Smartphone-Based Pelvic Movement Asymmetry Measures for Clinical Decision Making in Equine Lameness Assessment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Lameness Scoring
2.3. Equipment and Setup
2.4. Data Collection
2.5. Data Processing
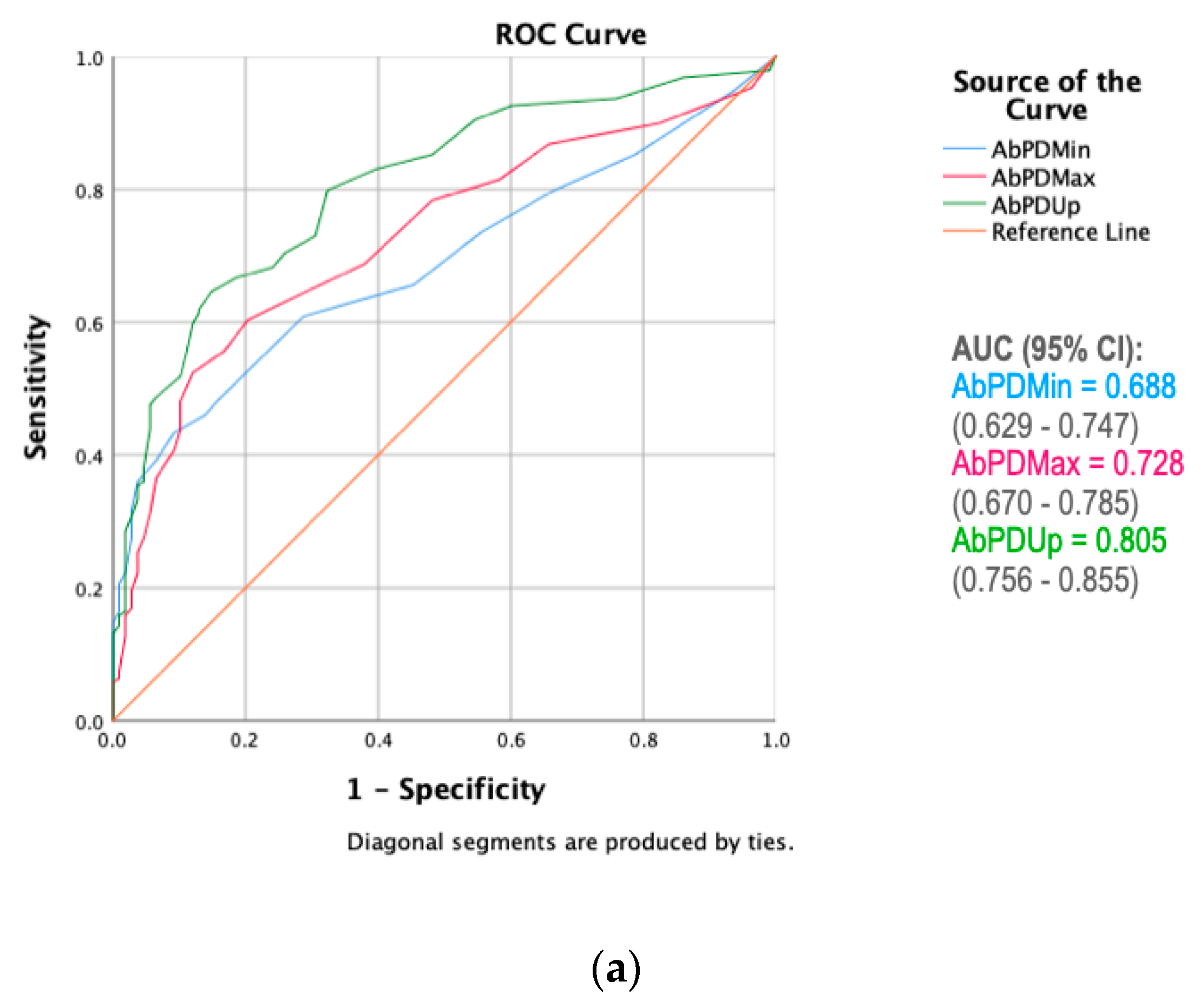
3. Results
4. Discussion
4.1. Comparison of Lameness Grades
4.2. Thresholds When Applying Minimum Specificity
4.3. Thresholds When Applying Minimum Sensitivity
4.4. Summary of the Discriminative Power of Pelvic Asymmetry Measures
4.5. Limitations of Smartphone-Based Pelvic Symmetry Measures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuller, C.J.; Bladon, B.M.; Driver, A.J.; Barr, A.R.S. The Intra- and Inter-Assessor Reliability of Measurement of Functional Outcome by Lameness Scoring in Horses. Vet. J. 2006, 171, 281–286. [Google Scholar] [CrossRef]
- Serra Bragança, F.M.; Rhodin, M.; van Weeren, P.R. On the Brink of Daily Clinical Application of Objective Gait Analysis: What Evidence Do We Have so Far from Studies Using an Induced Lameness Model? Vet. J. 2018, 234, 11–23. [Google Scholar] [CrossRef]
- Egan, S.; Brama, P.; McGrath, D. Research Trends in Equine Movement Analysis, Future Opportunities and Potential Barriers in the Digital Age: A Scoping Review from 1978 to 2018. Equine Vet. J. 2019, 51, 813–824. [Google Scholar] [CrossRef]
- Keegan, K.G.; Kramer, J.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Dent, E.V.; Kellerman, T.E.; Wilson, D.A.; Reed, S.K. Assessment of Repeatability of a Wireless, Inertial Sensor-Based Lameness Evaluation System for Horses. Am. J. Vet. Res. 2011, 72, 1156–1163. [Google Scholar] [CrossRef]
- Pfau, T.; Weller, R. Comparison of a Standalone Consumer Grade Smartphone with a Specialist Inertial Measurement Unit for Quantification of Movement Symmetry in the Trotting Horse. Equine Vet. J. 2017, 49, 124–129. [Google Scholar] [CrossRef]
- Starke, S.D.; Oosterlinck, M. Reliability of Equine Visual Lameness Classification as a Function of Expertise, Lameness Severity and Rater Confidence. Vet. Rec. 2019, 184, 63. [Google Scholar] [CrossRef]
- Baxter, G.M. Adams and Stashak’s Lameness in Horses; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 978-1-119-27668-5. [Google Scholar]
- May, S.A.; Wyn-Jones, G. Identification of Hindleg Lameness. Equine Vet. J. 1987, 19, 185–188. [Google Scholar] [CrossRef]
- Uhlir, C.; Licka, T.; Kübber, P.; Peham, C.; Scheidl, M.; Girtler, D. Compensatory Movements of Horses with a Stance Phase Lameness. Equine Vet. J. 1997, 29, 102–105. [Google Scholar] [CrossRef]
- Buchner, F.; Kastner, J.; Girtler, D.; Knezevic, P.F. Quantification of Hind Limb Lameness in the Horse. CTO 1993, 146, 196–199. [Google Scholar] [CrossRef]
- Kramer, J.; Keegan, K.G.; Kelmer, G.; Wilson, D.A. Objective Determination of Pelvic Movement during Hind Limb Lameness by Use of a Signal Decomposition Method and Pelvic Height Differences. Am. J. Vet. Res. 2004, 65, 741–747. [Google Scholar] [CrossRef]
- McCracken, M.J.; Kramer, J.; Keegan, K.G.; Lopes, M.; Wilson, D.A.; Reed, S.K.; LaCarrubba, A.; Rasch, M. Comparison of an Inertial Sensor System of Lameness Quantification with Subjective Lameness Evaluation: Comparison of Inertial System with Subjective Lameness Evaluation. Equine Vet. J. 2012, 44, 652–656. [Google Scholar] [CrossRef]
- Bell, R.P.; Reed, S.K.; Schoonover, M.J.; Whitfield, C.T.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Keegan, K.G. Associations of Force Plate and Body-Mounted Inertial Sensor Measurements for Identification of Hind Limb Lameness in Horses. Am. J. Vet. Res. 2016, 77, 337–345. [Google Scholar] [CrossRef]
- Rhodin, M.; Egenvall, A.; Haubro Andersen, P.; Pfau, T. Head and Pelvic Movement Asymmetries at Trot in Riding Horses in Training and Perceived as Free from Lameness by the Owner. PLoS ONE 2017, 12, e0176253. [Google Scholar] [CrossRef]
- Sepulveda Caviedes, M.F.; Forbes, B.S.; Pfau, T. Repeatability of Gait Analysis Measurements in Thoroughbreds in Training. Equine Vet. J. 2018, 50, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Boultbee, H.; Davis, H.; Walker, A.; Rhodin, M. Agreement between Two Inertial Sensor Gait Analysis Systems for Lameness Examinations in Horses. Equine Vet. Educ. 2016, 28, 203–208. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Persson, A.B.; Jensen, A.T.; Sørensen, H.; Andersen, P.H. Agreement between Accelerometric Symmetry Scores and Clinical Lameness Scores during Experimentally Induced Transient Distension of the Metacarpophalangeal Joint in Horses. Equine Vet. J. 2010, 42, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Sepulveda Caviedes, M.F.; McCarthy, R.; Cheetham, L.; Forbes, B.; Rhodin, M. Comparison of Visual Lameness Scores to Gait Asymmetry in Racing Thoroughbreds during Trot In-Hand. Equine Vet. Educ. 2018, 32, 191–198. [Google Scholar] [CrossRef]
- Maliye, S.; Voute, L.; Lund, D.; Marshall, J.F. An Inertial Sensor-Based System Can Objectively Assess Diagnostic Anaesthesia of the Equine Foot: Inertial Sensor-Based Objective Analysis of Diagnostic Anaesthesia of the Foot. Equine Vet. J. 2013, 45, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Pfau, T.; Spicer-Jenkins, C.; Smith, R.K.; Bolt, D.M.; Fiske-Jackson, A.; Witte, T.H. Identifying Optimal Parameters for Quantification of Changes in Pelvic Movement Symmetry as a Response to Diagnostic Analgesia in the Hindlimbs of Horses: Quantification of Pelvic Movement Symmetry after Diagnostic Analgesia. Equine Vet. J. 2014, 46, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Starke, S.D.; Witte, T.H.; May, S.A.; Pfau, T. Accuracy and Precision of Hind Limb Foot Contact Timings of Horses Determined Using a Pelvis-Mounted Inertial Measurement Unit. J. Biomech. 2012, 45, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Hardeman, A.M.; Bragança, F.M.S.; Swagemakers, J.H.; van Weeren, P.R.; Roepstorff, L. Variation in Gait Parameters Used for Objective Lameness Assessment in Sound Horses at the Trot on the Straight Line and the Lunge. Equine Vet. J. 2019, 51, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.G.; Wilson, D.A.; Kramer, J.; Reed, S.K.; Yonezawa, Y.; Maki, H.; Pai, P.F.; Lopes, M.A.F. Comparison of a Body-Mounted Inertial Sensor System–Based Method with Subjective Evaluation for Detection of Lameness in Horses. Am. J. Vet. Res. 2013, 74, 17–24. [Google Scholar] [CrossRef]
- Hewetson, M.; Christley, R.M.; Hunt, I.D.; Voute, L.C. Investigations of the Reliability of Observational Gait Analysis for the Assessment of Lameness in Horses. Vet. Rec. 2006, 158, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.G.; Dent, E.V.; Wilson, D.A.; Janicek, J.; Kramer, J.; Lacarrubba, A.; Walsh, D.M.; Cassells, M.W.; Esther, T.M.; Schiltz, P.; et al. Repeatability of Subjective Evaluation of Lameness in Horses: Repeatability of Subjective Evaluation of Lameness in Horses. Equine Vet. J. 2010, 42, 92–97. [Google Scholar] [CrossRef]
- Weeren, P.R.; Pfau, T.; Rhodin, M.; Roepstorff, L.; Serra Bragança, F.; Weishaupt, M.A. What Is Lameness and What (or Who) Is the Gold Standard to Detect It? Equine Vet. J. 2018, 50, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, M.A.; Wiestner, T.; Hogg, H.P.; Jordan, P.; Auer, J.A. Compensatory Load Redistribution of Horses with Induced Weightbearing Hindlimb Lameness Trotting on a Treadmill. Equine Vet. J. 2010, 36, 727–733. [Google Scholar] [CrossRef]
- Maliye, S.; Marshall, J.F. Objective Assessment of the Compensatory Effect of Clinical Hind Limb Lameness in Horses: 37 Cases (2011–2014). J. Am. Vet. Med. Assoc. 2016, 249, 940–944. [Google Scholar] [CrossRef]
- Leelamankong, P.; Estrada, R.; Mählmann, K.; Rungsri, P.; Lischer, C. Agreement among Equine Veterinarians and between Equine Veterinarians and Inertial Sensor System during Clinical Examination of Hindlimb Lameness in Horses. Equine Vet. J. 2020, 52, 326–331. [Google Scholar] [CrossRef]
- Marshall, J.F.; Lund, D.G.; Voute, L.C. Use of a Wireless, Inertial Sensor-Based System to Objectively Evaluate Flexion Tests in the Horse: Sensor-Based System to Objectively Evaluate Flexion Tests in Horses. Equine Vet. J. 2012, 44, 8–11. [Google Scholar] [CrossRef]
- Rhodin, M.; Pfau, T.; Roepstorff, L.; Egenvall, A. Effect of Lungeing on Head and Pelvic Movement Asymmetry in Horses with Induced Lameness. Vet. J. 2013, 198, e39–e45. [Google Scholar] [CrossRef]
- Maliye, S.; Voute, L.C.; Marshall, J.F. Naturally-Occurring Forelimb Lameness in the Horse Results in Significant Compensatory Load Redistribution during Trotting. Vet. J. 2015, 204, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, M.; Persson-Sjodin, E.; Egenvall, A.; Serra Bragança, F.M.; Pfau, T.; Roepstorff, L.; Weishaupt, M.A.; Thomsen, M.H.; van Weeren, P.R.; Hernlund, E. Vertical Movement Symmetry of the Withers in Horses with Induced Forelimb and Hindlimb Lameness at Trot. Equine Vet. J. 2018, 50, 818–824. [Google Scholar] [CrossRef] [PubMed]

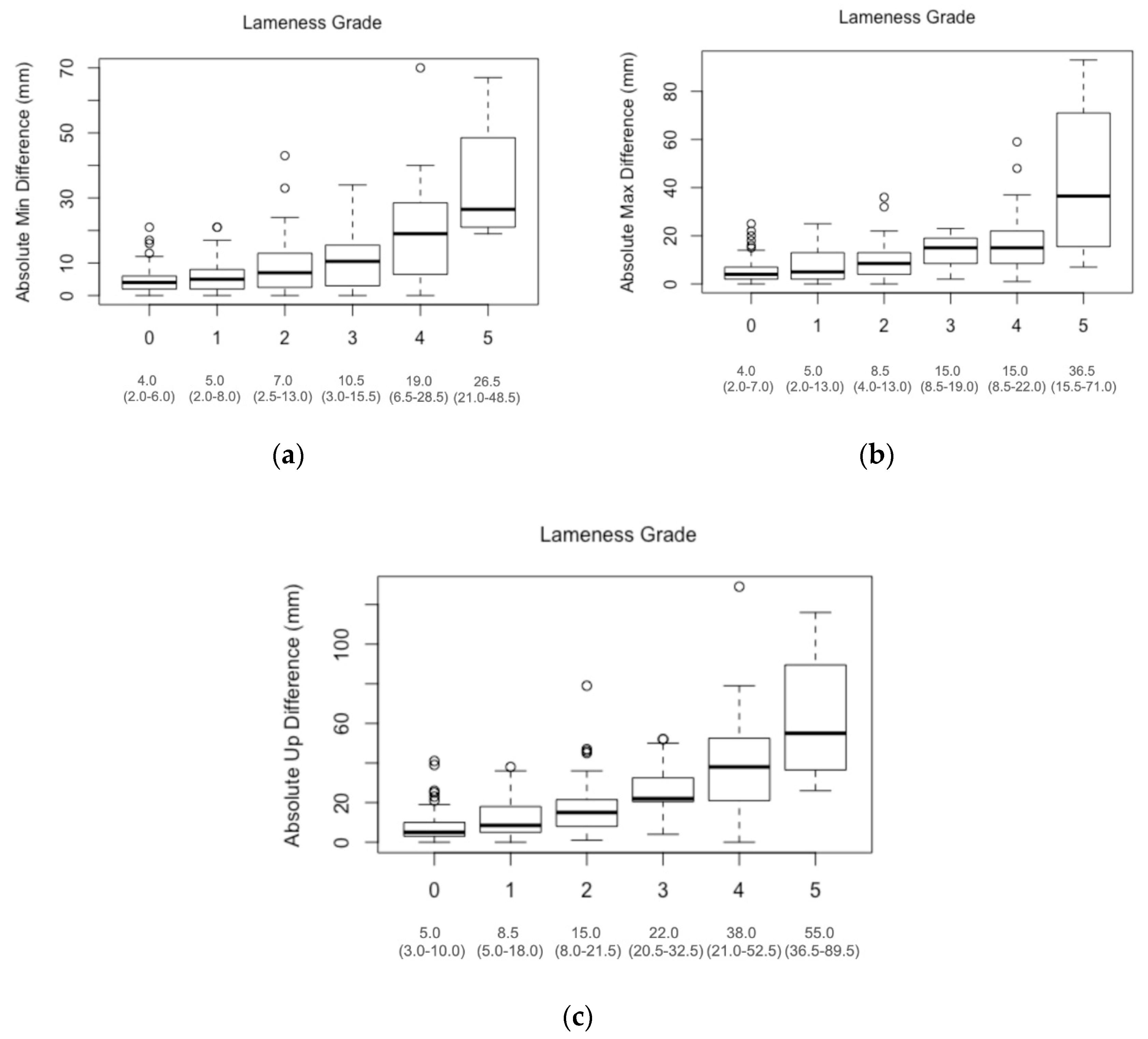


| Lameness Grades | AbPDMin | AbPDMax | AbPDUp |
|---|---|---|---|
| 0–1 | 1 | 1 | 0.02 |
| 0–2 | 0.02 | 0.001 | <0.001 |
| 0–3 | 0.001 | <0.001 | <0.001 |
| 0–4 | <0.001 | <0.001 | <0.001 |
| 1–2 | 1 | 0.6 | 0.5 |
| 1–3 | 0.2 | <0.001 | <0.001 |
| 1–4 | <0.001 | <0.001 | <0.001 |
| 2–3 | 1 | 0.03 | 0.08 |
| 2–4 | 0.002 | 0.002 | 0.001 |
| 3–4 | 0.4 | 1 | 1 |
| Asymmetry Measure | Lameness Grade (AUC *) | Specificity Benchmark | Threshold ≥ (mm) | Sensitivity | Specificity |
|---|---|---|---|---|---|
| AbPDMin | Grade 1 | 75% | 6.5 | 54.5% | 77.8% |
| (0.688) | 80% | 7.5 | 48.1% | 84.3% | |
| 85% | 8.5 | 46.0% | 86.1% | ||
| Grade 2 | 75% | 7.5 | 55.6% | 79.6% | |
| (0.710) | 80% | 8.5 | 54.8% | 82.7% | |
| 85% | 9.5 | 51.1% | 85.8% | ||
| Grade 3 | 75% | 8.5 | 65.3% | 76.1% | |
| (0.747) | 80% | 9.5 | 64.0% | 80.2% | |
| 85% | 11.5 | 58.7% | 86.0% | ||
| Grade 4 | 75% | 9.5 | 72.1% | 76.0% | |
| (0.785) | 80% | 11.5 | 69.8% | 82.3% | |
| 85% | 13.5 | 67.4% | 86.6% | ||
| AbPDMax | Grade 1 | 75% | 7.5 | 60.3% | 79.6% |
| (0.728) | 80% | 8.5 | 55.6% | 83.3% | |
| 85% | 9.5 | 52.4% | 88.0% | ||
| Grade 2 | 75% | 8.5 | 63.7% | 77.2% | |
| (0.764) | 80% | 9.5 | 60.0% | 80.9% | |
| 85% | 13.5 | 43.0% | 88.9% | ||
| Grade 3 | 75% | 10.5 | 68.0% | 77.0% | |
| (0.813) | 80% | 12.5 | 60.0% | 81.1% | |
| 85% | 13.5 | 58.7% | 85.6% | ||
| Grade 4 | 75% | 12.5 | 60.5% | 76.0% | |
| (0.786) | 80% | 14.5 | 53.5% | 83.5% | |
| 85% | 15.5 | 48.8% | 85.8% | ||
| AbPDUp | Grade 1 | 75% | 10.5 | 68.3% | 75.9% |
| (0.805) | 80% | 11.5 | 66.7% | 81.5% | |
| 85% | 12.5 | 64.6% | 85.2% | ||
| Grade 2 | 75% | 12.5 | 73.3% | 75.9% | |
| (0.818) | 80% | 16.5 | 64.4% | 81.5% | |
| 85% | 18.5 | 58.5% | 87.7% | ||
| Grade 3 | 75% | 17.5 | 81.3% | 78.4% | |
| (0.853) | 80% | 18.5 | 78.7% | 82.0% | |
| 85% | 20.5 | 76.0% | 85.6% | ||
| Grade 4 | 75% | 19.5 | 79.1% | 75.6% | |
| (0.837) | 80% | 21.5 | 72.1% | 81.9% | |
| 85% | 24.5 | 72.1% | 85.8% |
| Asymmetry Measure | Lameness Grade (AUC *) | Sensitivity Benchmark | Threshold ≥ (mm) | Sensitivity | Specificity |
|---|---|---|---|---|---|
| AbPDMin | Grade 1 | 75% | 2.5 | 79.9% | 33.3% |
| (0.688) | 80% | 1.5 | 85.2% | 21.3% | |
| 85% | 1.5 | 85.2% | 21.3% | ||
| Grade 2 | 75% | 3.5 | 77.0% | 41.4% | |
| (0.710) | 80% | 2.5 | 82.2% | 30.9% | |
| 85% | 1.5 | 86.7% | 20.4% | ||
| Grade 3 | 75% | 4.5 | 77.3% | 48.2% | |
| (0.747) | 80% | 3.5 | 81.3% | 37.8% | |
| 85% | 2.5 | 88.0% | 29.3% | ||
| Grade 4 | 75% | 5.5 | 76.7% | 55.5% | |
| (0.785) | 80% | 4.5 | 83.7% | 46.1% | |
| 85% | 3.5 | 88.4% | 36.6% | ||
| AbPDMax | Grade 1 | 75% | 4.5 | 78.3% | 51.9% |
| (0.728) | 80% | 3.5 | 81.5% | 41.7% | |
| 85% | 2.5 | 86.8% | 34.3% | ||
| Grade 2 | 75% | 5.5 | 77.0% | 58.6% | |
| (0.764) | 80% | 4.5 | 85.9% | 48.1% | |
| 85% | 4.5 | 85.9% | 48.1% | ||
| Grade 3 | 75% | 7.5 | 81.3% | 66.2% | |
| (0.813) | 80% | 7.5 | 81.3% | 66.2% | |
| 85% | 5.5 | 85.3% | 51.8% | ||
| Grade 4 | 75% | 7.5 | 81.4% | 60.2% | |
| (0.786) | 80% | 7.5 | 81.4% | 60.2% | |
| 85% | 4.5 | 97.7% | 37.8% | ||
| AbPDUp | Grade 1 | 75% | 7.5 | 79.9% | 67.6% |
| (0.805) | 80% | 6.5 | 83.1% | 60.2% | |
| 85% | 5.5 | 85.2% | 51.9% | ||
| Grade 2 | 75% | 11.5 | 75.6% | 72.8% | |
| (0.818) | 80% | 8.5 | 82.2% | 63.0% | |
| 85% | 7.5 | 88.9% | 59.3% | ||
| Grade 3 | 75% | 20.5 | 76.0% | 85.6% | |
| (0.852) | 80% | 17.5 | 81.3% | 78.4% | |
| 85% | 12.5 | 85.3% | 66.7% | ||
| Grade 4 | 75% | 20.5 | 76.7% | 78.0% | |
| (0.837) | 80% | 19.5 | 81.4% | 70.9% | |
| 85% | 12.5 | 86.0% | 60.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marunova, E.; Dod, L.; Witte, S.; Pfau, T. Smartphone-Based Pelvic Movement Asymmetry Measures for Clinical Decision Making in Equine Lameness Assessment. Animals 2021, 11, 1665. https://doi.org/10.3390/ani11061665
Marunova E, Dod L, Witte S, Pfau T. Smartphone-Based Pelvic Movement Asymmetry Measures for Clinical Decision Making in Equine Lameness Assessment. Animals. 2021; 11(6):1665. https://doi.org/10.3390/ani11061665
Chicago/Turabian StyleMarunova, Eva, Leea Dod, Stefan Witte, and Thilo Pfau. 2021. "Smartphone-Based Pelvic Movement Asymmetry Measures for Clinical Decision Making in Equine Lameness Assessment" Animals 11, no. 6: 1665. https://doi.org/10.3390/ani11061665
APA StyleMarunova, E., Dod, L., Witte, S., & Pfau, T. (2021). Smartphone-Based Pelvic Movement Asymmetry Measures for Clinical Decision Making in Equine Lameness Assessment. Animals, 11(6), 1665. https://doi.org/10.3390/ani11061665






