | 20–28 | 4.9–5.7 | 8.7–12 | – | 67–72 | 8.3–9.2 | – | 78 | 4 | Lima and Siddiqi [6] |
| 33 B. zaini | Spiral | Continuous, high, smooth, truncated | Conoid, terminus rounded | 87 | 640–670 | 27–30 | 5.7–7.2 | 7.6–9.5 | 6.5–7.3 | 65.2–67.3 | 12.6 | 4 | – | 6 | Maqbool [31] |
1 Oral aperture; 2 Distance from anterior end to excretory pore; 3 Number of lateral lines in lateral field.

Table 2.
Main morphological characters and morphometrics of male Boleodorus species. All measurements are in µm.
Table 2.
Main morphological characters and morphometrics of male Boleodorus species. All measurements are in µm.
| Species Name | n | L | a | b | c | Stylet Length | Spicule Length | Spicule Morphology | Gubernaculum | Bursa | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B. acurvus | 1 | 460 | 38 | 4.2 | 5 | – | 16 | Cephalated, arcuate | – | Adanal | Jairajpuri [44] |
| 1 | 520 | 37 | 4.4 | 6.1 | 10.5 | 12.5 | – | 5.0 | Adanal | Zeidan and Geraert [50] | ||
| 2 | B. azadkashmirensis | 10 | 400–440 | 25–44 | 4.0–4.6 | 6.6–7.8 | 10.4–11.2 | 12.8–16 | Cephalated, arcuate | 4.0–4.8 | Crenated, adanal | Maqbool, Shahina, and Firoza [46] |
| 3 | B. clavicaudatus | 1 | 635 | 39 | 5.6 | 9.6 | 11.5 | 14 | – | – | – | Sturhan and Hohberg [52] |
| 4 | B. cylindricus | – | – | – | – | – | – | 16 | – | 3–6 | – | Dhanachand, Renubala, and Anandi [36] |
| 5 | B. cynodoni | 4 | 390–450 | 32–35 | 4.5–6.0 | 6–7 | 8 | 10–11 | – | 4 | Crenate, short | Fotedar and Mahajan [47] |
| 6 | B. filiformis | 1 | 460 | 38 | 4 | 6 | 9 | 13 | Paired, cephalated, arcuate | 3 | Crenate, adanal | Husain and Khan [33] |
| 7 | B. flexuosus | – | – | – | – | – | – | 14 | – | – | – | Eroshenko [49] |
| 8 | B. hyderi | 1 | 380 | 22 | 4.1 | 7.0 | 10 | 12 | – | 5 | – | Husain and Khan [53] |
| 9 | B. impar | 6 | 520–560 | 37–47 | 4–6 | 5–6 | 12–14 | 18–20 | Paired, cephalated, arcuate | 7–8 | Adanal | Khan and Basir [34] |
| 10 | B. minustylus | 2 | 660–670 | 52–53 | 5.3–5.8 | 11–15 | 3–4 | 14–16 | Sclerotized and arcuate | 4.8 | – | Mohilal, Anandi, and Dhanachand [43] |
| 11 | B. mirus | 5 | 525–590 | 37–47 | 5–6 | 4–4.8 | 10–11 | 19–22 | Paired, cephalated, arcuate | 9–10 | Crenated | Khan [35] |
| 12 | B. neosimilis | – | – | – | – | – | – | – | Cephalated | 14 | – | Geraert [54] |
| 13 | B. punici | 2 | 470–500 | 34–35 | 5.2–5.8 | 5.0–5.7 | 7–9 | 13–16 | – | 2–3 | – | Gambhir and Dhanachand [39] |
| 14 | B. similis | 1 | 380 | 26 | 4.2 | 5 | 11 | 15 | Cephalated, arcuate | 3 | Crenated | Khan and Basir [48] |
| 15 | B. solomonensis | 6 | 505–590 | 25.5–35.0 | 4.2–5.4 | 5.7–6.6 | 12–13 | 14–17 | Paired, cephalated | – | Adanal | Ye and Geraert [40] |
| 16 | B. thylactus | 1 | 500 | 33 | 5.0 | 7.2 | – | – | – | – | – | Thorne [5] |
| 14 | 535–662 | 30.2–42.3 | 4.8–6.3 | 8.2–11.5 | 10.9–12.4 | 8.8–11.1 | – | – | – | Deimi and Mitkowski [58] | ||
| 17 | B. tenuis | 10 | 440–530 | 25.0–31.6 | 4.5–5.0 | 6.5–6.9 | 10.8–12.0 | 12–14.2 | Arcuate | 5.0 | – | Lal and Khan [38] |
| 18 | B. teres | – | – | – | – | – | – | 13 | Cephalated | 6 | Crenate | Nanjappa and Khan [57] |
| 19 | B. typicus | 5 | 520–560 | 33–47 | 4.5–5.3 | 7 | 13–14 | 18–20 | Paired, cephalated | 3–5 | Adanal | Husain and Khan [30] |
| 20 | B. volutus | 1 | 440 | 28 | 4.9 | 9.6 | 9.0 | 14–15 | Cephalated, arcuate | 4.8–5.1 | Adanal | Lima and Siddiqi [6] |
| 21 | B. zaini | 9 | 480–600 | 30–33 | 5.3–6.2 | 7.5–9 | 12.6 | 16–18 | Paired, arcuate | 6.7 | Short, adanal | Maqbool [31] |
3.1.2. Description of B. thylactus Thorne, 1941
Female: Body habitus ventrally arcuate to C-shaped after heat relaxed. Lateral field with four equidistant lines, inner lines not as faint as described in the majority of other species. Lip region slightly offset, conical rounded in shape, anteriorly flattened with depressions at oral aperture. Stylet short, delicate with flange-like knobs, pharyngeal dorsal gland orifice (DGO) close to the stylet knob base. Pharynx composed of long and narrow corpus, metacorpus (i.e., median bulb) indistinct, valveless, irregularly shaped. Isthmus narrow, encircled with nerve ring ending in pyriform basal pharyngeal bulb. Excretory pore located in the pharyngeal bulb region, hemizonid indistinct, located 2–3 body annuli anterior to excretory pore, visible in fixed specimens. Cardia hemispherical. Intestine thick, appears filled with granules. Gonad monoprodelphic, vulva a transverse slit, vagina extending into uterine sac, crustaformeria indistinct, columnar arrangements of cells not clearly distinguishable, spermatheca offset, filled with sperm, oviduct composed of large cells, ovary short and composed of multiple rows. Post-vulval uterine sac small, composed of undifferentiated cells, half of the maximum body diameter long. Anus distinct, appears as oblique line. Tail region slender, posterior half of the tail curved ventrally to form hooked shape, tail tip rounded in the majority of specimens, pointed tip also observed in a few.
Male: Body habitus and anterior region similar to females. Spicule arcuate to curved, 16–19 µm long. Tail morphology similar to that of females. Bursa adanal, starting at the head of spicule and ending one spicule length posterior to cloaca. Phasmids not found in any of the specimens.
Juveniles: Juveniles are present in each studied sample. Only a few juveniles were handpicked and observed under the light microscope. They are similar to adults in general appearance except for under-developed pharyngeal and reproductive components. Since immature forms do not have enough characters to enable diagnosis, they were therefore excluded from the morphometrical analyses.
Remarks: Though B. thylactus is reported in different countries, morphometrics were provided for only a few populations (Table 1, Table 2 and Table 3, Figure 1, Figure 2 and Figure 3).
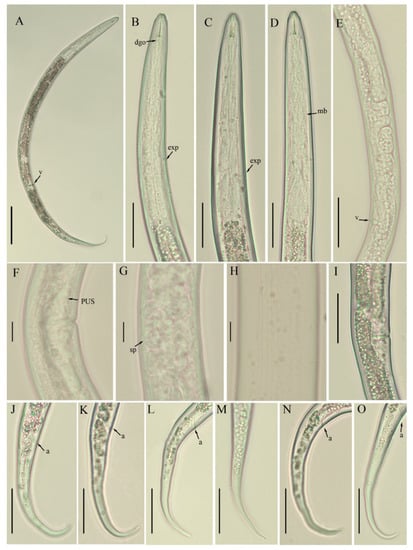
Figure 1.
Photomicrographs of B. thylactus female, Canadian population 61. (A) Entire body; (B–D) pharyngeal region; (E) gonad; (F) vulval region; (G) spermatheca; (H) lateral lines; (I) vulval region; (J–O) tail regions. Scale bars: (A) 50 µm; (B–E,I–O) 20 µm; (F–H) 5 µm. Arrowheads: (a) anus; (exp) excretory pore; (dgo) dorsal esophageal gland orifice; (mb) median bulb; (sp) spermatheca; (PUS) post uterine sac; (v) vulva.
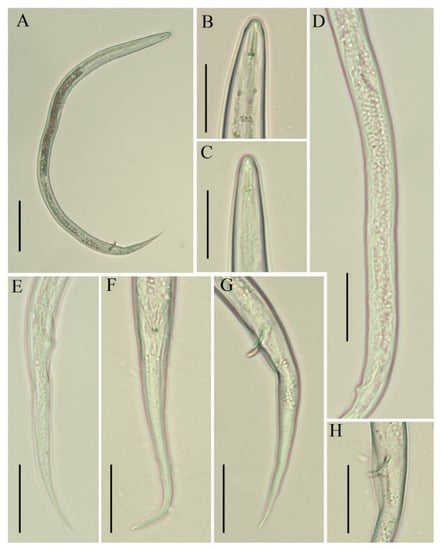
Figure 2.
Photomicrographs of B. thylactus male, Canadian population 61. (A) Entire body; (B,C) lip region; (D) gonad; (E–G) tails; (H) cloacal region with bursa. Scale bars: (A) 50 µm; (B–H) 20 µm.
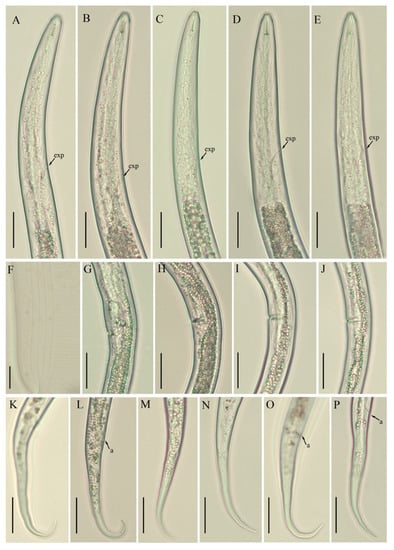
Figure 3.
Photomicrographs of B. thylactus female, Canadian populations 40, 50, and 62. (A,B; C; D,E) pharyngeal region of population 40; 50; and 62, respectively; (F) lateral lines; (G; H,I; J) vulval regions of populations 40; 50; and 62, respectively; (K,L; M,N; O,P) tail regions of populations 40; 50; and 62, respectively. Scale bars: (A–E, G–P) 20 µm; (F) 5 µm. Arrowheads: (a) anus; (exp) excretory pore.
The morphology and morphometry of Alberta populations of B. thylactus agree well with the original and other reports, except for the Brazilian population which is the smallest amongst all the reported populations (Table 3). The presence of males was reported in the original and Belgian populations, but a formal characterization was not provided in any of the reports. The population from Iran supplied the detailed morphometrics of males but no morphological characterization was provided by the authors [58]. In our study, males were present and were characterized both morphologically and morphometrically; however, males were not abundant in each studied sample. Considering this, we speculate that males have little diagnostic importance in this species. B. thylactus was originally described in the USA and was later reported from Afghanistan, Belgium, Brazil, Germany, India, Iran, Slovakia, and Spain in the rhizosphere of cultivated plants, grasses, and fruit trees. This species was also reported from meadows and arable lands, which indicates its ability to survive in any soil type and vegetation (Table 3). In the present study, we recovered four populations of B. thylactus from the cultivated areas of southern Alberta, making ours the first report of B. thylactus from Canada.

Table 3.
Morphometrics of B. thylactus from Canada and other countries. All measurements are in µm and presented as mean ± standard deviation (range).
Table 3.
Morphometrics of B. thylactus from Canada and other countries. All measurements are in µm and presented as mean ± standard deviation (range).
| Canada (Present Study) | Thorne [5] | Geraert [59] | Rashid et al. [60] | Lal and Khan [38] | Deimi and Mitkowski [58] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Populations | 61 | 40 | 50 | 62 | USA 1 | Belgium | Brazil | India | Iran | ||
| Characters | Females | Males | Females | Females | Females | Female | Females 2 | Female | Females 3 | Females | Male |
| n | 15 | 5 | 10 | 10 | 10 | – | – | 1 | – | 12 | 14 |
| Body length | 525.3 ± 31.5 (483–602.0) | 477.3 ± 23.7 (450.0–500.0) | 501.3 ± 32.7 (446–552) | 527.1 ± 28.9 (490.0–576.0) | 507.5 ± 34.3 (450.0–558.0) | 600 | 380–590 | 390 | 430–580 | 598 (535–662) | 544 (501–614) |
| a | 32.5 ± 2.3 (28.5–35.9) | 38.5 ± 1.9 (36.9–41.2) | 33.0 ± 22.1 (37.2–31.5) | 31.8 ± 2.5 (27.3–34.4) | 32.8 ± 2.8 (27.8–37.9) | 31 | 21–39 | 25 | 23–29 | 35.4 (30.2–42.3) | 41.3 (37.6–47.2) |
| b | 4.8 ± 0.3 (4.3–5.5) | 4.4 ± 0.2 (4.2–4.6) | 4.7 ± 7.5 (4.5–4.9) | 4.9 ± 0.3 (4.6–5.5) | 4.8 ± 0.3 (4.3–5.3) | 5.5 | 4–6.4 | 4.4 | 4.8–6.2 | 5.2 (4.8–6.3) | 5.6 (4.6–5.8) |
| c | 9.0 ± 1.1 (7.0–10.2) | 6.8 ± 0.2 (6.6–7.0) | 8.1 ± 5.1 (8.1–7.7) | 8.7 ± 1.1 (7.0–10.1) | 8.0 ± 0.9 (6.6–9.1) | 8 | 5.6–8.7 | 7.4 | 6.7–8.2 | 9.7 (8.2–11.5) | 8.8 (7.2–9.4) |
| c′ | 5.9 ± 0.8 (5.0–8.0) | 7.9 ± 0.7 (6.9–8.4) | 6.4 ± 9.2 (6.1–6.7) | 6.2 ± 0.8 (5.2–7.7) | 6.5 ± 1.0 (5.0–8.1) | – | – | 6.4 | 5.9–7.8 | 6.4 (4.6–7.9) | 7.4 (6.4–9.1) |
| V | 67.0 ± 1.3 (65.0–69.0) | 31.2 ± 1.7 (29.2–32.4) | 66.2 ± 1.1 (65.0–68.0) | 66.8 ± 1.5 (65.0–69.0) | 66.5 ± 1.4 (65.0–69.0) | – | 62–71.5 | 65 | 64–68 | 64.1 (61.8–65.9) | – |
| MB | 41.9 ± 4.2 (37.5–48.6) | 56 | – | – | – | – | – | – | – | – | – |
| G1 | 25.7 ± 2.2 (23.1–30.6) | – | 22.0 ± 24.8 (22.2–21.2) | 22.4 ± 1.9 (21.1–23.8) | 21.5 ± 1.7 (19.5–22.6) | – | – | – | – | – | – |
| Lip height | 2.8 ± 0.2 (2.4–3.0) | 3.0 ± 0.1 (3.0–3.1) | 2.9 ± 0.2 (2.6–3.3) | 2.7 ± 0.2 (2.4–2.9) | 3.0 ± 0.2 (2.5–3.3) | – | – | – | – | – | – |
| Lip width | 5.5 ± 0.3 (5.1–6.0) | 5.3 ± 0.3 (5.0–5.7) | 5.9 ± 0.2 (5.4–6.0) | 5.4 ± 0.3 (5.1–5.9) | 5.6 ± 0.3 (5.0–5.9) | – | – | – | – | – | – |
| Stylet length | 9.3 ± 0.8 (8.2–10.7) | 9.0 ± 1.2 (7.5–10.3) | 9.4 ± 0.5 (8.5–10.6) | 9.6 ± 0.8 (8.6–10.8) | 9.6 ± 0.3 (9.2–10.0) | 12 | 8.5–12 | 10 | 10.5–12 | 10.9 (10.1–12.3) | 11.1 (10.9–12.4) |
| Anterior end to excretory pore | 84.8 ± 5.1 (74.0–92.0) | 82.3 ± 3.9 (77.0–86.0) | 85.1 ± 4.5 (79.0–92.0) | 88.3 ± 4.2 (85.0–96.0) | 82.2 ± 3.1 (79.0–87.0) | – | – | – | – | 82.6 (80.7–87.8) | 80.2 (78.7–84.1) |
| Pharynx length | 109.1 ± 3.9 (103.0–117.0) | 109.5 ± 1.7 (108.0–111.0) | 107.3 ± 4.4 (99.0–113.0) | 107.2 ± 6.0 (99.0–117.0) | 105.0 ± 5.1 (99.0–113.0) | – | – | 88.5 | – | 108 (100–119) | 101 (99–107) |
| Maximum body width | 16.3 ± 1.9 (13.8–20.0) | 12.4 ± 0.6 (12.0–13.3) | 15.2 ± 1.5 (12.0–17.5) | 16.6 ± 1.2 (15.0–18.0) | 15.6 ± 1.4 (13.5–18.0) | – | – | 16 | – | 15.7 (13.4–17.5) | 12.6 (12.1–14.3) |
| Vulva body width | 14.8 ± 1.6 (13.0–18.5) | – | 14.4 ± 1.3 (12.0–17.0) | 15.3 ± 1.1 (14.0–17.0) | 14.4 ± 1.1 (13.0–16.0) | – | – | – | – | – | – |
| Post uterine sac length | 11.1 ± 0.9 (9.7–13.0) | – | 10.2 ± 1.1 (9.0–12.0) | 9.7 ± 1.1 (8.2–11.6) | 9.4 ± 0.8 (8.2–10.8) | – | – | – | – | 10.7 (8.5–14.6) | – |
| Distance from vulva to anus | 111.6 ± 9.0 (102.0–131.0) | – | 106.9 ± 4.2 (101.0–115.0) | 112.7 ± 4.1 (106.0–118.0) | 104.8 ± 6.3 (101.0–119.0) | – | – | – | – | 107.8 (98–119) | – |
| Anal/cloacal body width | 10.2 ± 1.4 (8.8–13.0) | 8.9 ± 1.0 (8.1–10.3) | 9.7 ± 0.7 (9.0–10.8) | 10.0 ± 0.9 (8.1–10.9) | 9.8 ± 0.8 (9.0–11.0) | – | – | – | – | – | – |
| Spicule length | – | 17.0 ± 1.4 (16.0–19.0) | – | – | – | – | – | – | – | – | 9.5 (8.8–11.1) |
| Tail length | 59.4 ± 9.5 (50.0–77.0) | 70.3 ± 1.7 (68.0–72.0) | 61.9 ± 6.4 (55.0–72.0) | 61.5 ± 6.3 (52.0–71.0) | 63.9 ± 7.7 (55.0–75.0) | – | – | 53 | – | 67 (59–82) | 68.6 (66.8–70.7) |
1 Original description; 2 Composite values of two populations consisting of 15 and 9 females; 3 Composite values of two populations consisting of 20 and 10 females.
3.1.3. Description of B. volutus Lima and Siddiqi, 1963
Female: Body habitus ventrally arcuate to close C-shaped after heat relaxed. Lateral field with four equidistant lines. Lip region elevated, slightly offset, conical rounded in shape, anteriorly flattened with slight depressions at oral aperture. Stylet short, delicate with flange-like knobs, DGO close to the stylet knob base. Pharynx composed of long and narrow corpus, metacorpus (i.e., median bulb) indistinct, valveless, irregularly shaped. Isthmus narrow, encircled with nerve ring ending in pyriform basal pharyngeal bulb. Excretory pore located in the region of pharyngeal bulb, hemizonid indistinct, located 2–3 body annuli anterior to excretory pore. Intestine thick, appears filled with granules. Gonad monoprodelphic, vulva a transverse slit, vagina extending into uterine sac, crustaformeria indistinct, spermatheca offset. Post uterine sac small, composed of undifferentiated cells, half of the maximum body diameter long. Anus distinct, appears as oblique line. Tail ventrally curved, hook-shaped ending in rounded tip.
Male and juveniles: Not found.
Remarks: B. volutus was originally described from England in the rhizosphere of grass [6]. Subsequently, it was reported from the Netherlands [61] and Poland [62]. Unfortunately, both references are inaccessible, so it is not possible to compare the morphometrics or additional details associated with these reports. Recently, B. volutus was reported from Afghanistan [63] and Germany [52] in the rhizosphere of cultivated crops and grasslands, but without any photo documentation or morphometric data. The scarcity of morphometric and image data explains the difficulties in dealing with Boleodorus species. In the present study, we found four populations or B. volutus in cultivated areas of southern Alberta; however, we only studied the morphology of two populations. The other two populations did not contain sufficient adult individuals to carry out morpho-molecular studies. Morphometrically, the Canadian population of B. volutus is slightly longer and wider than the original description (Table 4). The rest of the characters, such as lip morphology, stylet length, and tail shape, agree well with the original description. B. volutus is morphologically very similar to B. thylactus, however, both species can be differentiated from each other by lip and tail morphology. In addition, our sequence analyses indicate that both species are molecularly distant (Figure 4).
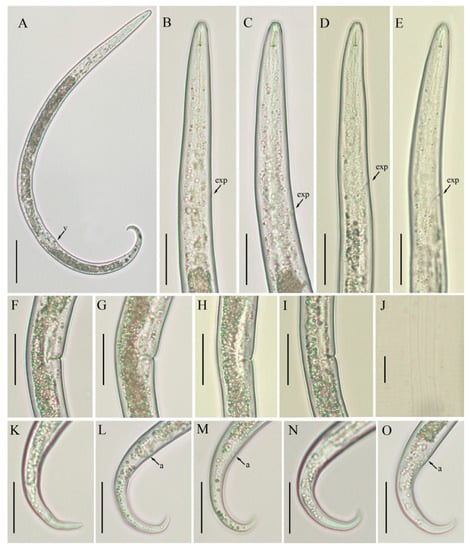
Figure 4.
Photomicrographs of B. volutus female, Canadian populations 61 and 40. (A) Entire body, population 61; (B,C; D,E) pharyngeal region of populations 61 and 40, respectively; (F,G; H,I) vulval regions of populations 61 and 40, respectively; (J) lateral lines; (K–M; N,O) tail regions of populations 61 and 40, respectively. Scale bars: (A) 50 µm; (B–I,K–O) 20 µm; (J) 5 µm. Arrowheads: (a) anus; (exp) excretory pore; (v) vulva.
3.2. Habitat and Locality
Both populations of B. thylactus and B. volutus were present in all four fields. The geographical locations of each field are as follows. Population 40: latitude 49°46′3.684″ N; longitude −112°24′13.3056″ W; Municipal District of Taber, Alberta, Canada. Population 50: latitude 49°47′22.7256″ N; longitude −111°59′50.082″ W; Municipal District of Taber, Alberta, Canada. Population 61: latitude 49°50′52.4868″ N; longitude −111°22′34.0752″ W; Rural Municipality of Forty Mile County, Alberta, Canada. Population 62: latitude 49°57′24.6276″ N; longitude −111°18′24.5556″ W; Rural Municipality of Forty Mile County, Alberta, Canada. Fields 50 and 61 were covered with grasses, whereas fields 40 and 62 had green manure cover crop and canola, respectively. The soil type of fields ranged from sandy to clayey. Regardless of the soil type, B. thylactus was the dominant species compared with B. volutus.
3.3. Distribution and Associated Hosts of Boleodorus Species
Boleodorus species appear to have a diverse host range and geographical distribution. The majority of species of this genus were originally described from India (20 spp.), the USA (4 spp.), Pakistan (3 spp.), Hungary, the Solomon Islands, New Zealand, Nigeria, Russia, and the UK (Table 5; [7]). Once formally described, only B. acurvus, B. clavicaudatus, B. pakistanensis Siddiqi [55], B. thylactus, and B. volutus were reported outside of their type locality [3,12,50,52,58].
There are some additional reports in which species level identification was not carried out and only generic presence was reported [64,65]. Species level resolution is imperative, as each species has a different ecological role and properties [10,66]. The associated hosts of Boleodorus species range from agronomic/horticultural crops to grasses, perennial plants, meadows, and arable lands. Based on these reports, we speculate that the diversity of Boleodorus species has not been fully explored; if more attention is given to this genus, together with other principle parasitic species, there will likely be more reports of Boleodorus species.

Table 4.
Morphometrics of B. volutus female. All measurements are in µm and presented as mean ± standard deviation (range).
Table 4.
Morphometrics of B. volutus female. All measurements are in µm and presented as mean ± standard deviation (range).
| Character | Canadian Populations | Original Description | |
|---|---|---|---|
| Populations | 61 | 40 | Lima and Siddiqi [6] |
| n | 10 | 8 | 16 |
| Body length | 547.4 ± 31.5 (501.0–581.0) | 533.0 ± 30.7 (487.0–577.0) | 480 (450–510) |
| a | 27.4 ± 2.5 (24.5–31.3) | 28.9 ± 2.6 (24.9–32.1) | 26 (20–28) |
| b | 4.6 ± 0.2 (4.2–4.8) | 4.5 ± 0.2 (4.2–4.9) | 5.3 (4.9–5.7) |
| c | 9.6 ± 0.8 (8.5–10.6) | 10.0 ± 1.0 (8.8–11.5) | 10 (8.7–12) |
| c′ | 5.4 ± 0.8 (4.3–6.3) | 5.2 ± 0.5 (4.6–5.8) | – |
| V | 68.9 ± 0.7 (68.0–70.0) | 69.0 ± 1.2 (68.0–71.0) | 69 (67–72) |
| Lip height | 2.7 ± 0.2 (2.4–3.0) | 2.3 ± 0.3 (2.1–2.8) | – |
| Lip width | 5.9 ± 0.2 (5.4–6.3) | 5.6 ± 0.1 (5.5–5.8) | – |
| Stylet length | 8.5 ± 0.4 (8.0–9.3) | 9.2 ± 0.6 (8.2–9.8) | 8.7 (8.3–9.2) |
| Anterior end to excretory pore | 90.7 ± 4.2 (86.0–97.0) | 88.4 ± 3.8 (85.0–95.0) | 78 |
| Pharynx | 118.5 ± 4.7 (109.0–125.0) | 119.0 ± 7.3 (108.0–129.0) | – |
| Maximum body width | 20.1 ± 2.4 (16.0–22.7) | 18.6 ± 2.3 (16.0–22.4) | – |
| Vulva body width | 17.4 ± 1.7 (15.0–19.8) | 16.9 ± 1.4 (15.0–19.0) | – |
| Post uterine sac length | 10.4 ± 1.7 (7.2–12.6) | 9.6 ± 1.2 (8.0–12.1) | – |
| Distance from vulva to anus | 118.1 ± 8.1 (105.0–129.0) | 110.5 ± 7.3 (102.0–121.0) | – |
| Anal body width | 10.9 ± 1.3 (9.5–13.2) | 10.3 ± 0.7 (9.2–11.0) | – |
| Tail length | 57.8 ± 3.0 (52.0–62.0) | 53.9 ± 4.8 (46.0–59.0) | – |

Table 5.
Distribution and associated hosts of Boleodorus species.
Table 5.
Distribution and associated hosts of Boleodorus species.
| Species Name | Country | Host | References | |
|---|---|---|---|---|
| 1 | B. acurvus | Nigeria | Saccharum officinarum L. | Jairajpuri [44] |
| Sudan | Citrus limon | Zeidan and Geraert [50] | ||
| 2 | B. acutus | USA | – | Thorne and Malek [51] |
| 3 | B. azadkashmirensis | Pakistan | Allium cepa, Zea mays Fragaria ananassa | Maqbool, Shahina, and Firoza [46] |
| 4 | B. bambosus | India | Bambusa tuida | Mohilal, Anandi, and Dhanachand [43] |
| 5 | B. citri | India | Citrus reticulata | Edward and Rai [42] |
| 6 | B. clavicaudatus | USA | Alfalfa crowns | Thorne [5] |
| German | Loamy soil from meadows | Sturhan and Hohberg [52] | ||
| 7 | B. constrictus | India | Carica papaya | Rahman and Ahmad [32] |
| 8 | B. cylindricus | India | Saccharum officinarum L. | Dhanachand, Renubala, and Anandi [36] |
| India | Brassica oleracea | Hassan and Ahangar [67] | ||
| 9 | B. cynodoni | India | Cynodon dactylon Pers. | Fotedar and Mahajan [47] |
| 10 | B. filiformis | India | Solanum melongena | Husain and Khan [33] |
| 11 | B. flexuosus | Russia | – | Eroshenko [49] |
| 12 | B. hyderi | India | Mangifera indica L. | Husain and Khan [53] |
| 13 | B. impar | India | Cynodon dactylon Pers. | Khan and Basir [34] |
| 14 | B. innuptus | Hungary | – | Andrassy [37] |
| 15 | B. longicaudatus | India | Morus alba L. | Bina, Mohilal, Pramodini, and Majur-Shah [45] |
| 16 | B. minustylus | India | Banana roots | Mohilal, Anandi, and Dhanachand [43] |
| 17 | B. mirus | India | Cynodon dactylon Pers. | Khan [35] |
| 18 | B. modicus | India | Populus sp. and Myrica sapida | Lal and Khan [38] |
| 19 | B. neosimilis | USA | - | Geraert [54] |
| 20 | B. pakistanensis | Pakistan | Pinus excelsa Wall. | Siddiqi [55] |
| Egypt | Polypogon monspeliensis L., Solanum nigrum L., | Ibrahim et al. [3] | ||
| 21 | B. punici | India | Punica granatum | Gambhir and Dhanachand [39] |
| 22 | B. rafiqi | India | Pyrus communis L. | Husain and Khan [53] |
| 23 | B. seshadrii | India | Glycine max L. | Handoo et al. [56] |
| 24 | B. similis | India | Plumeria acutifolia Poir. | Khan and Basir [48] |
| 25 | B. spinocaudatus | India | Morus alba L. | Bina, Mohilal, Pramodini, and Majur-Shah [45] |
| 26 | B. solomonensis | Solomon Islands | Moist soil in tropical forest, host unknown | Ye and Geraert [40] |
| 27 | B. spiralis | New Zealand | Dominant vegetation composed of Leptospermum scoparium, Weinmannia racemose, and Psuedopanax arboreum | Egunjobi [41] |
| 28 | B. thylactus | USA | Cultivated soil, alfalfa crown roots | Thorne [5] |
| Belgium | Meadows, arable land | Geraert [59] | ||
| Brazil | Theobroma cacao | Rashid et al. [60] | ||
| India | Pinus sp., Artocarpus integrifolia | Lal and Khan [38] | ||
| Spain | Natural plant communities | Castillo et al. [13] | ||
| Iran | Grapes | Karegar et al. [68] Deimi and Mitkowski [58] | ||
| Afghanistan | Clover | Asghari et al. [63] | ||
| Germany | Arable soil, cherry tree plantation | Sturhan and Hohberg [52] | ||
| Iran | Polianthes tuberosa | Husseinvand et al. [69] | ||
| Slovakia | Solidago gigantea | Čerevková et al. [12] | ||
| 29 | B. tenuis | India | Casurina equsitifolia | Lal and Khan [38] |
| 30 | B. teres | India | – | Nanjappa and Khan [57] |
| 31 | B. typicus | India | Narcissus sp. | Husain and Khan [30] |
| 32 | B. volutus | UK | Grass species | Lima and Siddiqi [6] |
| Netherlands | – | Bongers [61] | ||
| Poland | – | Brzeski [62] | ||
| Afghanistan | Potato, tomato | Asghari et al. [63] | ||
| Germany | Meadow soil | Sturhan and Hohberg [52] | ||
| 33 | B. zaini | Pakistan | Citrus aurantium | Maqbool [31] |
3.4. Molecular Characterization of B. thylactus and B. volutus with Phylogenetic Relationships of Boleodorus with Related Genera
Both Boleodorus species were sequenced for partial 18S (MZ081056–MZ081059), D2–D3 of 28S (MZ081091–MZ081097), and ITS (MZ099822–MZ099823) regions. Thirteen new sequences were obtained in the present study.
The partial 18S sequences of Canadian B. thylactus (MZ081056–MZ081059) showed 98–99% (1–11 bp and 0–1 indels difference,) sequence identity with the B. thylactus (MW716329, KJ869348, AY993976, KT709462, MK639396, AY593915, MW056180) sequences deposited in NCBI (Figure 5). In addition to that, Canadian B. thylactus showed 99% (4 bp difference and 0 indels) sequence identity with the B. volutus (FJ969117) from the Netherlands.
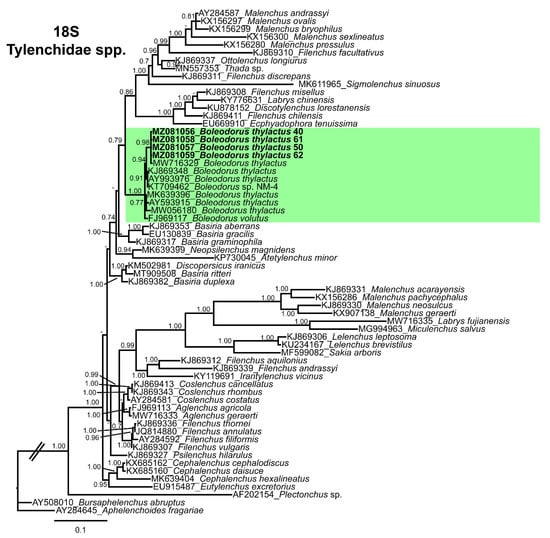
Figure 5.
Phylogenetic relationships of the genus Boleodorus spp. within Tylenchidae. Bayesian 50% majority rule consensus tree as inferred from 18S rRNA gene sequence alignment under the transition model with a gamma-shaped distribution (TIM1 + G). Posterior probabilities of more than 0.70 are given for appropriate clades. Sequences newly obtained in this study are shown in bold. The scale bar indicates expected changes per site. Boleodorus spp. clade in green color.
The D2–D3 of 28S (MZ081091–MZ081094) obtained for Canadian B. thylactus showed 95–98% (8–37 bp and 0–1 indels difference) sequence identity with the B. thylactus (MW716281, MW716282, KP313830, MW056183) sequences deposited in NCBI (Figure 6). Moreover, Canadian B. thylactus showed 94–99% (10–39 bp and 0–1 indels difference) sequence similarity with unidentified Boleodorus spp. (JQ005001–JQ005003, JQ005021, MK639378, MK639377, DQ328718) and 96% (28 and 0 indels difference) sequence similarity with B. volutus (MT994501) from the USA. The D2–D3 of 28S (MZ081095–MZ081097) sequences obtained for Canadian populations of B. volutus showed 99% (8 bp and 0 indels difference,) sequence identity with the B. volutus (MT994501) from the USA.
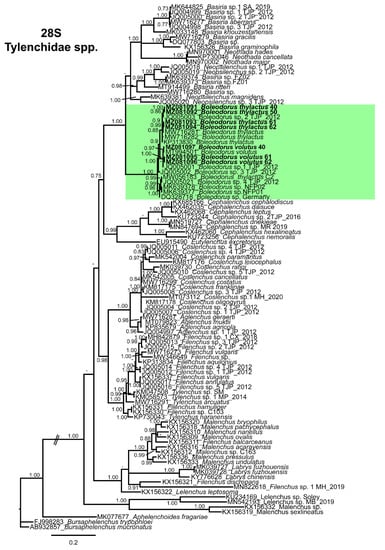
Figure 6.
Phylogenetic relationships of the genus Boleodorus spp. within Tylenchidae. Bayesian 50% majority rule consensus tree as inferred from D2–D3 expansion domains of the 28S rRNA sequence alignment under the transversion model with invariable sites and a gamma-shaped distribution (TVM + I + G). Posterior probabilities of more than 0.70 are given for appropriate clades. Sequences newly obtained in this study are shown in bold. The scale bar indicates expected changes per site. Boleodorus spp. clade in green color.
Phylogenetic analyses were performed for only two markers (18S and D2–D3 of 28S). ITS sequences were obtained for B. thylactus, however, due to lack of similarity with other Tylenchidae genera, phylogenetic analysis cannot be performed for this marker. The nucleotide BLAST results of ITS sequences showed 84–87% similarity with Ditylenchus sp. (MF669512, MF669513 from Taiwan), Filenchus sp. (MH842880 from China), and Coslenchus rhombus (MK874505 from South Africa), with very low sequence coverage of 26–56% and poor E-value.
The 18S tree was performed with 64 sequences of Tylenchidae species (four of them new, belonging to B. thylactus) and three outgroup taxa Plectonchus sp. (AF202154), Bursaphelenchus abruptus Giblin-Davis, Mundo-Ocampo, Baldwin, Norden, and Batra [70] (AY508010), and Aphelenchoides fragariae (Ritzema Bos) Christie [71,72] (AY284645). The Bayesian 50% majority rule consensus tree inferred from the partial 18S alignment is given in Figure 5. The tree contained a highly supported major clade (PP = 1.0) comprising all the species of the genus Boleodorus, including the B. thylactus and B. volutus from Alberta, Canada (Figure 5). The D2–D3 domains of the 28S rRNA gene alignment (699 bp long) included 98 sequences of Tylenchidae species (four of them new, belonging to B. thylactus, and three others from B. volutus) and three outgroup species, Bursaphelenchus trypophloei Tomalak [73] (FJ998283), Bursaphelenchus mucronatus Mamiya and Enda [74] (AB932857), and Aphelenchoides fragariae (MK077677). The Bayesian 50% majority rule consensus tree inferred from the D2–D3 alignment shows one highly supported major clade (PP = 1.0) comprising species of Boleodorus, including B. thylactus and B. volutus from Alberta, Canada (Figure 6). In both our phylogenetic analyses, none of the Boleodorus species held doubtful positions; both our species grouped within the Boleodorus clade. From these results, it seems that Boleodorus is a monophyletic genus, but we also consider the possibility that its phylogenetic positioning could change after more Boleodorus species sequences become available for phylogenetic study.
4. Discussion
The present study focuses on morphology/morphometry and phylogenetics of the least studied tylenchid nematode, namely, Boloeodorus species. Several studies have been published on different Tylenchidae genera, such as Basiria [75,76], Malenchus [22,77] Filenchus [78], and Cephalenchus [79], to improve our knowledge and understanding of this complex group of nematodes. However, no detailed studies were carried out for Boleodorus.
In general, Boleodorus species plays an essential role in soil ecological studies. Moreover, these species were detected in the rhizosphere of agricultural and horticultural plants [9,10,58,65]. The association of Boleodorus with plants has not been clearly demonstrated or assessed except by Geraert [59], who reported 150 individuals of B. thylactus in 100 mL of soil collected from arable lands of Belgium, and Deimi and Mitkowski [58], who reported the same species in a vineyard of Iran with a frequency as high as 80%. Since then, such a high density of nematodes has not been observed in subsequent reports; B. thylactus and B. volutus were found in clover and potato fields of Afghanistan at a frequency of 36 and 12%, respectively [63]. Boleodorus pakistanensis was frequently found present in the rhizosphere of grass and flowering plants in Egypt [3].
Boleodorus cylindricus was detected in cauliflower with a 90% frequency and infestation rate of 36% [67]. Similarly, Boleodorus was found to be the most abundant genus present in commercial vegetable fields of Srinagar, India [64]. Additionally, Pan et al. [65] conducted studies on the nematode communities in the black soil region of China, reporting that Boleodorus was the most abundant genus in both grass and bare lands. Moreover, Čerevková et al. [12] carried out a study in Slovakia to assess the effect of an invasive plant (Solidago gigantean Aiton) on the soil nematode communities. They found that B. thylactus and some other nematodes were abundantly present in the invasive sites as compared with noninvasive ones, suggesting that vegetation type partly shapes nematode communities. In our study, we found mixed populations of B. thylactus and B. volutus in the cultivated areas of southern Alberta. We detected adults and juveniles of B. thylactus in each sample with a density of 15–40 individuals/100g of soil. Despite the presence of a B. volutus in each sample, the number of individuals was very low. Because composite samples were prepared from a mixture of 30 core samples collected at a depth of 30 cm within each field, it is likely that areas with a high density of B. volutus either were missed or diluted significantly with other soils. Other than Boleodorus, we also detected root-lesion, spiral, and pin nematodes; the identification of other detected nematodes will be part of our future studies.
While analyzing published data on Boleodorus species, we found that some Boleodorus species were inadequately described, requiring synonymization or further detailed studies to retain valid taxa status. In our work, we do not propose any taxonomical revisions, rather we list all the important characters that one should consider while performing Boleodorus identification. Moreover, we agree with several nematologists [2,52,80] that such actions should only be performed after recollection of type material and conducting a detailed molecular study using ribosomal and mitochondrial markers [81]. Like other Tylenchidae nematodes, the current sequence-based data for Boleodorus species are insufficient. Very few Boleodorus sequences are present in NCBI for comparative and phylogenetic studies; most of the sequences consist of unidentified Boleodorus species. In our phylogenetic analyses, none of the Boleodorus species held a doubtful position, consequently allowing us to conclude that Boleodorus species may be monophyletic. However, it is prudent to consider that only a small portion of Boleodorus species have been discovered or are available for sequence-based study; the phylogenetic positioning will likely change in the future.
5. Conclusions
Surprisingly, no Boleodorus species were previously reported from Alberta, Canada. The latest reports on nematode studies focus on those groups of nematodes that are considered pests of economic relevance [1,2,4]. In this study, we presented morphological data and distribution of a nematode genus which, so far, does not hold pest status. Knowledge of nematode species present (or absent) in a plant growing area is important to the growers, because each cropping system hosts different nematode species and may require different soil and crop management strategies. Moreover, the results of our study will aid researchers to correctly identify species that were previously unknown or escaped detection in prior field surveillance programs.
Author Contributions
Conceptualization, M.M. and D.P.Y.; methodology, M.M. and D.P.Y.; software, M.M. and P.C.; validation, M.M., D.P.Y., and P.C.; formal analysis, M.M. and P.C.; investigation, M.M. and D.P.Y.; resources, D.P.Y.; data curation, M.M., D.P.Y., and P.C.; writing—original draft preparation, M.M., D.P.Y., and P.C.; writing—review and editing, M.M., D.P.Y., and P.C.; visualization, M.M., D.P.Y., and P.C.; supervision, D.P.Y.; project administration, D.P.Y.; funding acquisition, D.P.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Potato Early Dying Complex project funded by the University of Lethbridge Research Operating Fund, and the Canadian Potato Early Dying Network project funded by the Canadian Agri-Science Cluster for Horticulture 3 grant to D.P.Y., in collaboration with the Potato Growers of Alberta, McCain Foods Canada Ltd., Cavendish Farms Corp. and Lamb Weston Inc.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank potato growers in Alberta, Canada, for providing access to their fields, and Mariana Vetrici (University of Lethbridge, AB, Canada) for the collection of soil samples. We thank Akbar Karegar from Shiraz University, Iran, for providing electronic copies of research articles. We also thank Carolina Cantalapiedra-Navarrete (Institute for Sustainable Agriculture (IAS), CSIC, Spain), for the excellent technical assistance in molecular analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Munawar, M.; Yevtushenko, D.P.; Castillo, P. Integrative taxonomy, distribution, and host associations of Geocenamus brevidens and Quinisulcius capitatus from southern Alberta, Canada. J. Nematol. 2021, 53. [Google Scholar] [CrossRef]
- Munawar, M.; Yevtushenko, D.P.; Palomares-Rius, J.E.E.; Castillo, P. Species diversity of pin nematodes (Paratylenchus spp.) from potato growing regions of southern Alberta, Canada. Plants 2021, 10, 188. [Google Scholar] [CrossRef]
- Ibrahim, I.; Handoo, Z.; El-Sherbiny, A. A survey of phytoparasitic nematodes on cultivated and non-cultivated plants in Northwestern Egypt. J. Nematol. 2000, 32, 478. [Google Scholar] [PubMed]
- Forge, T.A.; Larney, F.J.; Kawchuk, L.M.; Pearson, D.C.; Koch, C.; Blackshaw, R.E. Crop rotation effects on Pratylenchus neglectus populations in the root zone of irrigated potatoes in southern Alberta. Can. J. Plant Pathol. 2015, 37, 363–368. [Google Scholar] [CrossRef]
- Thorne, G. Some nematodes of the family Tylenchidae which do not possess a valvular median esophageal bulb. Great Basin Nat. 1941, 2, 37–85. [Google Scholar]
- Lima, M.; Siddiqi, M.R. B. volutus n. sp.(Nematoda: Nothotylenchinae) found in soil about grass roots in England. Nematologica 1963, 9, 19–23. [Google Scholar] [CrossRef]
- Geraert, E. The Tylenchidae of the World: Identification of the Family Tylenchidae (Nematoda); Academia Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Geraert, E. Tylenchidae in agricultural soils. Man. Agric. Nematol. 1991, 795–825. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.; Freckman, D.W.; Georgieva, S. Feeding habits in soil nematode families and genera—an outline for soil ecologists. J. Nematol. 1993, 25, 315. [Google Scholar]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Čerevková, A.; Miklisová, D.; Bobuľská, L.; Renčo, M. Impact of the invasive plant Solidago gigantea on soil nematodes in a semi-natural grassland and a temperate broadleaved mixed forest. J. Helminthol. 2020, 94. [Google Scholar] [CrossRef]
- Castillo, P.; País, M.; Barcina, A.G. Nematodos de la Familia Tylenchidae Orley, 1880 (Nematoda: Tylenchida), en la sierra de Cazorla, España. Zool. Baetica 1991, 2, 137–161. [Google Scholar]
- Jenkins, W. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Report. 1964, 48, 692. [Google Scholar]
- Seinhorst, J. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef]
- De Grisse, A.T. Redescription ou Modifications de Quelques Technique Utilis [a] es Dan L’etude des n [a] Ematodes Phytoparasitaires; Mededelingen Rijksfakulteit Landbouwwetenschappen: Ghent, Belgium, 1969. [Google Scholar]
- Maria, M.; Powers, T.; Tian, Z.; Zheng, J. Distribution and description of criconematids from Hangzhou, Zhejiang Province, China. J. Nematol. 2018, 50, 183–206. [Google Scholar]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Holterman, M.; Rybarczyk, K.; Van den Elsen, S.; Van Megen, H.; Mooyman, P.; Santiago, R.P.; Bongers, T.; Bakker, J.; Helder, J. A ribosomal DNA-based framework for the detection and quantification of stress-sensitive nematode families in terrestrial habitats. Mol. Ecol. Resour. 2008, 8, 23–34. [Google Scholar] [CrossRef]
- Ferris, V. Variation in spacer ribosomal DNA in some cyst-forming species of plant parasitic nematodes. Fundam. Appl. Nematol. 1993, 16, 177–184. [Google Scholar]
- Curran, J.; Driver, F.; Ballard, J.; Milner, R. Phylogeny of Metarhizium: Analysis of ribosomal DNA sequence data. Mycol. Res. 1994, 98, 547–552. [Google Scholar] [CrossRef]
- Qing, X.; Bert, W. Redefinition of genus Malenchus Andrássy, 1968 (Tylenchomorpha: Tylenchidae) with additional data on ecology. J. Nematol. 2017, 49, 189. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT; Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1999; pp. 95–98. [Google Scholar]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1. 4.2, a Graphical Viewer of Phylogenetic Trees. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 21 April 2021).
- Geraert, E. Morphometric relations in nematodes. Nematologica 1968, 14, 171–183. [Google Scholar] [CrossRef]
- Husain, S.I.; Khan, A.M. Paurodontella n. gen. and three new species of nematodes from North India (Nematoda: Neotylenchidae). Nematologica 1968, 13, 493–500. [Google Scholar] [CrossRef]
- Maqbool, M. Three new species of the super family Neotylenchoidea (Nematoda: Tylenchida) from Pakistan. J. Nematol. 1982, 14, 317. [Google Scholar]
- Rahman, P.F.; Ahmad, I. Two new species of Tylenchoidea from Uttar Pradesh. Indian J. Nematol. 1995, 25, 136–141. [Google Scholar]
- Husain, S. Boleodorus filiformis nomen novum for B. acutus Husain and Khan, 1974. Indian J. Nematol. 1977, 5, 246–247. [Google Scholar]
- Khan, E.; Basir, M. Boleodorus impar n. sp.(Nematoda: Tylenchida) from India. Proc. Helminthol. Soc. Wash. 1964, 31, 187–190. [Google Scholar]
- Khan, E. Boleodorus mirus n. sp. (Tylenchida: Boleodorinae n. subfam.) from Kufri, Simla (HP) India, with a key to the species of the genus Boleodorus Thorne, 1941. Zool. Anz. 1964, 173, 336–341. [Google Scholar]
- Dhanachand, C.; Renubala, K.; Anandi, Y. Three new species of Tylenchida associated with sugarcane from Manipur. Curr. Nematol. 1993, 4, 45–52. [Google Scholar]
- Andrassy, I. Zur taxonomie der Neotylenchiden. Nematologica 1961, 6, 25–36. [Google Scholar] [CrossRef]
- Lal, A.; Khan, E. Nematodes Associated with Forest Trees in Northern India-Species of Boleodorus Thorne, 1941; My Forest 24: 301-306; Diversity of Plant and Soil Nematodes in Uttarakhand: Dehradun, India, 1988; p. 267. [Google Scholar]
- Gambhir, R.; Dhanachand, C. Nematodes of fruit plants in Manipur–five new species of tylenchids (Nematoda: Tylenchida). Indian J. Nematol. 1996, 26, 197–207. [Google Scholar]
- Ye, W.; Geraert, E. Plant parasitic nematodes from the Solomon Islands with a description of Boleodorus solomonensis. Nematologica 1997, 43, 431–454. [Google Scholar] [CrossRef]
- Egunjobi, O.A. Three new species of nematodes from New Zealand. N. Z. J. Sci. 1968, 11, 488–497. [Google Scholar]
- Edward, J.; Rai, B. Plant parasitic nematodes association with hill orange (Citrus reticulata Blanco) in Sikkim. Allahabad Farmer 1970, 44, 251–254. [Google Scholar]
- Mohilal, N.; Anandi, Y.; Dhanachand, C. Two new species of Boleodorus Thorne, 1941 and male report of Neopsilenchus affinis. Curr. Nematol. 1997, 8, 17–22. [Google Scholar]
- Shamim Jairajpuri, M. Three new species of nematodes from sugarcane fields in Nigeria. Rev. Nématol. 1982, 5, 241–246. [Google Scholar]
- Bina, L.; Mohilal, N.; Pramodini, M.; Shah, M.M. Two new species of Boleodorus (Nematoda: Tylenchida) from Manipur, India. Bioscan 2012, 7, 115–118. [Google Scholar]
- Maqbool, M.; Shahina, F.; Firoza, K. Description of Boleodorus azadkashmirensis n. sp.(Nematoda: Tylenchidae) and observation on Filenchus vulgaris (Brzeski, 1963) Lownsbery & Lownsbery, 1985 from Pakistan. Pak. J. Nematol. 1990, 8, 43–48. [Google Scholar]
- Fotedar, D.; Mahajan, R. Two new nematode species (Nothotylenchidae) from Kashmir. Indian J. Nematol. 1972, 2, 169–172. [Google Scholar]
- Khan, E.; Basir, M. Boleodorus similis n. sp.(Nematoda: Nothotylenchinae) from India. Z. Parasitenkd. 1963, 23, 121–123. [Google Scholar] [CrossRef]
- Eroshenko, A. A new species of the genus Boleodorus (Nematoda, Nothotylenchidae) from the spruce rhizosphere in the Primorsky Region. Zool. Zhurnal 1982, 61, 135–137. [Google Scholar]
- Zeidan, A.; Geraert, E. The genera Filenchus Andrássy, 1954, Sakia Khan, 1964, Boleodorus Thorne, 1941 and Basiria Siddiqi, 1959 (Nemata: Tylenchida) from Sudan. Nematologica 1991, 37, 185–212. [Google Scholar]
- Thorne, G.; Malek, R.B. Nematodes of the northern Great Plains. Part I. Tylenchida (Nemata: Secernentea). Tech. Bull. S. Dak. Agric. Exp. Stn 1968, 31, 111. [Google Scholar]
- Sturhan, D.; Hohberg, K. Nematodes of the order Tylenchida in Germany–the non-phytoparasitic species. Soil Org. 2016, 88, 19–41. [Google Scholar]
- Husain, S.I.; Khan, A. Two new species of Boleodorus Thome, 1941 (Nematoda: Neotylenchidae) from India. Proc. Helm. Soc. Wash. 1965, 32, 176–179. [Google Scholar]
- Geraert, E. Observations on the genera Boleodorus and Boleodoroides (Nematoda: Tylenchida). Nematologica 1971, 17, 263–276. [Google Scholar] [CrossRef]
- Siddiqi, M. Boleodorus pakistanensis n. sp.(Nematoda: Tylenchida), found associated with pine roots in Abbottabad, Pakistan. Sci. Cult. 1963, 29, 562–563. [Google Scholar]
- Handoo, Z.A.; Kantor, M.R.; Khan, E. Description of seven new species and one new record of plant-parasitic nematodes (Nematoda: Tylenchida) associated with economically important crops of Kashmir valley, Jammu and Kashmir (Part-1 of the series). Pak. J. Nematol. 2020, 38. [Google Scholar] [CrossRef]
- Nanjappa, C.; Khan, E. Paurodontus indicus sp. nov. and Boleodorus teres sp. nov.(Nematoda: Tylenchida) from India. Bull. Entomol. 1970, 11, 138–142. [Google Scholar]
- Deimi, A.M.; Mitkowski, N. Nematodes associated with vineyards throughout Markazi province (Arak), Iran. Australas. Plant Pathol. 2010, 39, 571–577. [Google Scholar] [CrossRef]
- Geraert, E. On some Tylenchidae and Neotylenchidae from Belgium with the description of a new species, Tylenchorhynchus microdorus. Nematologica 1966, 12, 409–416. [Google Scholar] [CrossRef]
- Rashid, F.; Geraert, E.; Sharma, R. Seven species of Tylenchida from Brazil with description of a new species (Nematoda: Tylenchoidea). Nematol. Mediterr. 1987, 15, 29–45. [Google Scholar]
- Bongers, T. De Nematoden van Nederland: Een Identificatietabel voor de in Nederland Aangetroffen Zoetwater-en Bodembewonende Nematoden; Stichting Uitgeverij Koninklijke Nederlandse Natuurhistorische Vereniging: Voorschoten, The Netherlands, 1988. [Google Scholar]
- Brzeski, M.W. Nematodes of Tylenchina in Poland and Temperate Europe; Muzeum i Instytutu Zoologii, Polska Akademia Nauk (MiIZ PAN): Warsaw, Poland, 1998. [Google Scholar]
- Asghari, R.; Pourjam, E.; Mohamadi Goltapeh, E.; Latifi, A. Plant-parasitic nematodes from Afghanistan with discussion on the taxonomic status of Merlinius neohexagrammus Ivanova, 1978 (Nematoda: Dolichodoridae). J. Agric. Sci. Technol. 2012, 14, 1397–1404. [Google Scholar]
- Sheikh, J.H.; Chishti, M.; Rasheed, M.; Dar, S.A.; Lal, E.P.; Mohiuddin, D. Population analysis of the genera buildup on some commercially important vegetable crops grown in Kashmir Valley. J. Parasit. Dis. 2016, 40, 877–880. [Google Scholar] [CrossRef][Green Version]
- Pan, F.J.; Xu, Y.L.; McLaughlin, N.B.; Xue, A.G.; Yu, Q.; Han, X.-Z.; Liu, W.; Zhan, L.L.; Zhao, D.; Li, C.J. Response of soil nematode community structure and diversity to long-term land use in the black soil region in China. Ecol. Res. 2012, 27, 701–714. [Google Scholar] [CrossRef]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Hassan, J.; Ahangar, M. Phytonematode infestation on Brassica oleracea with special emphasis on Boleodorus cylindricus Dhanachand, Renubala & Anandi, 1993. Int. J. Adv. Educ. Res. 2018, 3, 157–159. [Google Scholar]
- Karegar, A.; Geraert, E.; Kheiri, A. Tylenchs associated with Grapevine in the province of Hamadan, Iran. Med. Fac. Landbouww. Univ. Gent. 1995, 6O/3b, 1063–1086. [Google Scholar]
- Husseinvand, M.; Abdollahi, M.; Karegar, A. Description of some nematode species of Tylenchidae, associated with Polianthes tuberosa from Iran. J. Agric. Sci. Technol. 2016, 18, 1953–1966. [Google Scholar]
- Giblin-Davis, R.; Mundo-Ocampo, M.; Baldwin, J.; Norden, B.; Batra, S. Description of Bursaphelenchus abruptus n. sp.(Nemata: Aphelenchoididae), an associate of a digger bee. J. Nematol. 1993, 25, 161. [Google Scholar] [PubMed]
- Ritzema Bos, J. De bloemkoolziekte der aardbeien, veroorzaakt door Aphelenchus fragariae nov. spec. Voorloopige mededeeling. Maanblad Nat. 1890, 16, 107–117. [Google Scholar]
- Christie, J. Recent observations on the strawberry dwarf neaiatode in Massachusetts. Plant Dis. Rep. 1932, 16, 113–114. [Google Scholar]
- Tomalak, M. Bursaphelenchus trypophloei sp. n. (Nematoda: Parasitaphelenchinae)–an associate of the bark beetle, Trypophloeus asperatus (Gyll.)(Coleoptera: Curculionidae, Scolytinae), in aspen, Populus tremula L. Nematology 2011, 13, 619–636. [Google Scholar] [CrossRef]
- Mamiya, Y.; Enda, N. Bursaphelenchus mucronatus n. sp. (Nematoda: Aphelenchoididae) from pine wood and its biology and pathogenicity to pine trees. Nematologica 1979, 25, 353–361. [Google Scholar] [CrossRef]
- Karegar, A.; Geraert, E. The genus Basiria Siddiqi, 1959 (Nematoda: Tylenchidae) I. Introduction and species with two lateral lines. Nematologica 1997, 43, 327–339. [Google Scholar] [CrossRef]
- Karegar, A.; Geraert, E. The genus Basiria Siddiqi, 1959 (Nematoda: Tylenchidae) IV. General discussion, genus diagnosis and key to the species. Nematologica 1998, 44, 1–13. [Google Scholar] [CrossRef]
- Qing, X.; Pereira, T.J.; Slos, D.; Couvreur, M.; Bert, W. A new species of Malenchus (Nematoda: Tylenchomorpha) with an updated phylogeny of the Tylenchidae. Nematology 2018, 20, 815–836. [Google Scholar] [CrossRef]
- Qing, X.; Decraemer, W.; Claeys, M.; Bert, W. Molecular phylogeny of Malenchus and Filenchus (Nematoda: Tylenchidae). Zool. Scr. 2017, 46, 625–636. [Google Scholar] [CrossRef]
- Pereira, T.J.; Qing, X.; Chang, K.F.; Mundo-Ocampo, M.; Cares, J.E.; Ragsdale, E.J.; Nguyen, C.N.; Baldwin, J.G. Phylogeny and biogeography of the genus Cephalenchus (Tylenchomorpha, Nematoda). Zool. Scr. 2017, 46, 506–520. [Google Scholar] [CrossRef]
- Van den Berg, E.; Tiedt, L.R.; Subbotin, S.A. Morphological and molecular characterisation of several Paratylenchus Micoletzky, 1922 (Tylenchida: Paratylenchidae) species from South Africa and USA, together with some taxonomic notes. Nematology 2014, 16, 323–358. [Google Scholar] [CrossRef]
- Qing, X.; Bert, W. Family Tylenchidae (Nematoda): An overview and perspectives. Org. Divers. Evol. 2019, 19, 391–408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).