The Effects of Vehicle Type, Transport Duration and Pre-Transport Feeding on the Welfare of Sheep Transported in Low Temperatures
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Facilities
Vehicles
2.2. Experimental Details
2.2.1. Experiment 1. Enclosed vs. Open Truck
2.2.2. Experiment 2. Journey Duration
2.2.3. Experiment 3 Feeding the Sheep before Transport
2.3. Measurements
2.3.1. Environmental Parameters
2.3.2. Physical Indices and Sheep Temperatures
2.3.3. Physiological Indices
2.4. Statistical Analysis
3. Results
3.1. Experiment 1
3.1.1. Indoor Average Temperatures
3.1.2. Outdoor Minimum Temperatures
3.1.3. Blood Parameters
3.1.4. Principal Components
3.2. Experiment 2
3.2.1. Indoor Average Temperatures
3.2.2. Outdoor Minimum Temperatures
3.2.3. Blood Parameters
3.2.4. Principal Components
3.3. Experiment 3
3.3.1. Indoor Average Temperatures and Outdoor Minimum Temperatures
3.3.2. Blood Parameters
3.3.3. Principal Components
4. Discussion
4.1. The Effect of Enclosing the Truck
4.2. Effect of Duration of Transport in Cold Conditions on Sheep Welfare
4.3. Effect of Pre-Transport Feeding on Sheep Welfare during Transport in Cold Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broom, D.M. The welfare of livestock during road transport. In Long Distance Transport and the Welfare of Farm Animals; Appleby, M., Cussen, V., Garcés, L., Lambert, L., Turner, J., Eds.; CABI: Wallingford, UK, 2008; Chapter 7; pp. 157–181. [Google Scholar]
- Freer, M.; Dove, H.; Nolan, J.V. Nutrient Requirements of Domesticated Ruminants; CSIRO Publishing: Melbourne, Australia, 2007. [Google Scholar]
- Messori, S.; Pedernera-Romano, C.; Rodriguez, P.; Barnard, S.; Giansante, D.; Magnani, D.; Dalmau, A.; Velarde, A.; Dalla Villa, P. Effects of different rest-stop durations at control posts during a long journey on the welfare of sheep. Vet. Ital. 2017, 53, 121–129. [Google Scholar] [PubMed]
- Fisher, A.D.; Niemeyer, D.O.; Lea, J.M.; Lee, C.; Paull, D.R.; Reed, M.T.; Ferguson, D.M. The effects of 12, 30, or 48 hours of road transport on the physiological and behavioural responses of sheep. J. Anim. Sci. 2010, 88, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Tarrant, P.V.; Kenny, F.J.; Harrington, D.; Murphy, M. Long distance transportation of steers to slaughter: Effect of stocking density on physiology, behaviour and carcass quality. Livest. Prod. Sci. 1992, 30, 223–238. [Google Scholar] [CrossRef]
- Cockram, M.S.; Baxter, E.M.; Smith, L.A.; Bell, S.; Howard, C.M.; Prescott, R.J.; Mitchell, M.A. Effect of driver behaviour, driving events and road type on the stability and resting behaviour of sheep in transit. Anim. Sci. 2004, 79, 165–176. [Google Scholar] [CrossRef]
- Ruiz-de-la-Torre, J.L.; Velarde, A.; Manteca, X.; Diestre, A.; Gispert, M.; Hall, S.J.G.; Broom, D.M. Effects of vehicle movements during transport on the stress responses and meat quality of sheep. Vet. Rec. 2001, 148, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Stockman, C.A.; Barnes, A.L.; Miller, D.W.; Wickham, S.L.; Fleming, P.A. Qualitative behavioural assessment as a method to identify potential stressors during commercial sheep transport. Animals 2018, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.M.; Goode, J.A.; Hall, S.J.G.; Lloyd, D.M.; Parrott, R.F. Hormonal and physiological effects of a 15h road journey in sheep: Comparison with the response to loading, handling and penning in the absence of transport. Br. Vet. J. 1996, 152, 593–604. [Google Scholar] [CrossRef]
- Parrott, R.F.; Hall, S.J.G.; Lloyd, D.M. Heart rate and stress hormone responses of sheep to road transport following two different loading procedures. Anim. Welf. 1998, 7, 257–267. [Google Scholar]
- Fisher, A.D.; Stewart, M.; Duganzich, D.M.; Tacon, J.; Matthews, L.R. The effects of stationary periods and external temperature and humidity on thermal stress conditions within sheep transport vehicles. N. Z. Vet. J. 2005, 53, 6–9. [Google Scholar] [CrossRef]
- Ames, D.R.; Insley, L.W. Wind-chill effect for cattle and sheep. J. Anim. Sci. 1975, 40, 161–165. [Google Scholar] [CrossRef]
- Wojtas, K.; Cwynar, P.; Kolacz, R. Effect of thermal stress on physiological and blood parameters in merino sheep. Bull. Vet. Inst. Pulawy 2014, 58, 283–288. [Google Scholar] [CrossRef]
- Liang, X.; Jin, J.; Bi, X.; Kamruzzaman, M.; Kudo, T.; Sano, H. Effects of Chinese herbal medicine and cold exposure on plasma glucose, leucine and energy metabolism in sheep. J. Anim. Physiol. Anim. Nutr. 2018, 102, e534–e541. [Google Scholar] [CrossRef]
- Cottle, D.J.; Pacheco, D. Prediction of fleece insulation after shearing and its impact on maintenance energy requirements of Romney sheep. Small Rumin. Res. 2017, 157, 14–22. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Kemp, D.; Ma, Y.B.; Jiang, C. Effect of warm-shed feeding on liveweight loss and lambing performance of ewes during winter–spring period. Acta Prataculturae Sin. 2017, 26, 203–209. [Google Scholar]
- Cockram, M.S.; Kent, J.E.; Goddard, P.J.; Waran, N.K.; McGilp, I.M.; Jackson, R.E.; Muwanga, G.M.; Prytherch, S. Effect of space allowance during transport on the behavioural and physiological responses of lambs during and after transport. Anim. Sci. 1996, 62, 461–477. [Google Scholar] [CrossRef]
- Pascual-Alonso, M.; Miranda-de la Lama, G.; Aguayo-Ulloa, L.A.; Villarroel, M.; Mitchell, M.; Maria Levrino, G. Thermophysiological, haematological, biochemical and behavioural stress responses of sheep transported on road. J. Anim. Physiol. Anim. Nutr. 2016, 101, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Miranda-de la Lama, G.; Rodríguez-Palomares, M.; Cruz Monterrosa, R.; Rayas-Amor, A.; Pinheiro, R.s.B.; Galindo, F.; Villarroel, M. Long-distance transport of hair lambs: Effect of location in pot-belly trailers on thermo-physiology, welfare and meat quality. Trop. Anim. Health Prod. 2018, 50, 327–336. [Google Scholar] [CrossRef]
- Hall, S.J.G.; Bradshaw, R.H. Welfare aspects of the transport by road of sheep and pigs. J. Appl. Anim. Welf. Sci. 1998, 1, 235–254. [Google Scholar] [CrossRef]
- Chambers, P.G.; Grandin, T.; Heinz, G.; Srisuvan, T. Guidelines for Humane Handling, Transport and Slaughter of Livestock, book chapter. FAO Regional Office for Asia and the Pacific; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2001. [Google Scholar]
- Rioja-Lang, F.C.; Brown, J.A.; Brockhoff, E.J.; Faucitano, L. A review of swine transportation research on priority welfare issues: A Canadian perspective. Front. Vet. Sci. 2019. [Google Scholar] [CrossRef]
- Guàrdia, M.D.; Gispert, M.; Diestre, A. Mortality rates during transport and lairage in pigs for slaughter. Meat Focus Inter. 1996, 10, 362–365. [Google Scholar]
- Peterson, E.; Remmenga, M.; Hagerman, A.D.; Akkina, J.E. Use of temperature, humidity, and slaughter condemnation data to predict increases in transport losses in three classes of swine and resulting foregone revenue. Front. Vet. Sci. 2017, 4, 67. [Google Scholar] [CrossRef]
- Gonyou, H.W.; Brown, J. Reducing stress and improving recovery from handling during loading and transport of market pigs. In Final Report Submitted to Alberta Livestock and Meat Agency; ALMA: Edmonton, AB, Canada, 2012; p. 40. [Google Scholar]
- Guàrdia, M.D.; Estany, J.; Balasch, S.; Oliver, M.A.; Gispert, M.; Diestre, A. Risk assessment of PSE condition due to pre-slaughter conditions and RYR1 gene in pigs. Meat Sci. 2004, 67, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.G.; Brown, S.N.; Warriss, P.D.; Phillips, A.J.; Dolan, S.K.; Hunt, P.; Ford, J.E.; Edwards, J.E.; Watkins, P.E. Effects on sheep of transport by road for up to 24 hours. Vet. Rec. 1995, 136, 431–438. [Google Scholar] [CrossRef]
- Knowles, T.G.; Warriss, P.D.; Brown, S.N.; Kestin, S.C. Long distance transport of export lambs. Vet. Rec. 1994, 134, 107–110. [Google Scholar] [CrossRef]
- Time and Date. Available online: https://www.timeanddate.com/weather/china/hohhot/historic?month=1&year=2020 (accessed on 1 November 2020).
- Taylor, R.E. Adaptation to the environment. In Scientific Farm Animal Production; Macmillan Publishing Company: New York, NY, USA, 1992; pp. 326–332. [Google Scholar]
- Hales, J.R.S.; Bennett, J.W.; Fawcett, A.A. Effects of acute cold exposure on the distribution of cardiac output in the sheep. Pflugers Archiv 1976, 366, 153–157. [Google Scholar] [CrossRef]
- Webster, A.; Hicks, A.; Hays, F. Cold climate and cold temperature induced changes in the heat production and thermal insulation of sheep. Can. J. Physiol. Pharmacol. 1969, 47, 553–562. [Google Scholar] [CrossRef]
- Ekesbo, I. Farm Animal Behaviour: Characteristic for Assessment of Health and Welfare; CAB International; Cambridge University Press: Cambridge, UK, 2011; p. 237. [Google Scholar]
- Dabiri, N.; Holmes, C.W.; McCutcheon, S.N.; Parker, W.J.; Morris, S.T. Resistance to cold stress in sheep shorn by covercomb or standard comb. Anim. Sci. 1995, 60, 451–456. [Google Scholar] [CrossRef]
- Piirsalu, P.; Kaart, T.; Nutt, I.; Marcone, G.; Arney, D. The effect of climate parameters on sheep preferences for outdoors or indoors at low ambient temperatures. Animals 2020, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- OMAFRA. Ministry of Agriculture, Food and Rural Affairs, Ontario Canada. Available online: http://www.omafra.gov.on.ca/english/livestock/sheep/facts/02-013.htm (accessed on 1 November 2020).
- Department of Primary Industries and Regional Development’s Agriculture and Food. Available online: https://www.agric.wa.gov.au/animal-welfare/hypothermia-sheep (accessed on 1 November 2020).
- Canadian Agri-Food Research Council. Recommended Code of Practice for the Care and Handling of Farm Animals—Transportation. Available online: http://nfacc.ca/pdf/english/Transportation2001.pdf (accessed on 1 November 2020).
- Obst, J.M.; Ellis, J.V. Weather, ewe behaviour and lamb mortality. Agric. Rec. 1977, 20, 44–49. [Google Scholar]
- Lynch, J.J.; Alexander, G. The effect of gramineous wind breaks on behaviour and lamb mortality among shorn and unshorn Merino sheep during lambing. Appl. Anim. Ethol. 1976, 2, 305–325. [Google Scholar] [CrossRef]
- Lynch, J.J.; Alexander, G. Sheltering behaviour of lambing Merino sheep in relation to grass hedges and artificial wind breaks. Aust. J. Agric. Res. 1977, 28, 691–701. [Google Scholar] [CrossRef]
- Younis, F. Expression pattern of heat shock protein genes in sheep. Mansoura Vet. Med. J. 2020, 21, 1–5. [Google Scholar] [CrossRef]
- Sejian, V.; Maurya, V.P.; Sharma, K.C.; Naqvi, S. Concept of multiple stresses and its significance on livestock productivity. In Environmental Stress and Amelioration in Livestock Production; Springer: Berlin/Heidelberg, Germany, 2012; pp. 129–150. [Google Scholar]
- Agnew, L.L.; Colditz, I.G. Development of a method of measuring cellular stress in cattle and sheep. Vet. Immunol. Immunopathol. 2008, 123, 197–204. [Google Scholar] [CrossRef]
- Kapila, R. Effect of low temperature on metabolic enzymes and HSP-70 expression of cold-water fish Barilius bendelisis. Asian Fish. Sci. 2009, 22, 125–136. [Google Scholar]
- Eng, J.W.; Reed, C.B.; Kokolus, K.M.; Repasky, E.A. Housing temperature influences the pattern of heat shock protein induction in mice following mild whole-body hyperthermia. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. North Am. Hyperth. Group 2014, 30, 540–546. [Google Scholar] [CrossRef]
- Parrott, R.F.; Misson, B.H.; de la Riva, C.F. Differential stressor effects on the concentrations of cortisol, prolactin and catecholamines in the blood of sheep. Vet. Sci. Res. 1994, 56, 234–239. [Google Scholar] [CrossRef]
- Fordham, D.P.; Lincoln, G.A.; Ssewannyna, E.; Rodway, R.G. Plasma β-endorphin and cortisol concentrations in lambs after handling, transport and slaughter. Anim. Prod. 1989, 49, 103–107. [Google Scholar]
- Androine, I.; Parvu, M.; Androine, V. The Effects of Transport Stress on Sheep Welfare; Lucrari Stiintifice—Zootehnie si Biotehnologii; Universitatea de Stiinte Agricole si Medicina Veterinara a Banatului Timisoara: Timisoara, Romania, 2008; Volume 41, pp. 729–734. [Google Scholar]
- Zhong, R.Z.; Liu, H.W.; Zhou, D.W.; Sun, H.X.; Zhao, C.S. The effects of road transportation on physiological responses and meat quality in sheep differing in age. J. Anim. Sci. 2011, 89, 3742–3751. [Google Scholar] [CrossRef]
- Braun, J.P.; Trumel, C.; Bézille, P. Clinical biochemistry in sheep: A selected review. Small Rumin. Res. 2010, 92, 10–18. [Google Scholar] [CrossRef]
- Earley, B.; Murray, M. The effect of road and sea transport on inflammatory, adrenocortical, metabolic and behavioural responses of weanling heifers. Vet. Res. 2010, 6, 36–48. [Google Scholar] [CrossRef]
- Miranda-De la Lama, G.; Monge, P.; Villarroel, M.; Olleta, J.; GarcãA-Belenguer, S.; Marã, A.G. Effects of road type during transport on lamb welfare and meat quality in dry hot climates. Trop. Anim. Health Prod. 2011, 43, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Beatty, D.T.; Blache, D.; Wemelsfelder, F.; Fleming, P.A. Flooring and driving conditions during road transport influence the behavioural expression of cattle. Appl. Anim. Behav. Sci. 2013, 143, 18–30. [Google Scholar]
- Walter, G.; Smith, G.; Walker, R. Interpretation of clinical pathology results in non-clinical toxicology testing. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology Book, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; Chapter 29. [Google Scholar]
- Hrkovic-Porobija, A.; Hodzić, A.; Hadzimusic, N. Functional liver stress in dairy sheep. Indian J. Small Rumin. 2017, 23, 194. [Google Scholar] [CrossRef]
- Ašimović, Z. Izabrani Parametri Metabolizma Kaoindikatori Specifičnosti Dubskog soja Pramenke. Ph.D. Thesis, University of Sarajevo, Sarajevo, Bosnia and Herzegovina, 2005. [Google Scholar]
- de Freitas, M.C.; Gerosa-Neto, J.; Zanchi, N.E.; Santos Lira, F.; Rossi, F.E. Role of metabolic stress for enhancing muscle adaptations: Practical applications. World J. Methodol. 2017, 7, 46–54. [Google Scholar] [CrossRef]
- Coffey, V.G.; Shield, A.; Canny, B.J.; Carey, K.A.; Cameron-Smith, D.; Hawley, J.A. Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E849–E855. [Google Scholar] [CrossRef] [PubMed]
- Bórnez, R.; Linares, M.B.; Vergara, H. Haematological, hormonal and biochemical blood parameters in lamb: Effect of age and blood sampling time. Livest. Sci. 2009, 121, 200–206. [Google Scholar] [CrossRef]
- Jones, A.R.; Prince, S.E. The Biology of Deer; Brown, R.D., Ed.; Springer: Berlin, Germany, 1992; pp. 211–216. [Google Scholar]
- Boccardo, A.; Belloli, A.; Locatelli, V.; Morandi, N.; Baggiani, L.; Cavallone, E.; Palma, A.; Pravettoni, D. Effect of lairage after transport in lambs slaughtered with islamic ritual. Large Anim. Rev. 2014, 20, 71–79. [Google Scholar]
- Proctor, H.S.; Carder, G. Can changes in nasal temperature be used as an indicator of emotional state in cows? Appl. Anim. Behav. Sci. 2016, 184, 1–6. [Google Scholar] [CrossRef]
- Alsaaod, M.; Syring, C.; Dietrich, J.; Moherr, M.G.; Gujan, T.; Steiner, A. A field trial of infrared thermography as a non-invasive diagnostic tool for early detection of digital dermatitis in dairy cows. Vet. J. 2014, 199, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Gómez, Y.; Bieler, R.; Hankele, A.K.; Zähner, M.; Savary, P.; Hillmann, E. Evaluation of visible eye white and maximum eye temperature as non-invasive indicators of stress in dairy cows. Appl. Anim. Behav. Sci. 2018, 198, 1–8. [Google Scholar] [CrossRef]
- Schaefer, A.; Cook, N.; Bench, C.; Chabot, J.; Colyn, J.; Liu, T.; Okine, E.; Stewart, M.; Webster, J. The non-invasive and automated detection of bovine respiratory disease onset in receiver calves using infrared thermography. Res. Vet. Sci. 2012, 93, 928–935. [Google Scholar] [CrossRef]
- Stokes, J.; Leach, K.; Main, D.; Whay, H. An investigation into the use of infrared thermography as a rapid diagnostic tool for foot lesions in dairy cattle. Vet. J. 2012, 193, 674–678. [Google Scholar] [CrossRef]
- Church, J.S.; Hegadoren, P.; Paetkau, M.; Miller, C.; Regev-Shoshani, G.; Schaefer, A.; Schwartzkopf-Genswein, K. Influence of environmental factors on infrared eye temperature measurements in cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.J.; Comline, R.S.; Dobson, A. Changes in the blood flow to the digestive organs of sheep induced by feeding. Q. J. Exp. Physiol. 1983, 68, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.; Ford, E. Normal variation in alanine amino transferase activity in sheep and cattle. J. Agric. Sci. 1967, 68, 385–389. [Google Scholar] [CrossRef]
- Krebs, H.A. Bovine ketosis. Vet. Rec. 1966, 78, 187. [Google Scholar] [CrossRef] [PubMed]
- Hewagalamulage, S.D.; Clarke, I.J.; Rao, A.; Belinda, A.H. Ewes with divergent cortisol responses to ACTH exhibit functional differences in the hypothalamo-pituitary-adrenal (HPA) axis. Endocrinology 2016, 157, 3540–3549. [Google Scholar] [CrossRef]
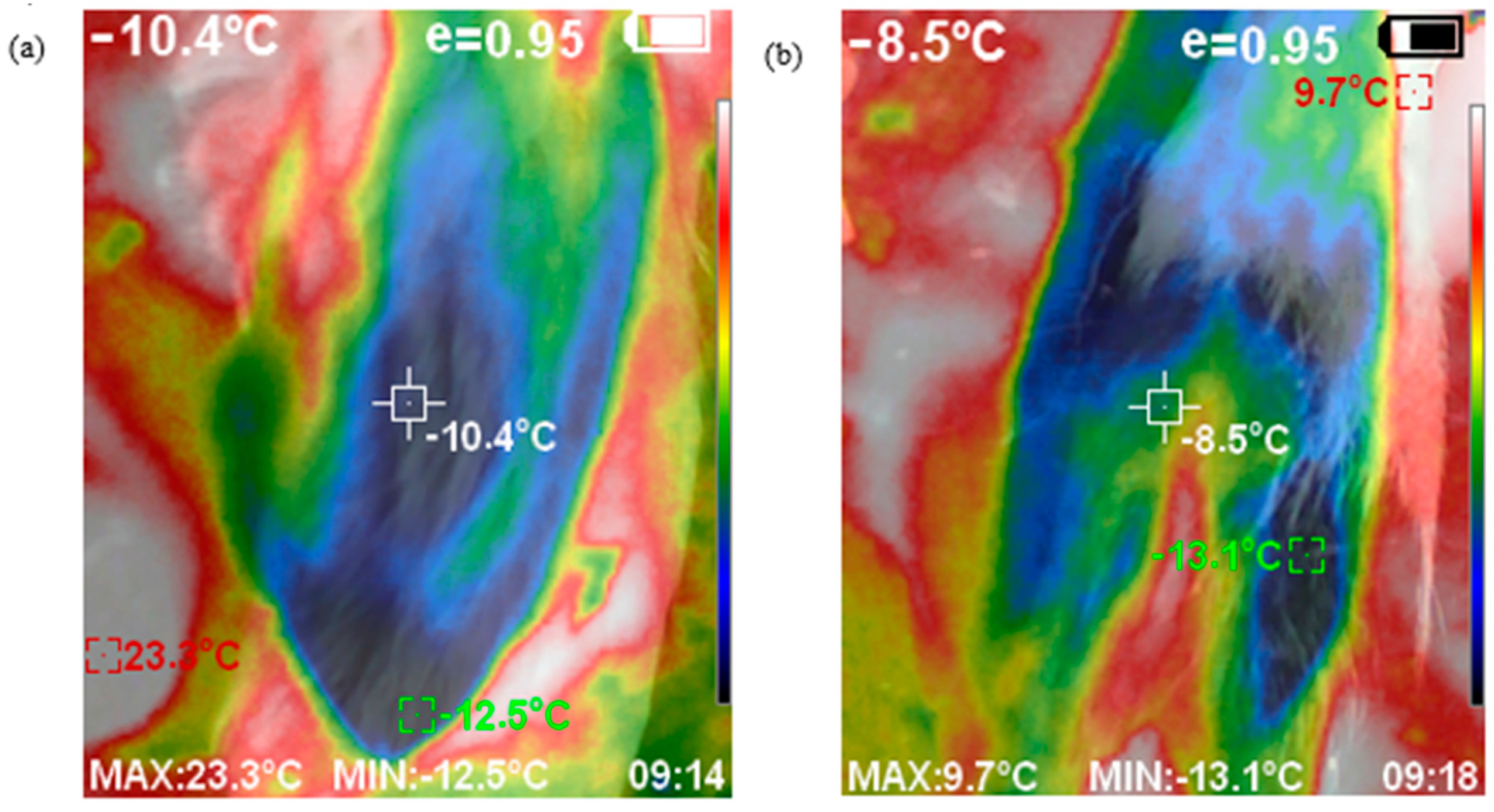
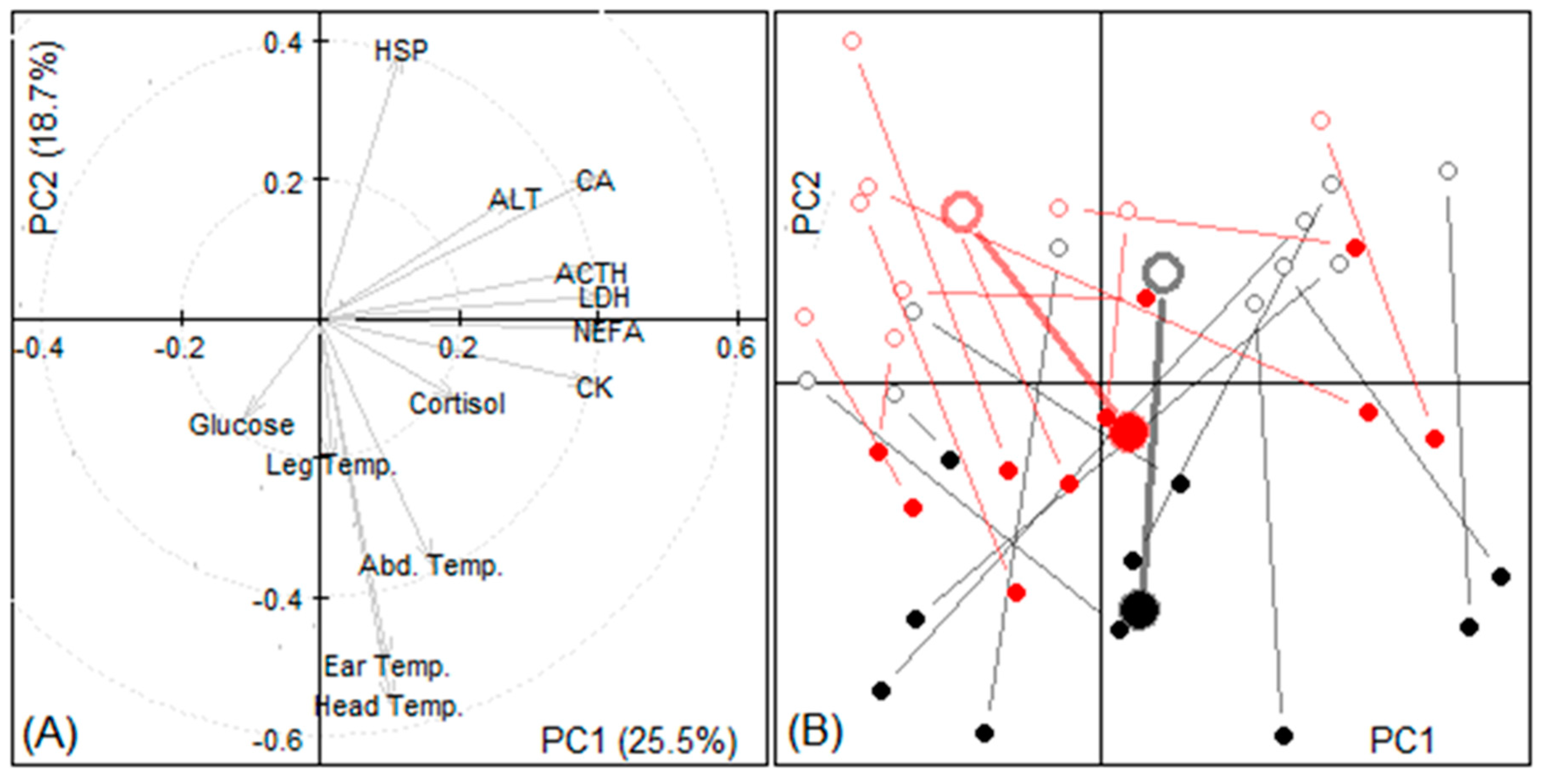
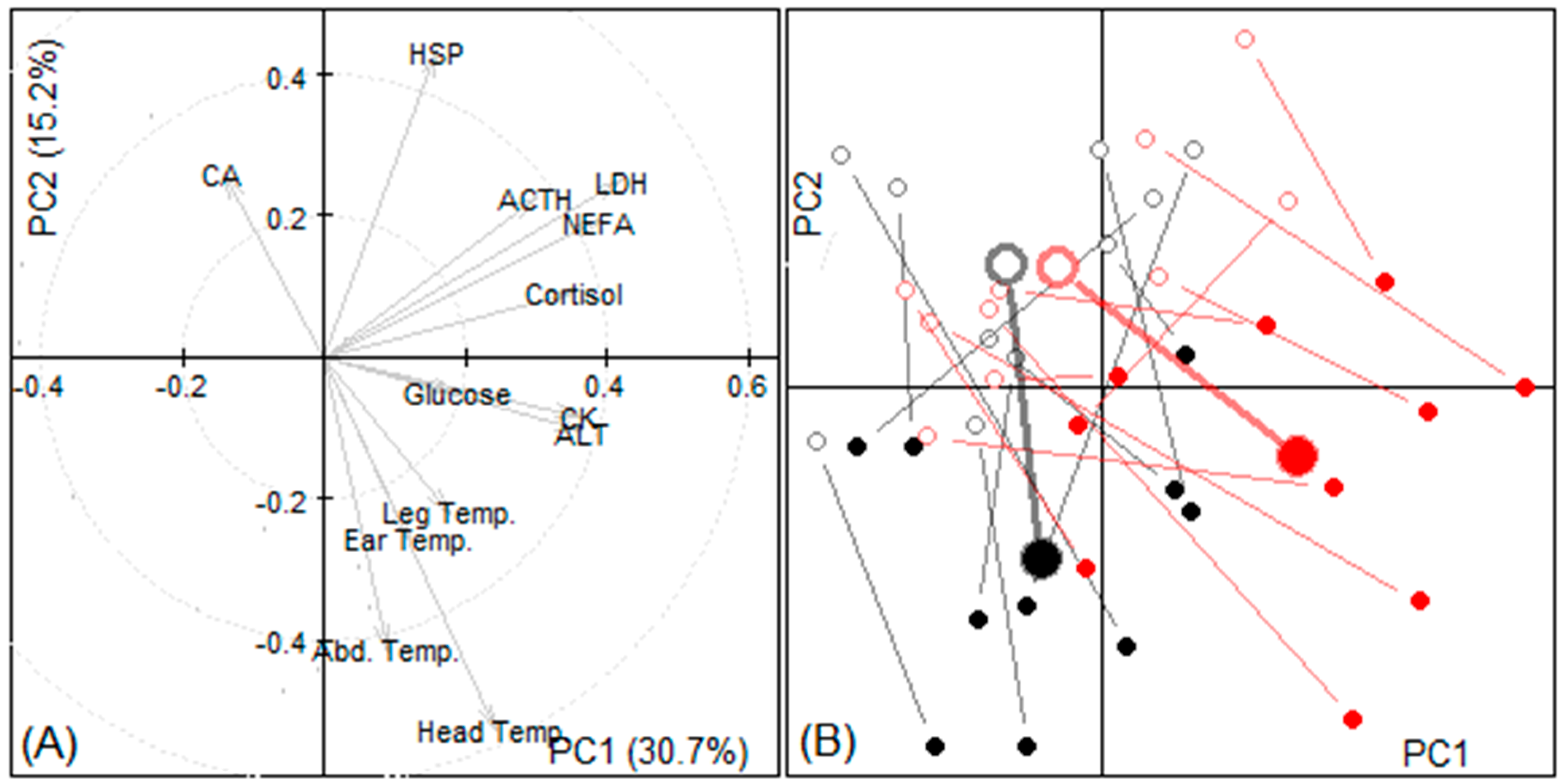
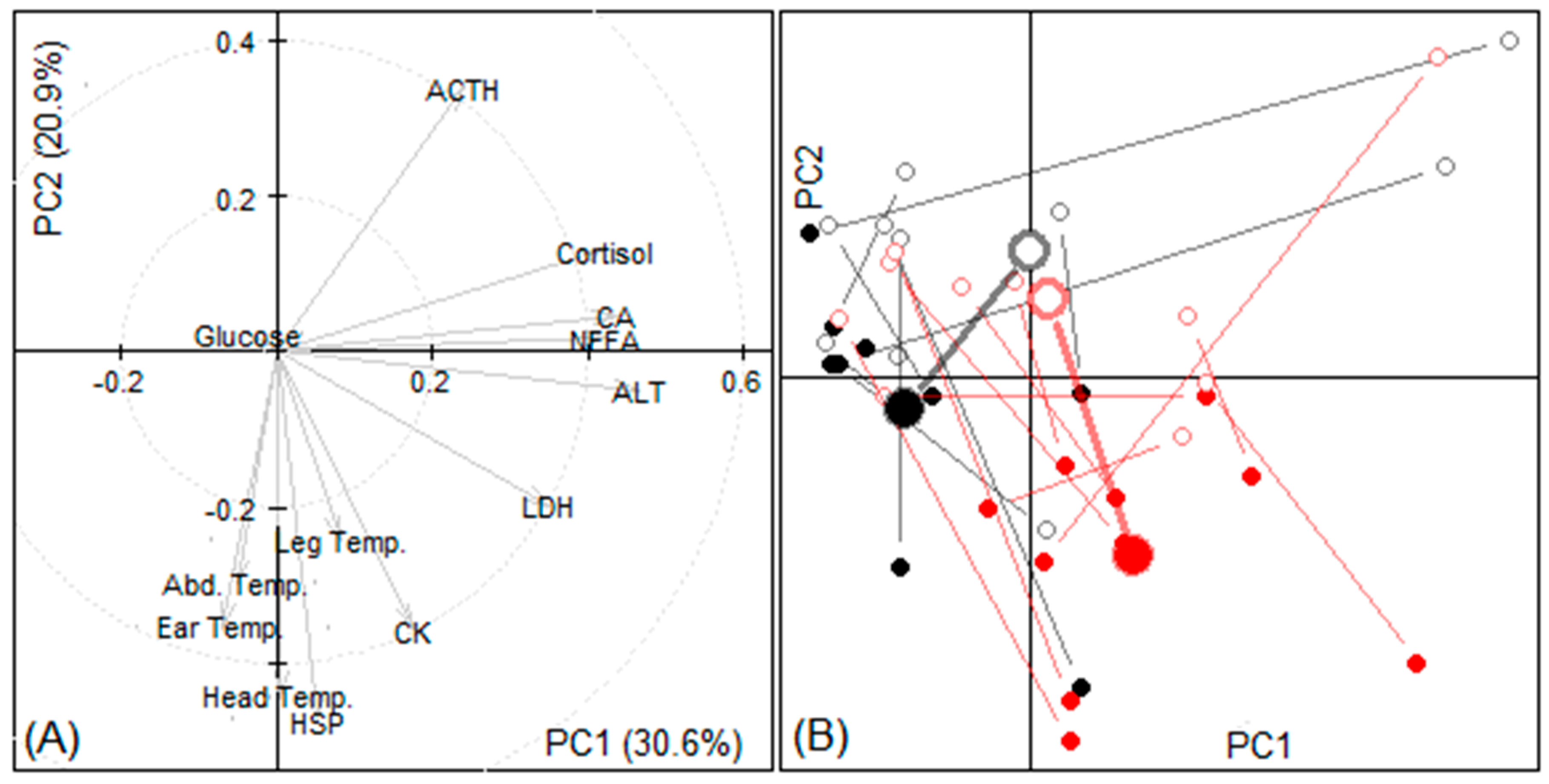
| Variable | Change During Transport | p-Value | |
|---|---|---|---|
| Enclosed Vehicle | Open Vehicle | ||
| Indoor temperature, °C | |||
| Head | +16.4 (14.8, 18.0) | +11.8 (10.3, 13.4) | <0.01 |
| Ear | +9.9 (8.0, 11.7) | +2.0 (0.1, 3.8) | <0.01 |
| Abdomen | +1.2 (0.3, 2.2) | +1.3 (0.3, 2.2) | 0.89 |
| Foot/pastern | +0.6 (−2.4, 3.6) | +0.2 (−2.8, 3.2) | 0.84 |
| Outdoor minimum temperature, °C | |||
| Ear | +9.7 (7.7;11.6) | +8.5 (6.5;10.4) | 0.39 |
| Foot/pastern | +5.8 (4.6;7.1) | +6.1 (4.7;7.3) | 0.85 |
| Blood parameters | |||
| ACTH, ng/L | +2.7 (−7.9, 13.3) | +0.2 (−10.3, 10.8) | 0.75 |
| ALT, U/L | −1.35 (−3.49, 0.79) | −0.15 (−2.29, 1.99) | 0.41 |
| HSP, ng/L | −303.9 (−495.7, −112.2) | −24.8 (−216.5, 166.9) | 0.04 |
| Cortisol, μg/L | +2.26 (−1.07, 5.58) | +2.96 (−0.37, 6.29) | 0.76 |
| NEFA, mmol/L | +7.0 (−10.1, 24.1) | +15.2 (−1.9, 32.3) | 0.51 |
| LDH, IU/L | −0.7 (−16.3, 14.8) | +7.1 (−8.4, 22.6) | 0.46 |
| CA, ng/L | −5.0 (−13.2, 3.2) | −0.8 (−9.0, 7.4) | 0.46 |
| CK, IU/L | +1.67 (−0.29, 3.63) | −0.48 (−2.44, 1.48) | 0.14 |
| Glucose, mmol/L | −0.01 (−0.83, 0.82) | +0.87 (0.04, 1.70) | 0.13 |
| Variable | Change During Transport | p-Value | |
|---|---|---|---|
| Duration of Transport 1 h | Duration of Transport 2 h | ||
| Indoor temperature, °C | |||
| Head | +10.0 (7.4, 12.6) | +9.4 (6.8, 12.0) | 0.73 |
| Ear | +3.3 (0.0, 6.7) | +1.3 (−2.0, 4.7) | 0.45 |
| Abdomen | +1.4 (0.7, 2.1) | +0.5 (−0.3, 1.2) | 0.08 |
| Foot/pastern | −0.5 (−1.7, 0.7) | +1.9 (0.7, 3.0) | 0.01 |
| Outdoor minimum temperature, °C | |||
| Ear | −2.2 (−4.3;−0.1) | +0.8 (−1.2;2.9) | 0.05 |
| Foot/pastern | −4.6 (−6.5;−2.8) | −1.1 (−2.9;0.7) | <0.01 |
| Blood parameters | |||
| ACTH, ng/L | −5.8 (−18.8, 7.1) | +10.7 (−2.2, 23.7) | 0.07 |
| ALT, U/L | +0.8 (−1.19, 2.86) | +6.5 (4.43, 8.48) | <0.01 |
| HSP, ng/L | −127.8 (−238.6, −17.1) | −126.6 (−237.3, −15.8) | 0.98 |
| Cortisol, μg/L | +0.1 (−2.25, 2.46) | +5.2 (2.87, 7.58) | <0.01 |
| NEFA, mmol/L | −19.7 (−40.0, 0.6) | +21.4 (1.1, 41.7) | <0.01 |
| LDH, IU/L | −18.5 (−43.2, 6.2) | +38.4 (13.8, 63.1) | <0.01 |
| CA, ng/L | −6.4 (−24.0, 11.3) | −14.4 (−32.0, 3.3) | 0.55 |
| CK, IU/L | +0.6 (−1.84, 3.02) | +5.3 (2.85, 7.71) | 0.01 |
| Glucose, mmol/L | +0.03 (−1.03, 1.08) | +0.74 (−0.31, 1.80) | 0.33 |
| Variable Pre-Transport Feeding | Change During Transport | p-Value | |
|---|---|---|---|
| Yes | No | ||
| Indoor temperature, °C | |||
| Head | +10.1 (6.8, 13.4) | +11.8 (8.5, 15.0) | 0.49 |
| Ear | +2.9 (−0.8, 6.6) | +6.6 (2.9, 10.3) | 0.16 |
| Abdomen | +0.7 (0.0, 1.3) | +1.4 (0.8, 2.1) | 0.13 |
| Foot/pastern | −0.2 (−2.1, 1.7) | +2.0 (0.1, 4.0) | 0.10 |
| Outdoor minimum temperature, °C | |||
| Ear | +5.2 (1.8;8.6) | +5.5 (2.09;8.9) | 0.90 |
| Foot/pastern | +6.1 (4.4;7.8) | +4.7 (2.9;6.4) | 0.24 |
| Blood parameters | |||
| ACTH, ng/L | −4.8 (−11.9, 2.3) | −8.6 (−15.7, −1.5) | 0.43 |
| ALT, U/L | −2.9 (−5, −0.93) | +4.6 (2.52, 6.59) | <0.01 |
| HSP, ng/L | +90.3 (−57.4, 238.1) | +309.7 (162.0, 457.5) | 0.04 |
| Cortisol, μg/L | −3.9 (−6.02, −1.88) | −0.2 (−2.25, 1.89) | 0.01 |
| NEFA, mmol/L | −20.1 (−27.3, −12.8) | +18.0 (10.8, 25.3) | <0.01 |
| LDH, IU/L | −18.5 (−48.1, 11.0) | +26.6 (−2.9, 56.1) | 0.03 |
| CA, ng/L | −8.5 (−18.1, 1.1) | +8.9 (−0.8, 18.5) | 0.01 |
| CK, IU/L | −1.7 (−5.81, 2.42) | +3.7 (−0.41, 7.82) | 0.06 |
| Glucose, mmol/L | +0.1 (−0.55, 0.63) | +0.2 (−0.37, 0.81) | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnovale, F.; Xiao, J.; Shi, B.; Kaart, T.; Arney, D.; Phillips, C.J.C. The Effects of Vehicle Type, Transport Duration and Pre-Transport Feeding on the Welfare of Sheep Transported in Low Temperatures. Animals 2021, 11, 1659. https://doi.org/10.3390/ani11061659
Carnovale F, Xiao J, Shi B, Kaart T, Arney D, Phillips CJC. The Effects of Vehicle Type, Transport Duration and Pre-Transport Feeding on the Welfare of Sheep Transported in Low Temperatures. Animals. 2021; 11(6):1659. https://doi.org/10.3390/ani11061659
Chicago/Turabian StyleCarnovale, Francesca, Jin Xiao, Binlin Shi, Tanel Kaart, David Arney, and Clive J. C. Phillips. 2021. "The Effects of Vehicle Type, Transport Duration and Pre-Transport Feeding on the Welfare of Sheep Transported in Low Temperatures" Animals 11, no. 6: 1659. https://doi.org/10.3390/ani11061659
APA StyleCarnovale, F., Xiao, J., Shi, B., Kaart, T., Arney, D., & Phillips, C. J. C. (2021). The Effects of Vehicle Type, Transport Duration and Pre-Transport Feeding on the Welfare of Sheep Transported in Low Temperatures. Animals, 11(6), 1659. https://doi.org/10.3390/ani11061659








