Proximal Perineural Femoral Nerve Injection in Pigs Using an Ultrasound-Guided Lateral Subiliac Approach—A Cadaveric Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Design
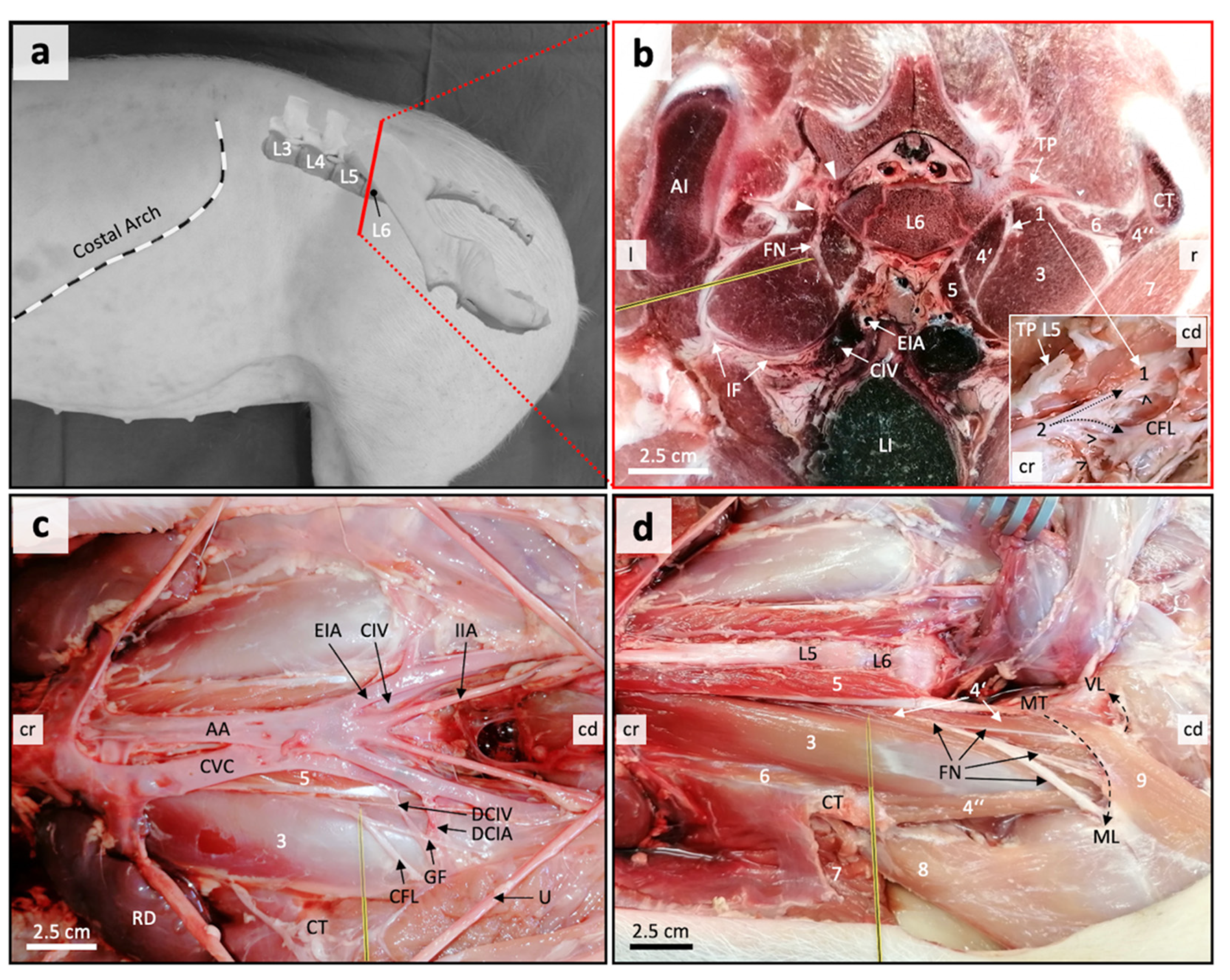
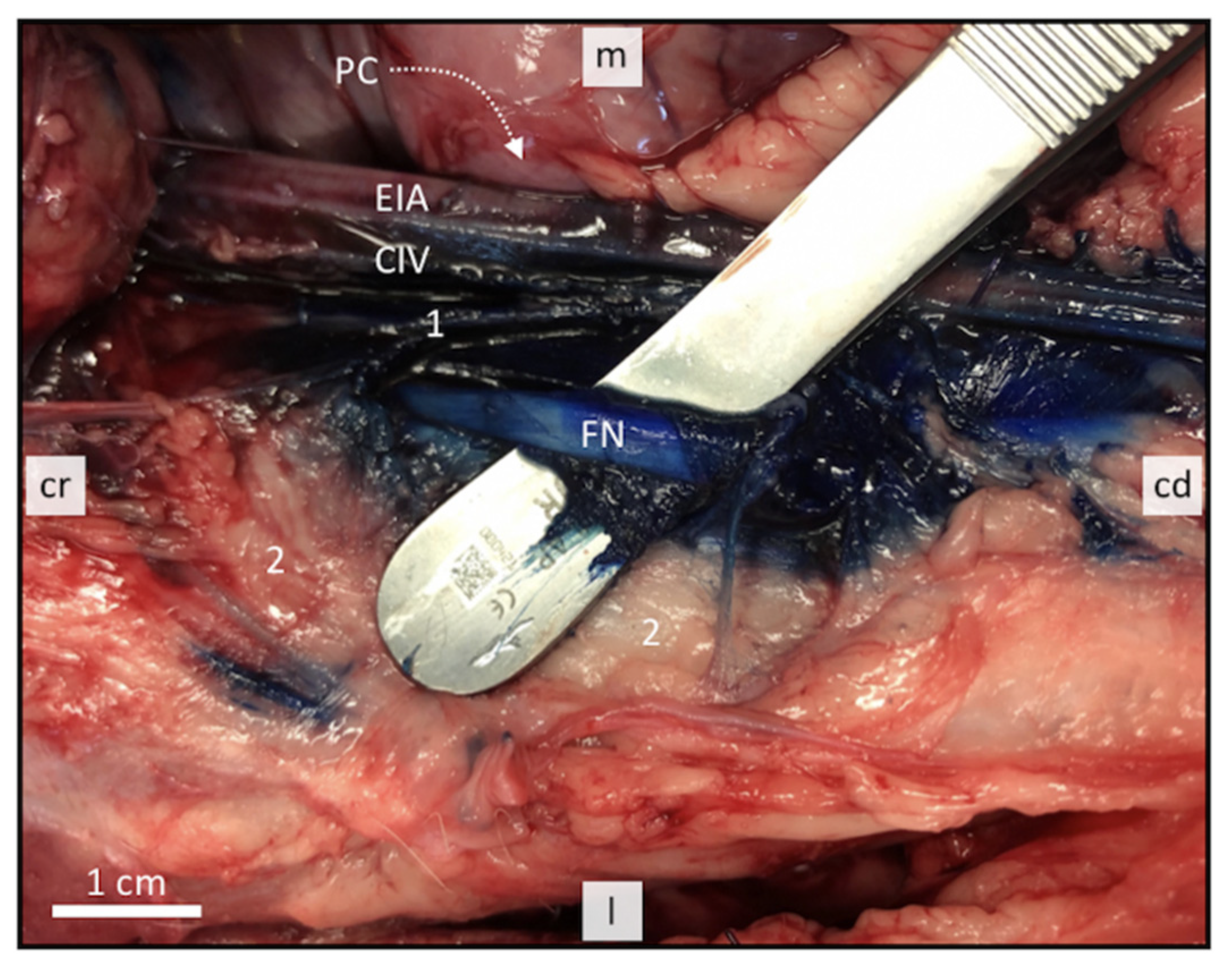
2.2. Sonoanatomical Study and Anatomical Dissection
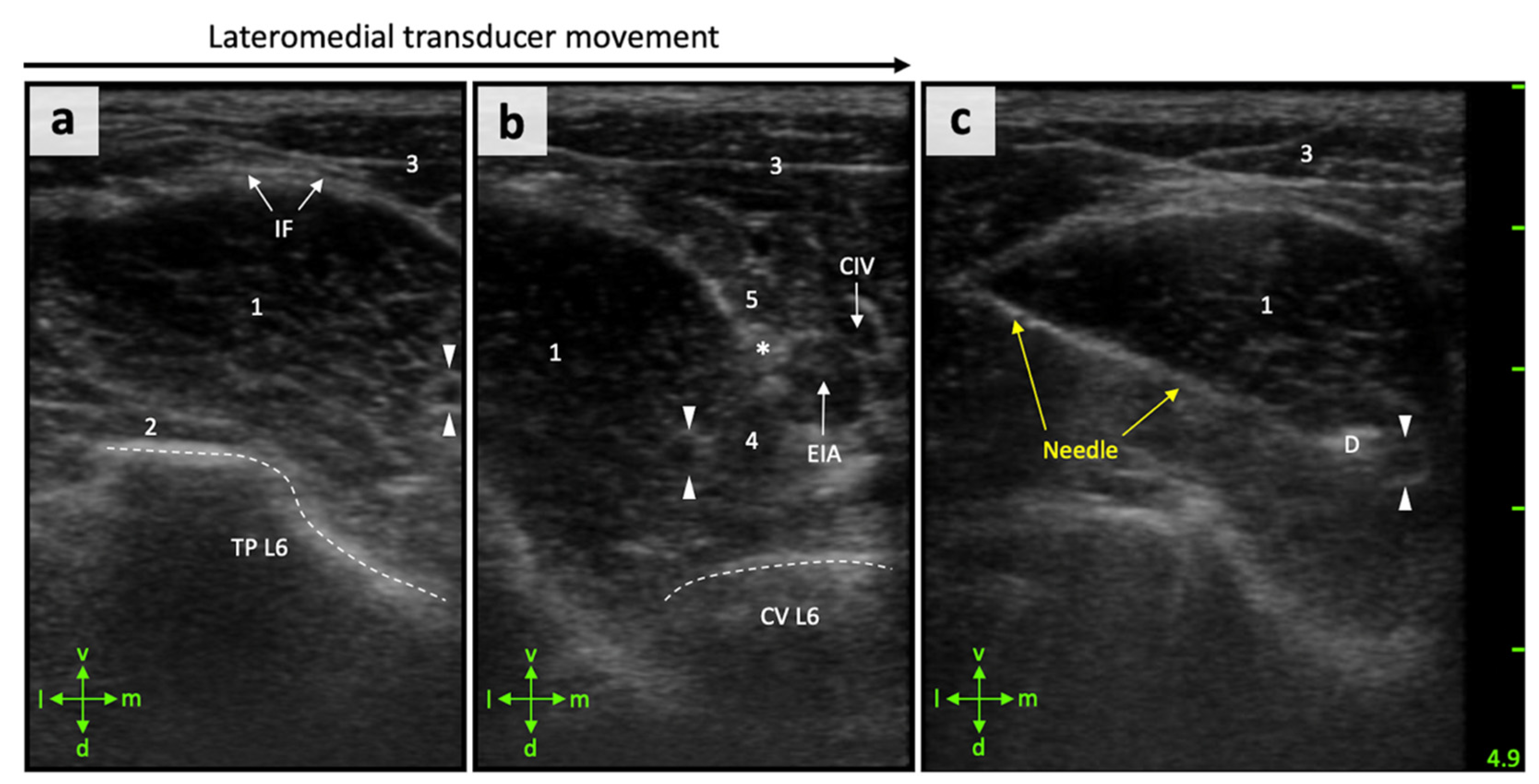
2.3. US-Guided Injection Procedure
3. Results
3.1. Anatomical Disection Helped Define a Suitable US Window for Proximal FN Injections
3.2. Anatomical Landmarks Can Be Depicted in Detail by Ultrasonography
3.3. Perineural FN Injections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goetz, J.E.; Fredericks, D.; Petersen, E.; Rudert, M.J.; Baer, T.; Swanson, E.; Roberts, N.; Martin, J.; Tochigi, Y. A clinically realistic large animal model of intra-articular fracture that progresses to post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1797–1805. [Google Scholar] [CrossRef]
- Visser, N.J.; Rezaie, E.S.; Friedrich, P.F.; Kotsougiani, D.; Shin, A.Y.; Bishop, A.T. Effects of Surgical Angiogenesis on Segmental Bone Reconstruction with Cryopreserved Massive-Structural Allografts in a Porcine Tibia Model. J. Orthop. Res. 2019, 37, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Kotsougiani, D.; Hundepool, C.A.; Bulstra, L.F.; Friedrich, P.F.; Shin, A.Y.; Bishop, A.T. Bone vascularized composite allotransplantation model in swine tibial defect: Evaluation of surgical angiogenesis and transplant viability. Microsurgery 2019, 39, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Dahl, J.B. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 2003, 362, 1921–1928. [Google Scholar] [CrossRef]
- Romano, M.; Portela, D.A.; Breghi, G.; Otero, P.E. Stress-related biomarkers in dogs administered regional anaesthesia or fentanyl for analgesia during stifle surgery. Vet. Anaesth. Analg. 2016, 43, 44–54. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Mabvuure, N.T.; Kozar, R.A.; Herndon, D.N. The Surgically Induced Stress Response. J. Parenter. Enter. Nutr. 2013, 37, 21S–29S. [Google Scholar] [CrossRef]
- Stundner, O.; Memtsoudis, S.G. Regional anesthesia and analgesia in critically ill patients: A systematic review. Reg. Anesth. Pain Med. 2012, 37, 537–544. [Google Scholar] [CrossRef]
- Bugada, D.; Ghisi, D.; Mariano, E.R. Continuous regional anesthesia: A review of perioperative outcome benefits. Minerva. Anestesiol. 2017, 83, 1089–1100. [Google Scholar]
- Mosing, M.; Reich, H.; Moens, Y. Clinical evaluation of the anaesthetic sparing effect of brachial plexus block in cats. Vet. Anaesth. Analg. 2010, 37, 154–161. [Google Scholar] [CrossRef]
- Congdon, J.M.; Boscan, P.; Goh, C.S.S.; Rezende, M. Psoas compartment and sacral plexus block via electrostimulation for pelvic limb amputation in dogs. Vet. Anaesth. Analg. 2017, 44, 915–924. [Google Scholar] [CrossRef]
- Portela, D.A.; Fuensalida, S.E.; Verdier, N. Peripheral nerve blocks of the pelvic limb. In Small Animal Regional Anesthesia, 2nd ed.; Otero, P.E., Portela, D.A., Eds.; Inter-Medica: Buenos Aires, Argentina, 2018; pp. 135–218. [Google Scholar]
- Albrecht, E.; Chin, K.J. Advances in regional anaesthesia and acute pain management: A narrative review. Anaesthesia 2020, 75, e101–e110. [Google Scholar] [CrossRef]
- Dimas, D. Lumb & Jones Veterinary Anesthesia and Analgesia, 5th ed.; John Wiley and Sons: Iowa City, IA, USA, 2015. [Google Scholar]
- Campoy, L.; Bezuidenhout, A.J.; Gleed, R.D.; Martin-Flores, M.; Raw, R.M.; Santare, C.L.; Jay, A.R.; Wang, A.L. Ultrasound-guided approach for axillary brachial plexus, femoral nerve, and sciatic nerve blocks in dogs. Vet. Anaesth. Analg. 2010, 37, 144–153. [Google Scholar] [CrossRef]
- Echeverry, D.F.; Laredo, F.G.; Gil, F.; Belda, E.; Soler, M.; Agut, A. Ventral ultrasound-guided suprainguinal approach to block the femoral nerve in the dog. Vet. J. 2012, 192, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Haro, P.; Laredo, F.; Gil, F.; Belda, E.; Ayala, M.D.; Soler, M.; Agut, A. Ultrasound-guided dorsal approach for femoral nerve blockade in cats: An imaging study. J. Feline Med. Surg. 2013, 15, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, M.S.; Aziz, M.F.; Fu, R.F.; Horn, J.L. Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: A systematic review and meta-analysis of randomized controlled trials. Br. J. Anaesth. 2009, 102, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.W.S.; Perlas, A.; McCartney, C.J.L.; Brull, R.; Xu, D.; Abbas, S. Ultrasounds guidance improves success rate of axillary brachial plexus block. Can. J. Anesth. 2007, 54, 176–182. [Google Scholar] [CrossRef]
- Mogicato, G.; Layssol-Lamour, C.; Mahler, S.; Charrouin, M.; Boyer, G.; Verwaerde, P.; Jourdan, G. Anatomical and ultrasonographic study of the femoral nerve within the iliopsoas muscle in beagle dogs and cats. Vet. Anaesth. Analg. 2015, 42, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Auroy, Y.; Benhamou, D.; Bargues, L.; Ecoffey, C.; Falissard, B.; Mercier, F.; Bouaziz, H.; Samii, K. Major complications of regional anesthesia in France: The SOS Regional Anesthesia Hotline Service. Anesthesiology 2002, 97, 1274–1280. [Google Scholar] [CrossRef]
- Koscielniak-Nielsen, Z.J. Ultrasound-guided peripheral nerve blocks: What are the benefits? Acta Anaesthesiol. Scand. 2008, 52, 727–737. [Google Scholar] [CrossRef]
- Lee, M.G.; Choi, S.U.; Lim, J.K.; Lee, M.J.; Hong, J.S.; Baek, M.O.; Yoon, S.Z.; Park, H.Y.; Shin, H.J. Ultrasound-guided sciatic nerve block at the midthigh level in a porcine model: A descriptive study. Vet. Med. Sci. 2020, 6, 543–549. [Google Scholar] [CrossRef]
- Raymond, S.A.; Steffensen, S.C.; Gugino, L.D.; Strichartz, G.R. The role of length of nerve exposed to local anesthetics in impulse blocking action. Anesth. Analg. 1989, 68, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Nickel, R.; Schummer, A.; Seiferle, E. Lehrbuch der Anatomie der Haustiere. Band IV: Nervensystem, Sinnesorgane, Endokrine Drüsen, 4th ed.; Verlag Paul Parey: Berlin/Hamburg, Germany, 2004; pp. 269–279. [Google Scholar]
- Herrera, G.C.; e Silva, F.O.C.; Dos Santos, L.A.; Menezes, L.T.; de MORAES, F.M. Origin and distribution of femoral nerves in swine (Sus scrofa domesticus linneaus, 1758) fetuses from crosses of dan bred and AGPIC-337 lines. Biosci. J. 2018, 34, 1334–1338. [Google Scholar] [CrossRef]
- Echeverry, D.F.; Gil, F.; Laredo, F.; Ayala, M.D.; Belda, E.; Soler, M.; Agut, A. Ultrasound-guided block of the sciatic and femoral nerves in dogs: A descriptive study. Vet. J. 2010, 186, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.; Peer, S.; Kovacs, P.; Marth, R.; Bodner, G. The ultrasonographic appearance of the femoral nerve and cases of iatrogenic impairment. J. Ultrasound Med. 2003, 22, 163–172. [Google Scholar] [CrossRef]
- Nielsen, K.C.; Guller, U.; Steele, S.M.; Klein, S.M.; Greengrass, R.A.; Pietrobon, R. Influence of obesity on surgical regional anesthesia in the ambulatory setting: An analysis of 9038 blocks. Anesthesiology 2005, 102, 181–187. [Google Scholar] [CrossRef]
- Marhofer, P.; Harrop-Griffiths, W.; Kettner, S.C.; Kirchmair, L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 1. Br. J. Anaesth. 2010, 104, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Marhofer, P.; Greher, M.; Kapral, S. Ultrasound guidance in regional anaesthesia. Br. J. Anaesth. 2005, 94, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Baldo, C.F.; Almeida, D.; Wendt-Hornickle, E.; Guedes, A. Transversus abdominis plane block in ponies: A preliminary anatomical study. Vet. Anaesth. Analg. 2018, 45, 392–396. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujanovic, R.; Otero, P.E.; Marhofer, P.; Auer, U.; Kau, S. Proximal Perineural Femoral Nerve Injection in Pigs Using an Ultrasound-Guided Lateral Subiliac Approach—A Cadaveric Study. Animals 2021, 11, 1759. https://doi.org/10.3390/ani11061759
Trujanovic R, Otero PE, Marhofer P, Auer U, Kau S. Proximal Perineural Femoral Nerve Injection in Pigs Using an Ultrasound-Guided Lateral Subiliac Approach—A Cadaveric Study. Animals. 2021; 11(6):1759. https://doi.org/10.3390/ani11061759
Chicago/Turabian StyleTrujanovic, Robert, Pablo E. Otero, Peter Marhofer, Ulrike Auer, and Silvio Kau. 2021. "Proximal Perineural Femoral Nerve Injection in Pigs Using an Ultrasound-Guided Lateral Subiliac Approach—A Cadaveric Study" Animals 11, no. 6: 1759. https://doi.org/10.3390/ani11061759
APA StyleTrujanovic, R., Otero, P. E., Marhofer, P., Auer, U., & Kau, S. (2021). Proximal Perineural Femoral Nerve Injection in Pigs Using an Ultrasound-Guided Lateral Subiliac Approach—A Cadaveric Study. Animals, 11(6), 1759. https://doi.org/10.3390/ani11061759






