The Pen Is Milder Than the Blade: Identification Marking Mice Using Ink on the Tail Appears More Humane Than Ear-Punching Even with Local Anaesthetic
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Effects of Ear-Punching on Mouse Welfare
1.2. Use of Local Anaesthetic for Identification Marking
1.3. Use of a Marker Pen for Identification Marking
1.4. Social Effects of Marking
1.5. Aims and Objectives
2. Materials and Methods
2.1. Animals and Housing
2.2. Weighing and Physical Health Checks
2.3. Treatment Procedure
- Control;
- Ear-punch (EP);
- Cagemate of EP (C-EP);
- Ear-punch with anaesthetic EMLATM cream (EP+A);
- Cagemate of EP+A (C-EP+A);
- Marker pen on the tail (MP);
- Cagemate of MP (C-MP).
2.4. Homecage Behaviour Recording after the Treatment Procedure
2.5. Elevated Plus Maze
2.6. Hyponeophagia Test
2.7. Interaction with Handler’s Hand
2.8. Faecal Corticosterone Measurement
2.9. Statistical Analysis
3. Results
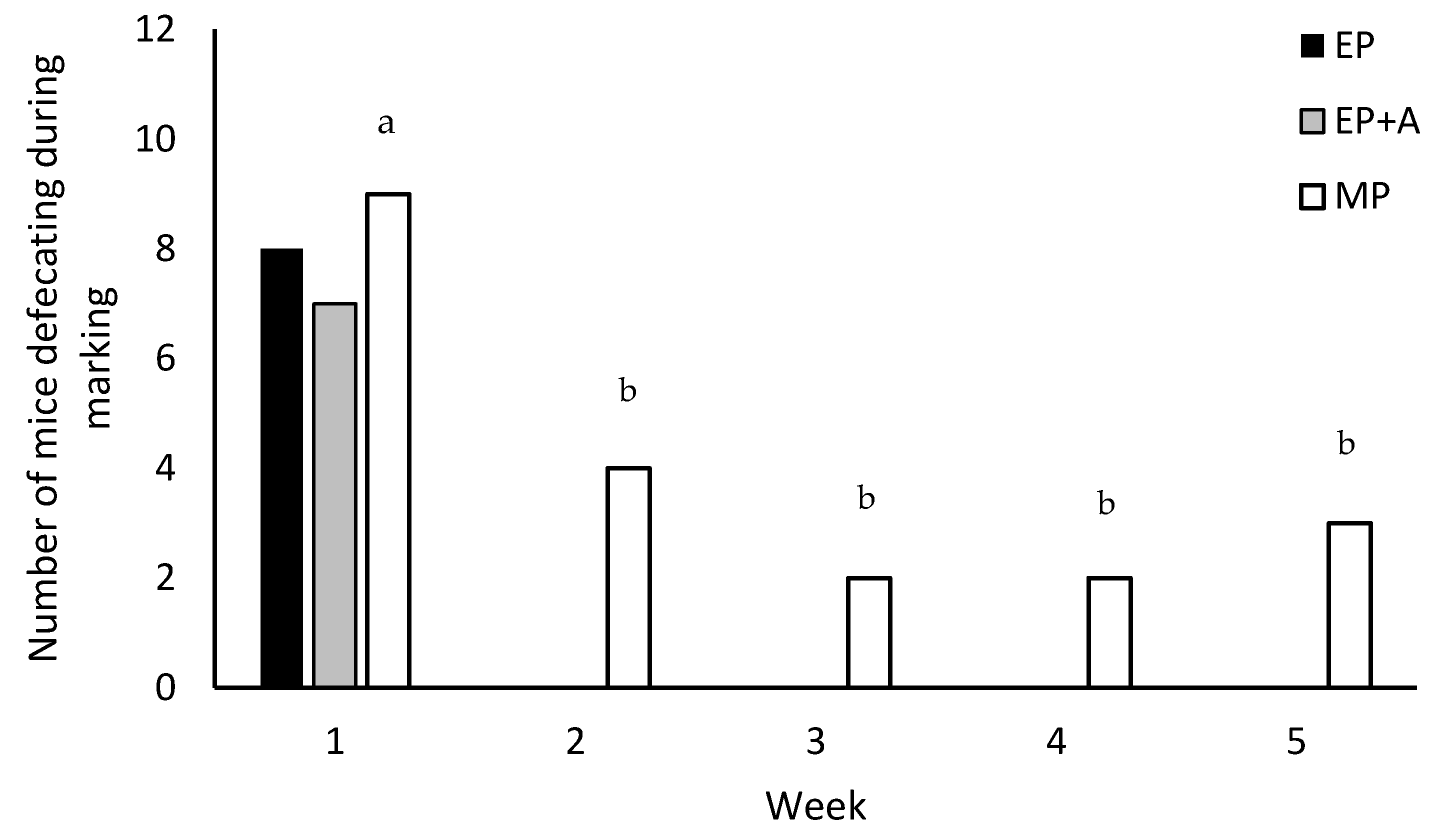
3.1. Immediate Responses to Identification Marking
3.2. Body Weight and Physical Health
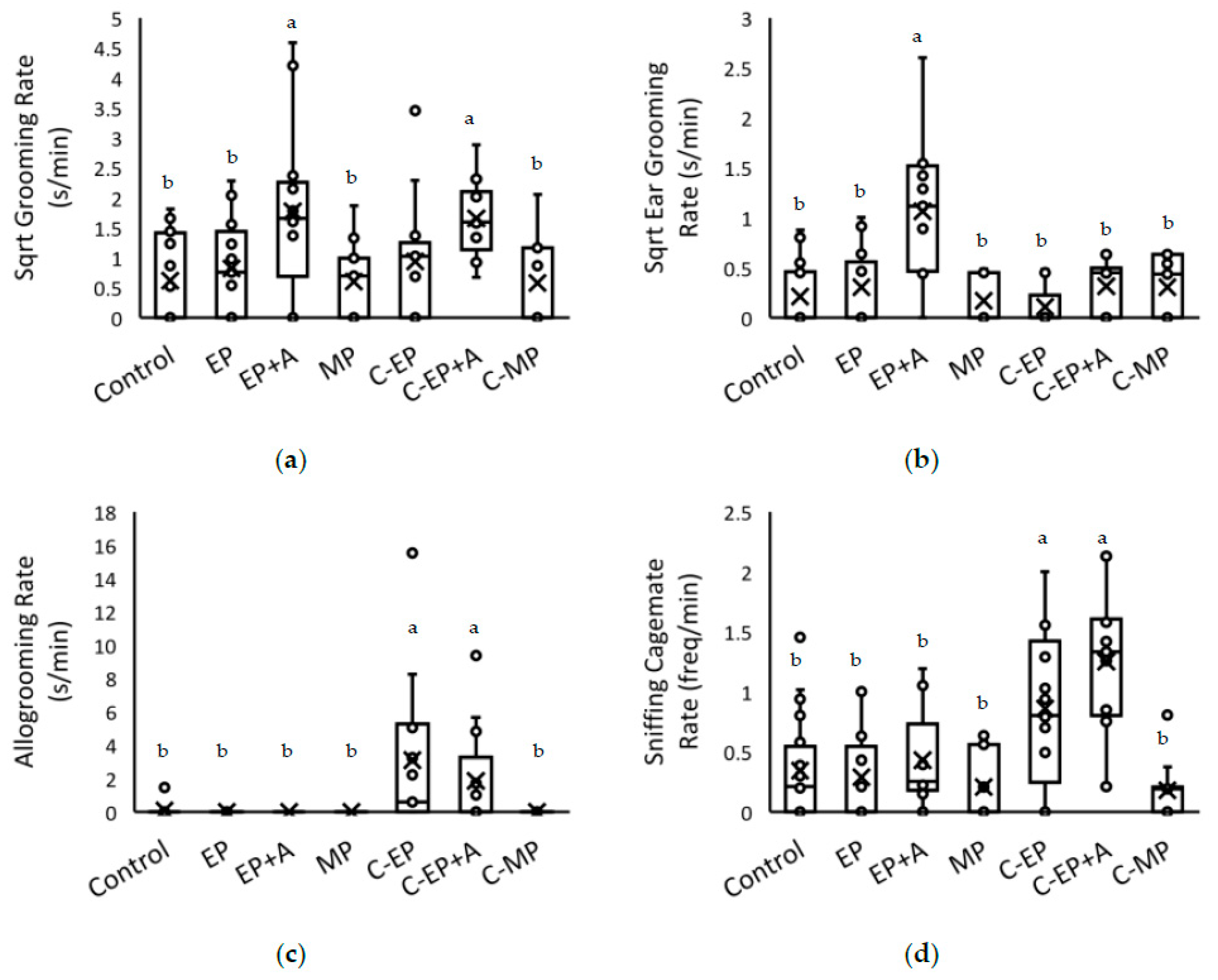
3.3. Homecage Behaviour after the Treatment Procedure
3.4. Elevated Plus Maze
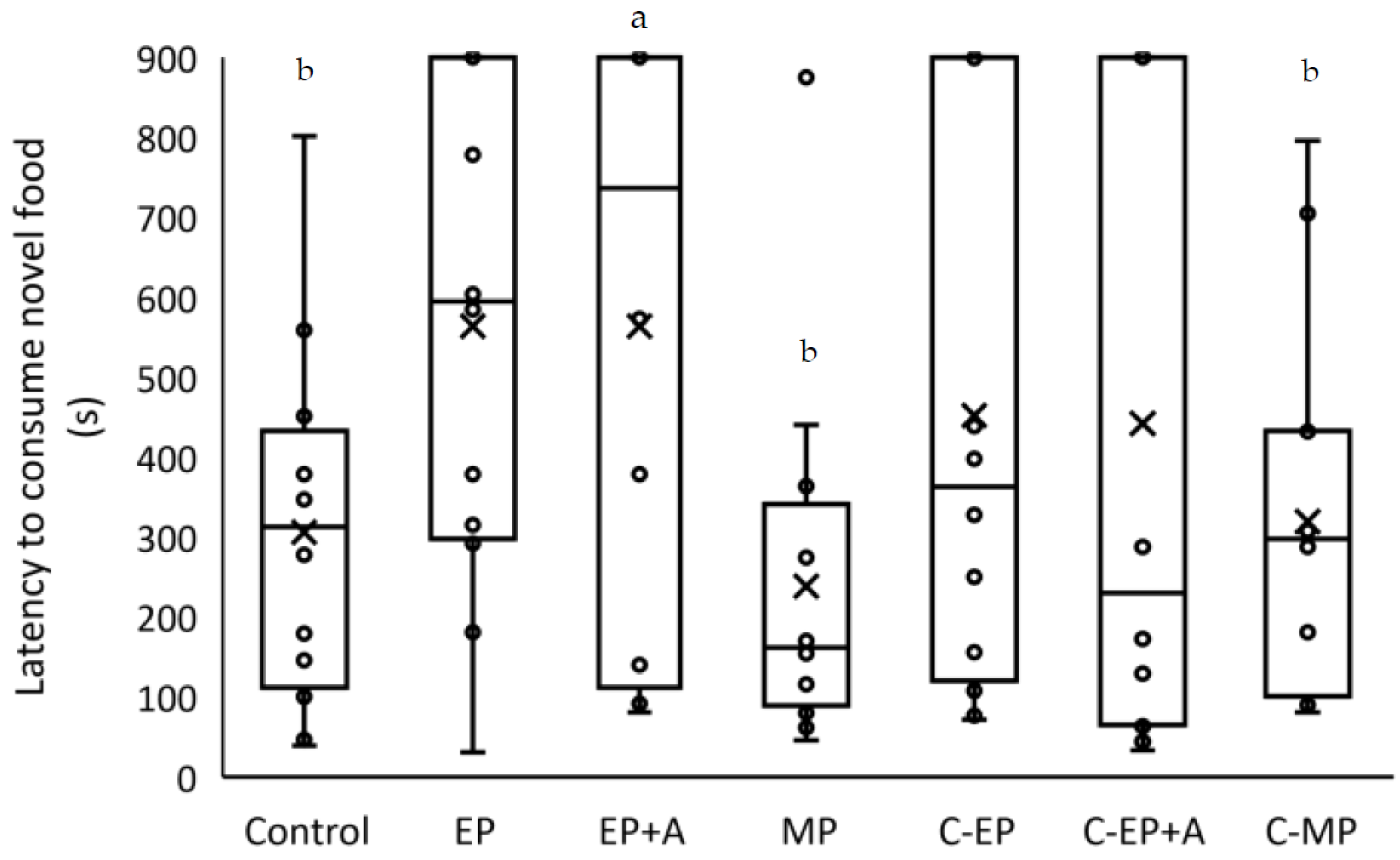
3.5. Hyponeophagia Test
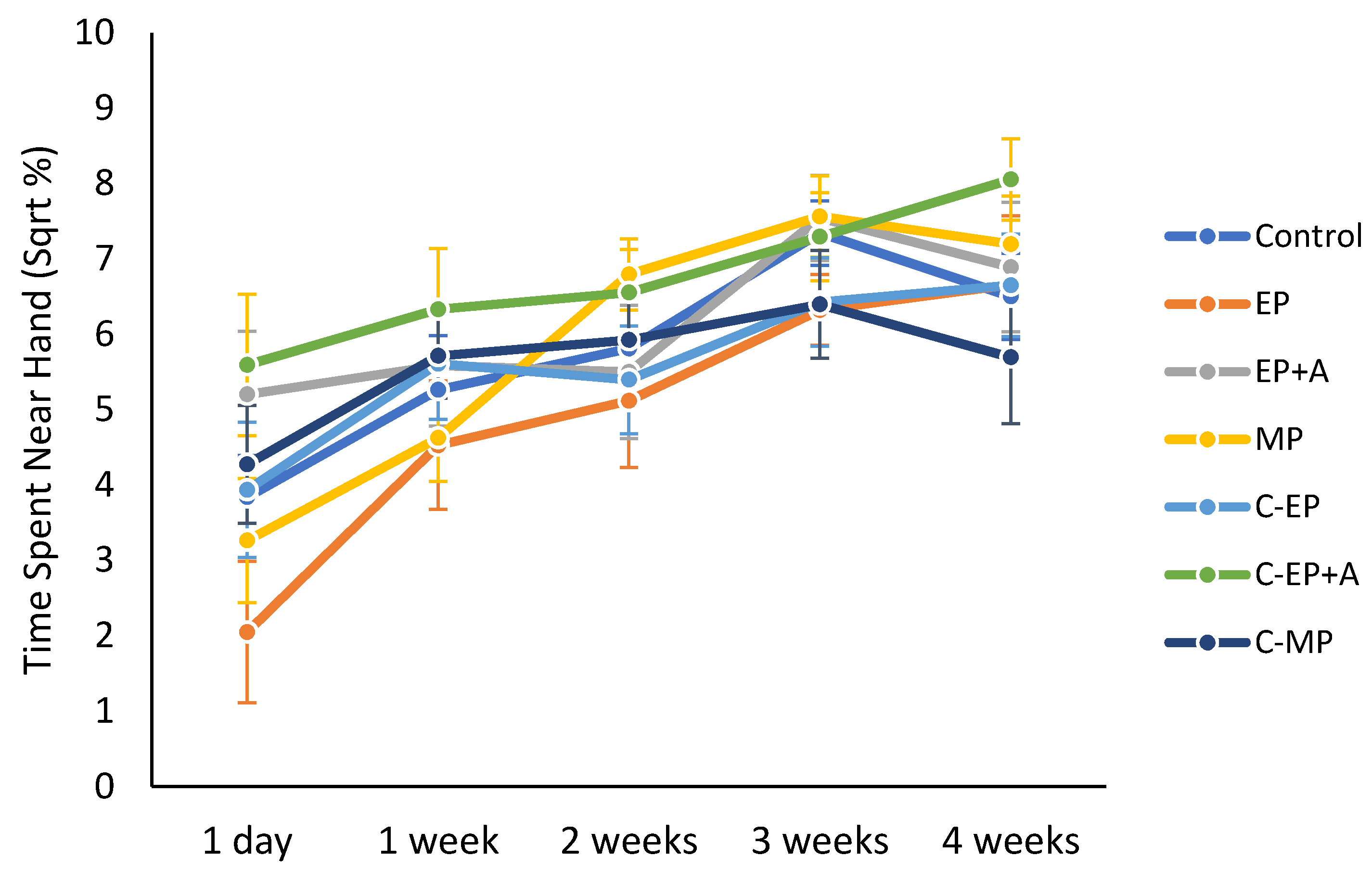
3.6. Interaction with Handler
3.7. Faecal Corticosterone Analysis
4. Discussion
4.1. Effects of Ear-Punching without EMLATM
4.2. Efficacy of EMLATM Cream
4.3. Tail-Marking Versus Ear-Punch
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazlan, N.H.; Lopez-Salesanksy, N.; Burn, C.C.; Wells, D.J. Mouse identification methods and potential welfare issues: A survey of current practice in the UK. Anim. Technol. Welf. 2014, 13, 1–10. [Google Scholar]
- Bonaparte, D.; Cinelli, P.; Douni, E.; Hérault, Y.; Maas, A.; Pakarinen, P.; Poutanen, M.; Lafuente, M.S.; Scavizzi, F. FELASA guidelines for the refinement of methods for genotyping genetically-modified rodents: A report of the Federation of European Laboratory Animal Science Associations Working Group. Lab. Anim. 2013, 47, 134–145. [Google Scholar] [CrossRef]
- Wever, K.E.; Geessink, F.J.; Brouwer MA, E.; Tillema, A.; Ritskes-Hoitinga, M. A systematic review of discomfort due to toe or ear clipping in laboratory rodents. Lab. Anim. 2017, 51, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Roughan, J.V.; Sevenoaks, T. Welfare and Scientific Considerations of Tattooing and Ear Tagging for Mouse Identification. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.C.; Main, D.C.J. An assessment of laboratory mouse welfare in UK animal units. Anim. Welf. 2008, 17, 171–187. [Google Scholar]
- Cinelli, P.; Rettich, A.; Seifert, B. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab. Anim. 2007, 41, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar] [PubMed]
- Hurst, J.L.; West, R.S. Taming anxiety in laboratory mice. Nat. Methods 2010, 7, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.O.; Riskin, D.K.; Mott, K.M. Ultrasonic sound as an indicator of acute pain in laboratory mice. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 8–10. [Google Scholar]
- Taitt, K.T.; Kendall, L.V. Physiologic Stress of Ear Punch Identification Compared with Restraint Only in Mice. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 438–442. [Google Scholar] [CrossRef]
- Mazlan, N.H.; Burn, C.C.; Wells, D.J. Marking mice: The humaneness of ear punching and ear notching versus ink marks on the tail. In Recent Advances in Animal Welfare Science VI-UFAW; Universities Federation for Animal Welfare: Newcastle, UK, 2018. [Google Scholar]
- Gaskill, B.N.; Stottler, A.M.; Garner, J.P.; Winnicker, C.W.; Mulder, G.B.; Pritchett-Corning, K.R. The effect of early life experience, environment, and genetic factors on spontaneous home-cage aggression-related wounding in male C57BL/6 mice. Lab Anim. 2017, 46, 176. [Google Scholar] [CrossRef]
- Wang, L. A primer on rodent identification methods. Lab. Anim. 2005, 34, 64–67. [Google Scholar] [CrossRef]
- Dahlborn, K.; Bugnon, P.; Nevalainen, T.; Raspa, M.; Verbost, P.; Spangenberg, E. Report of the Federation of European Laboratory Animal Science Associations Working Group on animal identification. Lab Anim. 2013, 47, 2–11. [Google Scholar] [CrossRef] [PubMed]
- AstraZeneca. EMLA Cream 5% Patient Information Leaflet. Available online: https://www.medicines.org.uk/emc/product/871/pil#gref (accessed on 29 April 2021).
- Keating SC, J.; Thomas, A.A.; Flecknell, P.A.; Leach, M.C. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: Changes in physiological, behavioural and facial expression responses. PLoS ONE 2012, 7, e44437. [Google Scholar] [CrossRef] [PubMed]
- Flecknell, P.A.; Liles, J.H.; Williamson, H.A. The use of lignocaine-prilocaine local anaesthetic cream for pain-free venepuncture in laboratory animals. Lab. Anim. 1990, 24, 142–146. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Duarte Vogel, S.; Longo, K.; Sanchez, D.; Lawson, G. The use of eutectic mixture of lidocaine and prilocaine in mice (Mus musculus) for tail vein injections. Vet. Anaesth. Analg. 2014, 41, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Dudley, E.S.; Johnson, R.A.; French, D.C.; Boivin, G.P. Effects of Topical Anesthetics on Behavior, Plasma Corticosterone, and Blood Glucose Levels after Tail Biopsy of C57BL/6NHSD Mice (Mus musculus). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 443–450. [Google Scholar]
- Miller, A.L.; Leach, M.C. Using the mouse grimace scale to assess pain associated with routine ear notching and the effect of analgesia in laboratory mice. Lab. Anim. 2014, 49, 117–120. [Google Scholar] [CrossRef]
- Burn, C.C.; Deacon, R.; Mason, G.J. Marked for life? Effects of early cage cleaning frequency, delivery batch and identification tail-marking on adult rat anxiety profiles. Dev. Psychobiol. 2008, 5, 266–277. [Google Scholar] [CrossRef]
- Anderson, R.C.; Anderson, J.H. Acute toxicity of marking pen emissions. J. Toxicol. Environ. Health Part A 2003, 66, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Gadek-Michalska, A.; Bugajski, J. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. J. Physiol. Pharmacol. 2003, 54, 449–460. [Google Scholar] [PubMed]
- Kramer, K.; van de Weerd, H.; Mulder, A.; van Heijningen, C.; Baumans, V.; Remie, R.; Voss, H.-P.; van Zutphen, B.F.M. Effect of Conditioning on the Increase of Heart Rate and Body Temperature Provoked by Handling in the Mouse. Altern. Lab. Anim. 2004, 32, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Cavigelli, S.A.; Ragan, C.M.; Barrett, C.E.; Michael, K.C. Within-litter variance in rat maternal behaviour. Behav. Process. 2010, 84, 696–704. [Google Scholar] [CrossRef]
- Bind, R.H.; Minney, S.M.; Rosenfeld, S.; Hallock, R.M. The Role of Pheromonal Responses in Rodent Behavior: Future Directions for the Development of Laboratory Protocols. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 124–129. [Google Scholar]
- Lacey, J.C.; Beynon, R.J.; Hurst, J.L. The importance of exposure to other male scents in determining competitive behaviour among inbred male mice. Appl. Anim. Behav. Sci. 2007, 104, 130–142. [Google Scholar] [CrossRef]
- Abatan, O.I.; Welch, K.B.; Nemzek, J.A. Evaluation of Saphenous Venipuncture and Modified Tail-clip Blood Collection in Mice. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 8–15. [Google Scholar]
- Vos, B.P.; Hans, G.; Adriaensen, H. Behavioral assessment of facial pain in rats: Face grooming patterns after painful and non-painful sensory disturbances in the territory of the rat’s infraorbital nerve. Pain 1998, 76, 173–178. [Google Scholar] [CrossRef]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; LaCroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef]
- Garner, J.P.; Gaskill, B.N.; Rodda, C.; Dufour, B.; Prater, A.; Klein, J.; Wurbel, H.; Mason, G.J.; Olsson, I.A.S.; Weber, E.M.; et al. A Wiki Ethogram for the Laboratory Mouse. Stanford: Stanford School of Medicine. 2014. Available online: www.mousebehavior.org (accessed on 1 May 2020).
- Touma, C.; Palme, R.; Sachser, N. Analyzing corticosterone metabolites in fecal samples of mice: A noninvasive technique to monitor stress hormones. Horm. Behav. 2004, 45, 10–22. [Google Scholar] [CrossRef]
- Touma, C.; Sachser, N.; Möstl, E.; Palme, R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen. Comp. Endocrinol. 2003, 130, 267–278. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Hyponeophagia: A Measure of Anxiety in the Mouse. J. Vis. Exp. 2011, 51, 2613. [Google Scholar] [CrossRef]
- Deacon RM, J.; Bannerman, D.M.; Rawlins, J.N.P. Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behav. Neurosci. 2002, 116, 494–497. [Google Scholar] [CrossRef]
- Alleva, E. Assessment of Aggressive Behavior in Rodents. Methods Neurosci. 1993, 14, 111–137. [Google Scholar]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Du, R.; Luo, W.-J.; Geng, K.-W.; Li, C.-L.; Yu, Y.; Wei, N.; Chen, J. Empathic Contagious Pain and Consolation in Laboratory Rodents: Species and Sex Comparisons. Neurosci. Bull. 2020, 36, 649–653. [Google Scholar] [CrossRef]
- Müller-Velten, H. Über den angstgeruch bei der hausmaus. Z. Für Vgl. Physiol. 1966, 52, 401–429. [Google Scholar] [CrossRef]
- Rottman, S.J.; Snowdon, C.T. Demonstration and analysis of an alarm pheromone in mice. J. Comp. Physiol. Psychol. 1972, 81, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Langford, D.J.; Crager, S.E.; Shehzad, Z.; Smith, S.B.; Sotocinal, S.G.; Levenstadt, J.S.; Chanda, M.L.; Levitin, D.J.; Mogil, J.S. Social modulation of pain as evidence for empathy in mice. Science 2006, 312, 1967–1970. [Google Scholar] [CrossRef]
- Lu, Y.-F.; Ren, B.; Ling, B.-F.; Zhang, J.; Xu, C.; Li, Z. Social interaction with a cagemate in pain increases allogrooming and induces pain hypersensitivity in the observer rats. Neurosci. Lett. 2018, 662, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, N.H. Assessment of The Welfare Consequences of Different Identification Methods in the Laboratory Mouse. Ph.D. Thesis, University of London, London, UK, 2017. [Google Scholar]
- Gray, S.; Hurst, J.L. The effect of cage cleaning on aggression within groups of male laboratory mice. Anim. Behav. 1995, 49, 821–826. [Google Scholar] [CrossRef]
- Gouveia, K.; Hurst, J.L. Optimising reliability of mouse performance in behavioural testing: The major role of non-aversive handling. Sci. Rep. 2017, 7, 44999. [Google Scholar] [CrossRef] [PubMed]
- Lezak, K.R.; Missig, G.; Carlezon, W.A., Jr. Behavioral methods to study anxiety in rodents. Dialogues Clin. Neurosci. 2017, 19, 181–191. [Google Scholar]
- O’Leary, T.P.; Gunn, R.K.; Brown, R.E. What are We Measuring When We Test Strain Differences in Anxiety in Mice? Behav. Genet. 2013, 43, 34–50. [Google Scholar] [CrossRef]
- Richter, S.H.; Garner, J.P.; Wurbel, H. Environmental standardization: Cure or cause of poor reproducibility in animal experiments? Nat. Methods 2009, 6, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C. Measuring hedonic impact in animals and infants: Microstructure of affective taste reactivity patterns. Neurosci. Biobehav. Rev. 2000, 24, 173–198. [Google Scholar] [CrossRef]
- Al-Musawi, A.; Matar, K.; Kombian, S.B.; Andersson, L. A pharmacokinetic study of a topical anesthetic (EMLA®) in mouse soft tissue laceration. Dent. Traumatol. 2012, 28, 483–487. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Tuohimaa, P. Grooming analysis algorithm for neurobehavioural stress research. Brain Res. Protoc. 2004, 13, 151–158. [Google Scholar] [CrossRef]
- Whittaker, A.L.; Liu, Y.; Barker, T.H. Methods Used and Application of the Mouse Grimace Scale in Biomedical Research 10 Years on: A Scoping Review. Animals 2021, 11, 673. [Google Scholar] [CrossRef] [PubMed]

| Behaviour | Behaviour Type | Description |
|---|---|---|
| Aggression | State | Mouse demonstrates one or more of the following behaviours: Chasing—focal mouse follows the same path as cagemate, cagemate is fleeing, both mice are running; Tail rattling—focal mouse waves tail side to side in fast almost vibrating motion; Biting—mouse attacks the cagemate with open mouth, appears to bite or latches on to recipient; Boxing/parrying/thrust—movements towards one another, kicking with forepaws. |
| Allogrooming | State | Focal mouse uses paws and/or mouth to brush, lick or nibble the fur of cagemate. |
| Body shake | Point event | Mouse rapidly shakes its whole body, as if to rid its coat of dust. |
| Climbing | State | Mouse hangs from the cage lid, with the forelimbs or all limbs gripping the lid bars. |
| Eating/Drinking | State | Mouse has its mouth touching food hopper or food pellet, with chewing movement of the jaw, or mouse has its mouth touching the water outlet. |
| Facial grimacing | Point event | Mouse demonstrates one or more of the following pain descriptors: Mouse shows orbital tightening/narrowing of the eyes (not in the context of grooming, because mice narrow/close their eyes to groom); Bulging on the bridge of the mouse’s nose and/or wrinkles on the side of the nose; Bulging of the cheeks; or Ears rotate outwards and/or backwards. Ears may fold to form a pointed shape. Space between the ear increases. (Whisker placement would normally also be included but it was not possible to see this level of detail in the footage.) |
| Freezing | State | Mouse makes no movement (except for slight head and/breathing movement), for at least 2 s. |
| Self-grooming (not including ears or tail) | State | Mouse demonstrates two or more of the following descriptors: 1. Mouse licks its forepaws; 2. Mouse makes repeated strokes along the snout with both forepaws; 3. Mouse makes semicircular movements over the top of its head and behind the ears with both forepaws; 4. Mouse licks or grooms its fur on its anterior part of the body and/or scratching with the hind paw; 5. Mouse licks or grooms its hind legs; 6. Mouse licks or grooms the genitals; 7. Mouse scratches any part of the body (other than the ears) with its hindpaw, a move isolated from the cephalocaudal grooming sequence. |
| Grooming left/right ear | Point event | Mouse strokes/scratches the left/right ear with its forepaw or hind paw. |
| Grooming tail | State | Mouse sniffs or licks its tail while holding it with both forepaws (isolated from the normal cephalocaudal grooming sequence). |
| In tunnel | State | Mouse has all four limbs in the shelter—behaviour is visible. |
| Not visible (tunnel) | State | Mouse is in the tunnel but detailed behaviour is not visible. |
| Not visible (other) | State | Mouse is located at a spot where it is hidden from scorer’s view (e.g., behind the food rack). |
| Other active behaviour | State | Collection of active behaviours not otherwise recorded, such as walking, sniffing, digging and gnawing. |
| Rapid shuttle | Point event | Mouse flits extremely fast from one location of the substrate to another. |
| Rearing | Point event | Mouse lifts its forepaws off the ground, mouse has its forepaws on the cage wall/shelter, only standing on its hind legs, |
| Sniffing cagemate | Point event | Mouse pauses and extends neck to touch cagemate with its nose. May also lightly touch the area with forepaws. |
| Response | Ear-Punch (n = 12) | Ear-Punch + EMLATM (n = 12) | Marker Pen (n = 12) | p-Value |
|---|---|---|---|---|
| Pulling head away | 6 | 2 | N/A | 0.097 |
| Vocalisation | 0 | 1 | 0 | N/A |
| Urination | 4 | 3 | 4 | >0.999 |
| Defaecation | 8 | 7 | 9 | 0.903 |
| Behaviour | Comparison | Effect Size | p-Value * |
|---|---|---|---|
| Allogrooming | Overall Treatment | H = 34.80 | <0.001 |
| C-EP > Control | 23.48 +/− 6.13 | <0.001 | |
| C-EP > EP | 27.15 +/− 6.98 | <0.001 | |
| C-EP > EP+A | 27.15 +/− 6.98 | <0.001 | |
| C-EP > MP | 27.15 +/− 7.29 | <0.001 | |
| C-EP > C-MP | 27.15 +/− 7.29 | <0.001 | |
| C-EP+A > Control | 18.94 +/− 6.13 | 0.002 | |
| C-EP+A > EP | 22.61 +/− 6.98 | 0.001 | |
| C-EP+A > EP+A | 22.61 +/− 6.98 | 0.001 | |
| C-EP+A > MP | 22.61 +/− 7.29 | 0.002 | |
| C-EP+A > C-MP | 22.61 +/− 7.30 | 0.002 | |
| Ear grooming | Overall Treatment | F6,89 = 10.10 | <0.001 |
| EP+A > Control | 0.90 +/− 0.13 | <0.001 | |
| EP+A > EP | 0.77 +/− 0.15 | <0.001 | |
| EP+A > MP | 0.91 +/− 0.15 | <0.001 | |
| EP+A > C-EP | 0.94 +/− 0.15 | <0.001 | |
| EP+A > C-EP+A | 0.75 +/− 0.15 | <0.001 | |
| EP+A > C-MP | 0.75 +/− 0.15 | <0.001 | |
| Facial grimacing | Overall Treatment | H = 18.90 | 0.004 |
| EP+A > MP | 15.23 +/− 7.50 | 0.042 FDR | |
| EP+A > C-EP | 15.23 +/− 7.18 | 0.034 FDR | |
| C-MP > Control | 21.41 +/− 6.66 | 0.001 | |
| C-MP > MP | 25.46 +/− 7.80 | 0.001 | |
| C-MP > C-EP | 25.46 +/− 7.50 | 0.001 | |
| C-MP > C-EP+A | 21.26 +/− 7.50 | 0.005 | |
| Other active behaviour | Overall Treatment | F6,89 = 2.22 | 0.048 FDR |
| Control > EP+A | 0.13 +/− 0.05 | 0.010 | |
| Control > C-EP | 0.12 +/− 0.05 | 0.019 FDR | |
| Control > C-EP+A | 0.12 +/− 0.05 | 0.017 | |
| Self-grooming | Overall Treatment | F6,89 = 4.74 | <0.001 |
| EP+A > Control | 0.15 +/− 0.04 | <0.001 | |
| EP+A > EP | 0.13 +/− 0.04 | 0.005 | |
| EP+A > MP | 0.16 +/− 0.05 | 0.001 | |
| EP+A > C-EP | 0.10 +/− 0.04 | 0.025 FDR | |
| EP+A > C-MP | 0.14 +/− 0.05 | 0.002 | |
| C-EP+A > Control | 0.13 +/− 0.04 | 0.001 | |
| C-EP+A > EP | 0.11 +/− 0.04 | 0.016 | |
| C-EP+A > MP | 0.14 +/− 0.05 | 0.003 | |
| C-EP+A > C-MP | 0.13 +/− 0.05 | 0.007 | |
| Sniffing cagemate | Overall Treatment | F6,89 = 13.39 | <0.001 |
| C-EP > Control | 1.02 +/− 0.21 | <0.001 | |
| C-EP > EP | 1.09 +/− 0.24 | <0.001 | |
| C-EP > EP+A | 1.04 +/− 0.24 | <0.001 | |
| C-EP > MP | 1.32 +/− 0.26 | <0.001 | |
| C-EP > C-MP | 1.02 +/− 0.25 | <0.001 | |
| C-EP+A > Control | 1.31 +/− 0.21 | <0.001 | |
| C-EP+A > EP | 1.38 +/− 0.24 | <0.001 | |
| C-EP+A > EP+A | 1.32 +/− 0.24 | <0.001 | |
| C-EP+A > MP | 1.60 +/− 0.25 | <0.001 | |
| C-EP+A > C-MP | 1.31 +/− 0.25 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burn, C.C.; Mazlan, N.H.B.; Chancellor, N.; Wells, D.J. The Pen Is Milder Than the Blade: Identification Marking Mice Using Ink on the Tail Appears More Humane Than Ear-Punching Even with Local Anaesthetic. Animals 2021, 11, 1664. https://doi.org/10.3390/ani11061664
Burn CC, Mazlan NHB, Chancellor N, Wells DJ. The Pen Is Milder Than the Blade: Identification Marking Mice Using Ink on the Tail Appears More Humane Than Ear-Punching Even with Local Anaesthetic. Animals. 2021; 11(6):1664. https://doi.org/10.3390/ani11061664
Chicago/Turabian StyleBurn, Charlotte C., Nur H. B. Mazlan, Natalie Chancellor, and Dominic J. Wells. 2021. "The Pen Is Milder Than the Blade: Identification Marking Mice Using Ink on the Tail Appears More Humane Than Ear-Punching Even with Local Anaesthetic" Animals 11, no. 6: 1664. https://doi.org/10.3390/ani11061664
APA StyleBurn, C. C., Mazlan, N. H. B., Chancellor, N., & Wells, D. J. (2021). The Pen Is Milder Than the Blade: Identification Marking Mice Using Ink on the Tail Appears More Humane Than Ear-Punching Even with Local Anaesthetic. Animals, 11(6), 1664. https://doi.org/10.3390/ani11061664






