Retriever and Pointer: Software to Evaluate Inbreeding and Genetic Management in Captive Populations
Abstract
Simple Summary
Abstract
1. Introduction
2. Software
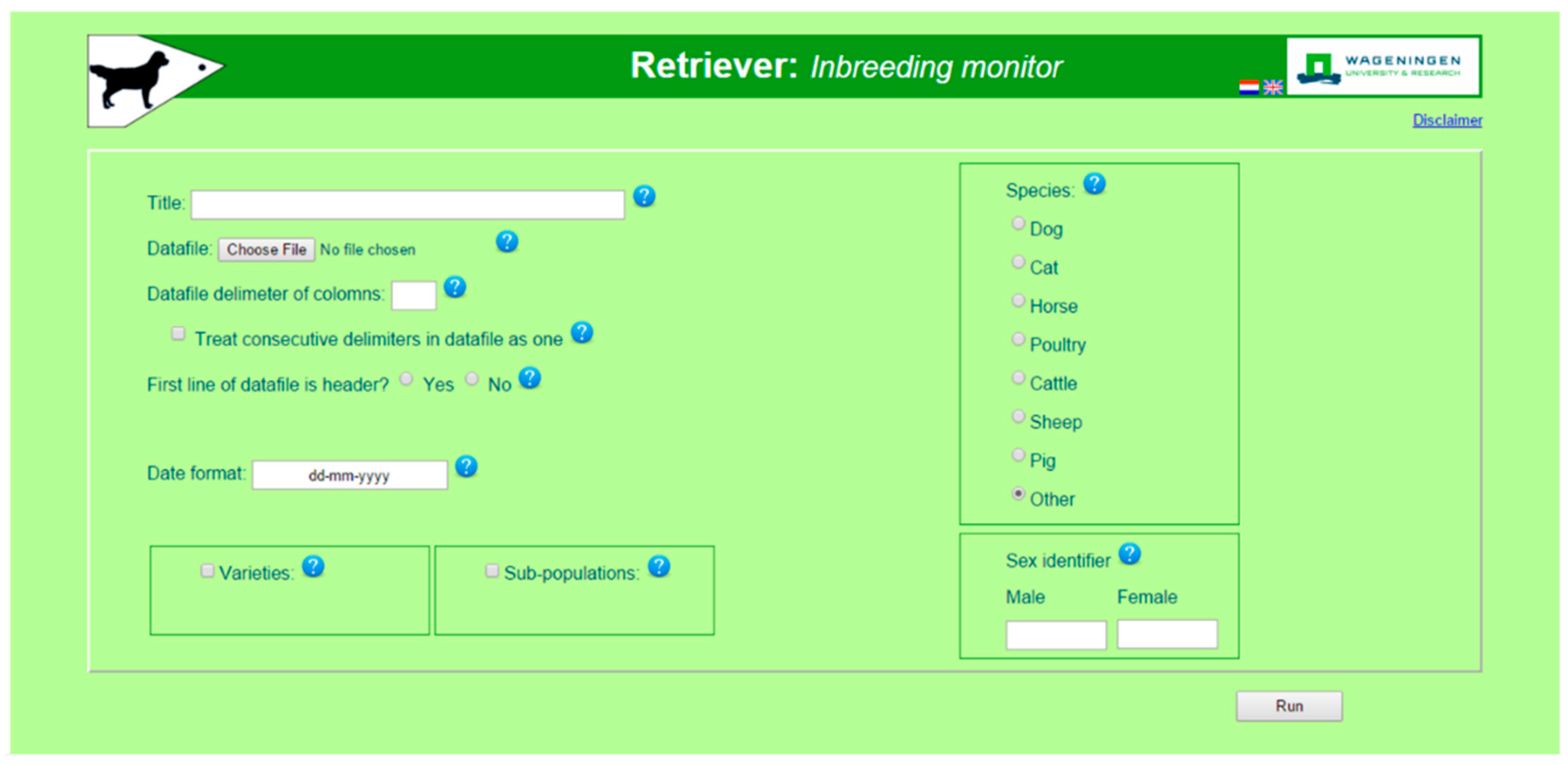
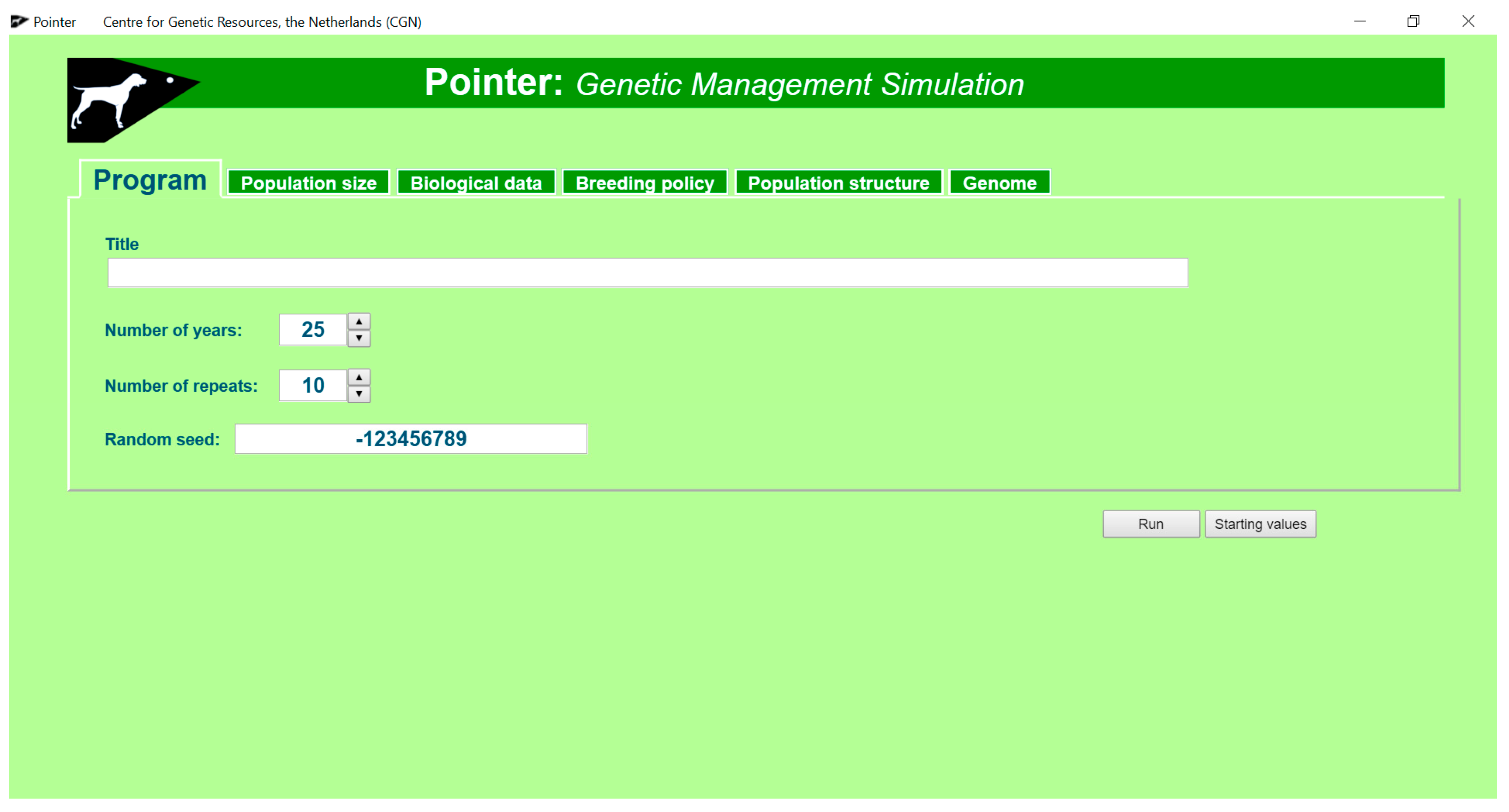
2.1. Operation and Graphical User Interface (GUI)
2.2. Retriever Software to Monitor Inbreeding and Population Structure
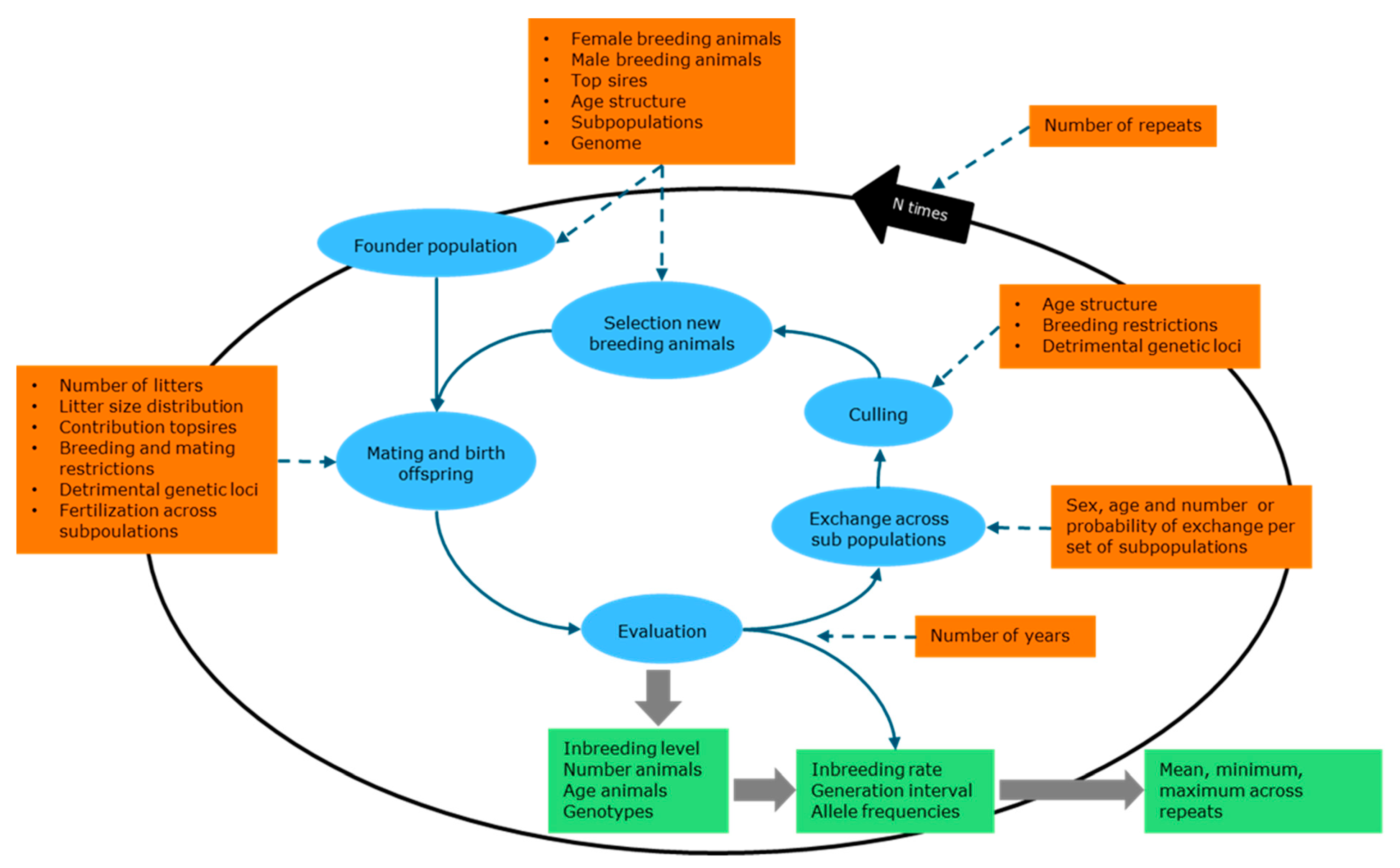
2.3. Pointer Software to Simulate Genetic Management
2.3.1. Mating and Birth
2.3.2. Subpopulations and Migration
2.3.3. Culling
2.3.4. Selection of New Breeding Animals
2.3.5. Genetic Management
2.3.6. Genome
2.3.7. Output
3. Examples
3.1. Blue Texel and Badger Face Sheep
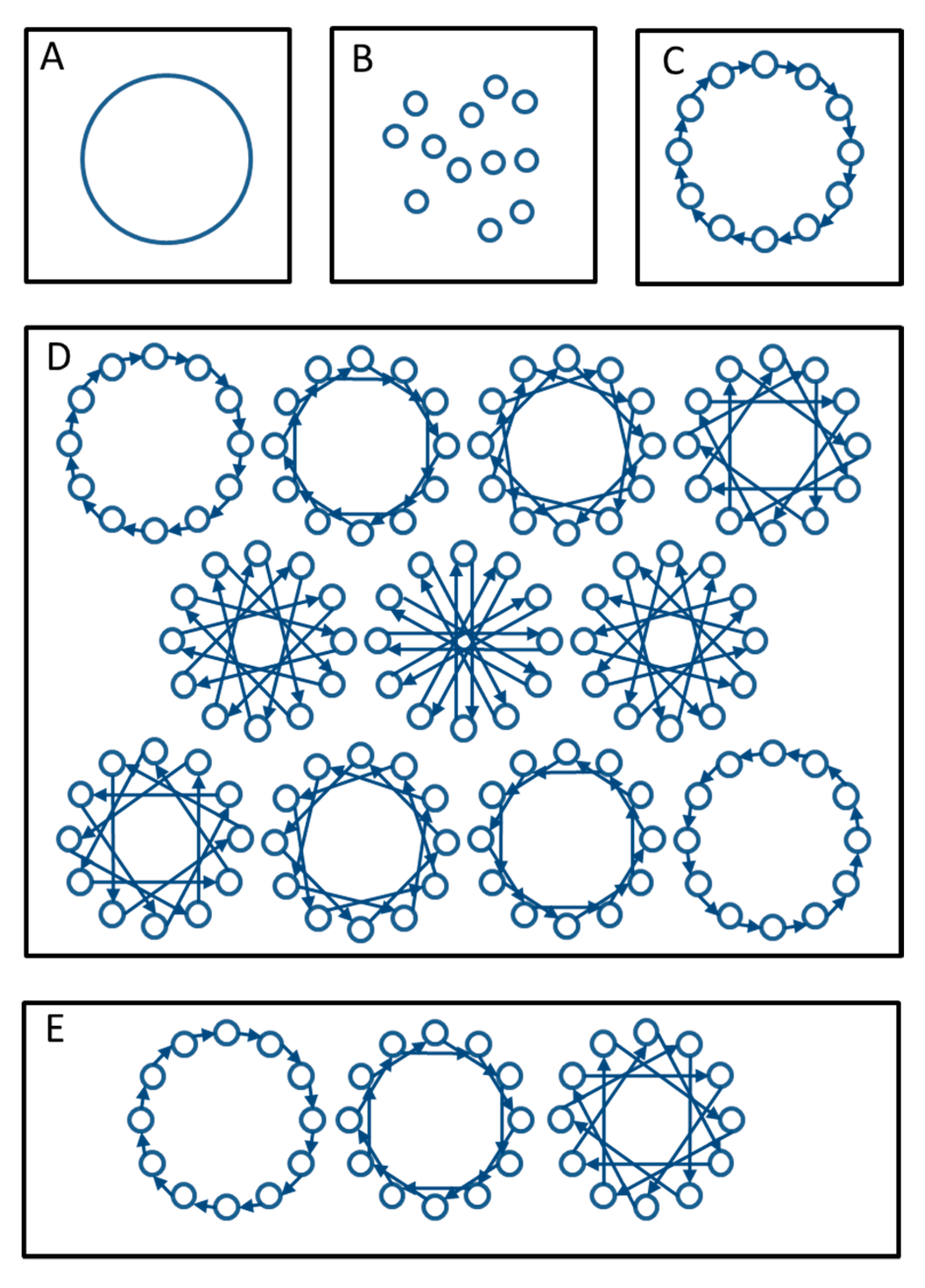
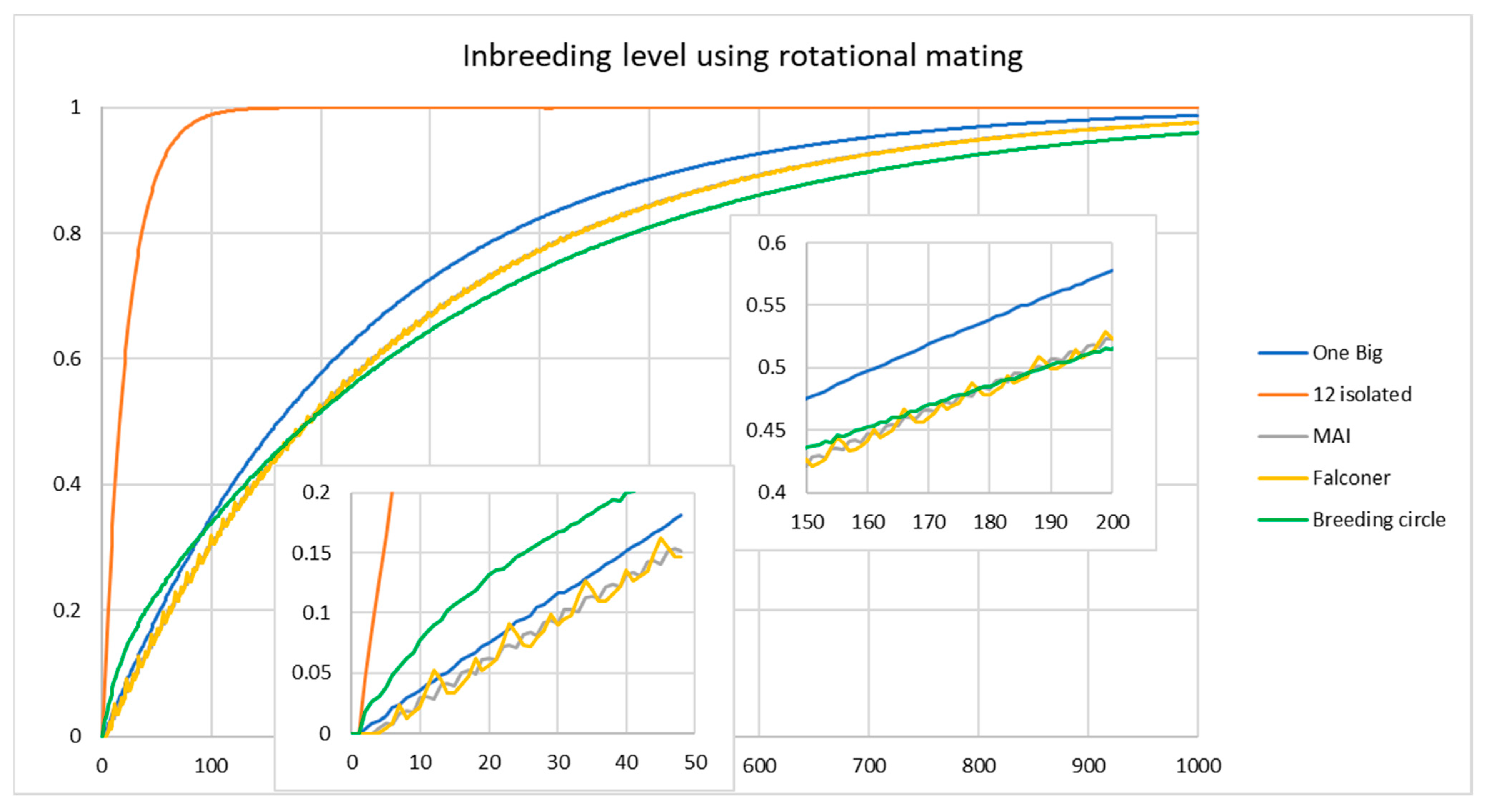
3.2. Rotational Mating to Manage Groups in Zoo Populations
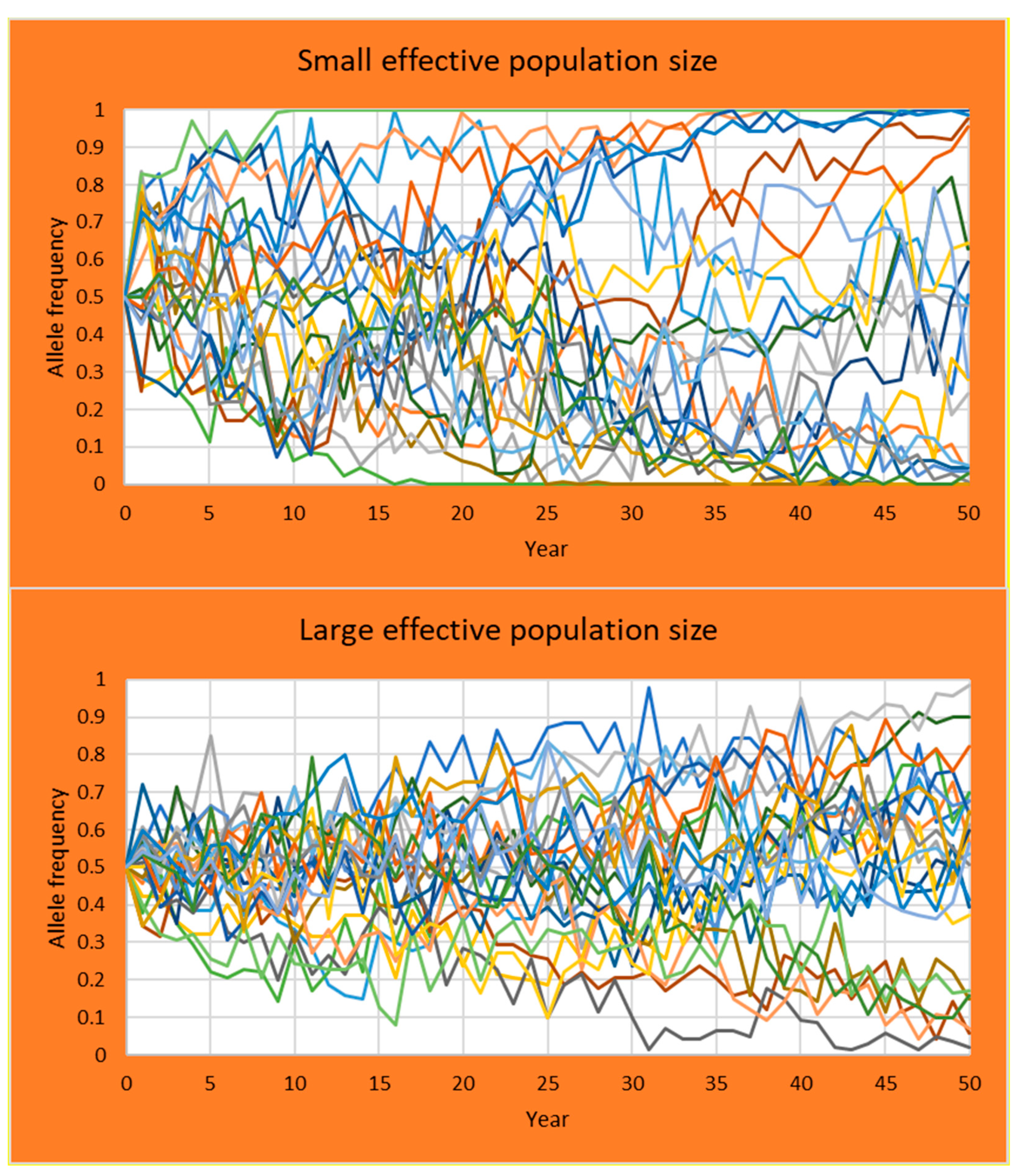
3.3. Student Practical on Allele Frequencies and Effective Population Size
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman Group: Harlow, UK, 1996; p. 464. [Google Scholar]
- Leroy, G.; Mary-Huard, T.; Verrier, E.; Danvy, S.; Charvolin, E.; Danchin-Burge, C. Methods to estimate effective population size using pedigree data: Examples in dog, sheep, cattle and horse. Genet. Sel. Evol. 2013, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Yonezawa, K. A comparison of four systems of group mating for avoiding inbreeding. Genet. Sel. Evol. 1996, 28, 141–159. [Google Scholar] [CrossRef]
- Boichard, D. Pedig: A fortran package for pedigree analysis suited for large populations. In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19 August 2002; pp. 13–28. [Google Scholar]
- Gutierrez, J.P.; Goyache, F. A note on ENDOG: A computer program for analysing pedigree information. J. Anim. Breed. Genet. 2005, 122, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Sargolzaei, M.; Iwaisaki, H.; Colleau, J. CFC: A tool for monitoring genetic diversity. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Brazil, 13–18 August 2006; pp. 27–28. [Google Scholar]
- Groeneveld, E.; Westhuizen, B.v.d.; Maiwashe, A.; Ferraz, J. POPREP: A generic report for population management. Genet. Mol. Res. 2009, 8, 1158–1178. [Google Scholar] [CrossRef] [PubMed]
- Sargolzaei, M.; Schenkel, F. QMSim: A large-scale genome simulator for livestock. Bioinformatics 2009, 25, 680–681. [Google Scholar] [CrossRef]
- Haller, B.C.; Messer, P.W. SLiM 3: Forward genetic simulations beyond the Wright–Fisher mode. Mol. Biol. Evol. 2019, 36, 632–637. [Google Scholar] [CrossRef]
- Lacy, R.C. Structure of the VORTEX simulation model for population viability analysis. Ecol. Bull. 2000, 48, 191–203. [Google Scholar]
- Meuwissen, T.H.E.; Luo, Z. Computing inbreeding coefficients in large populations. Genet. Sel. Evol. 1992, 24, 305. [Google Scholar] [CrossRef]
- Sargolzaei, M.; Iwaisaki, H.; Colleau, J.J. A fast algorithm for computing inbreeding coefficients in large populations. J. Anim. Breed. Genet. 2005, 122, 325–331. [Google Scholar] [CrossRef]
- Perez-Enciso, M. Use of the uncertain relationship matrix to compute effective population size. J. Anim. Breed. Genet. 1995, 112, 327–332. [Google Scholar] [CrossRef]
- Alderson, L. (Ed.) Genetic Conservation of Livestock; CAB International: Oxon, UK, 1990; p. 242. [Google Scholar]
- Derochambeau, H.; Chavalet, C. Minimizing inbreeding rates in small populations of domestic species. Genet. Sel. Evol. 1985, 17, 459–480. [Google Scholar] [CrossRef]
- Windig, J.J.; Verweij, M.J.W.; Oldenbroek, J.K. Reducing inbreeding rates with a breeding circle: Theory and practice in Veluws Heideschaap. J. Anim. Breed. Genet. 2019, 136, 51–62. [Google Scholar] [CrossRef]
- Kappelhof, J.; Windig, J.J. Controlling inbreeding rate in the European zoo population of Hamadryas baboons Papio hamadryas with a breeding circle. J. Zoo Aquar. Res. 2021, 9, 26–34. [Google Scholar] [CrossRef]
- Windig, J.J.; Doekes, H.P. Limits to genetic rescue by outcross in pedigree dogs. J. Anim. Breed. Genet. 2018, 135, 238–248. [Google Scholar] [CrossRef]
- Toro, M.A.; Villanueva, B.; Fernandez, F. The concept of effective population size loses its meaning in the context of optimal management of diversity using molecular markers. J. Anim. Breed. Genet. 2019, 134, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Windig, J.J.; Oldenbroek, J.K. Genetic management of Dutch golden retriever dogs with a simulation tool. J. Anim. Breed. Genet. 2015, 132, 428–440. [Google Scholar] [CrossRef]
- Windig, J.J.; Eding, H.; Moll, L.; Kaal, L. Effects on inbreeding of different strategies aimed at eliminating scrapie sensitivity alleles in rare sheep breeds in The Netherlands. Anim. Sci. 2004, 79, 11–20. [Google Scholar] [CrossRef]
- Windig, J.J.; Kaal, L. An effective rotational mating scheme for inbreeding reduction in captive populations illustrated by the rare sheep breed Kempisch Heideschaap. Animal 2008, 2, 1733–1741. [Google Scholar] [CrossRef]
- Windig, J.J.; Meuleman, H.; Kaal, L. Selection for scrapie resistance and simultaneous restriction of inbreeding in the rare sheep breed “Mergellander”. Prev. Vet. Med. 2007, 78, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, I.; Goyache, F.; Molina, A.; Valera, M.; Gutierrez, J.P. Application of individual increase in inbreeding to estimate realised effective sizes from real pedigrees. J. Anim. Breed. Genet. 2008, 125, 301–310. [Google Scholar] [CrossRef]
- Gutierrez, J.P.; Cervantes, I.; Goyache, F. Improving the estimation of realized effective population sizes in farm animals. J. Anim. Breed. Genet. 2009, 126, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Pook, T.; Schlather, M.; Simianer, H. MoBPS-Modular Breeding Program Simulator. G3 Genes Genomes Genet 2020, 10, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Strandberg, E.; Viklund, Å.; Windig, J.J.; Malm, S.; Lewis, T.; Laloë, D.; Leroy, G. Genetic improvement of canine hip dysplasia through sire selection across countries. Vet. J. 2019, 248, 18–24. [Google Scholar] [CrossRef] [PubMed]
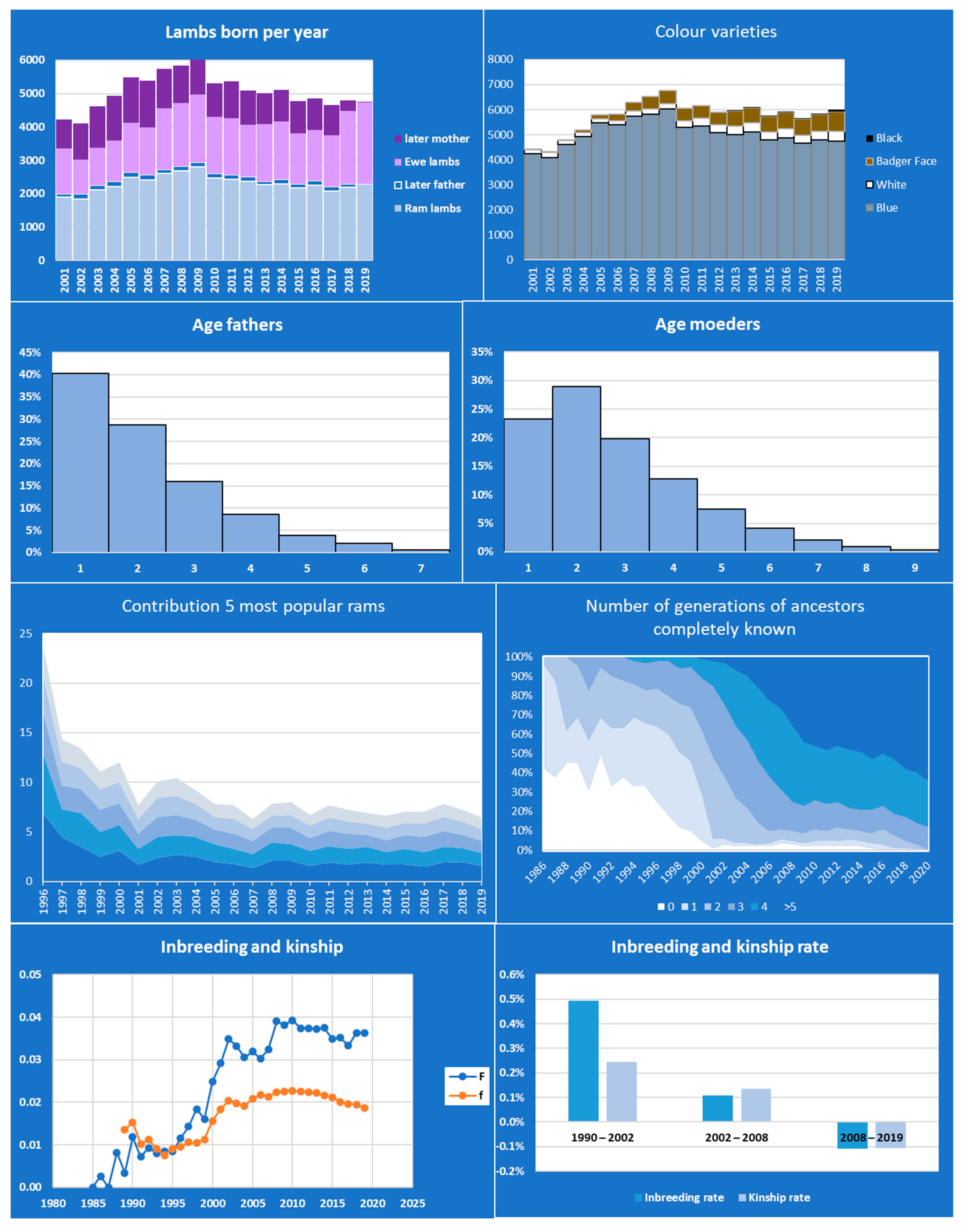
| Item | Description | Period | Columns |
|---|---|---|---|
| Population size | Number young born | Per year | Males, females, total not used and later used in breeding |
| Parents and offspring per year | Number of parents and offspring/parent per year | Per year | #mothers, #fathers, average litter size, #litter/father, #offspring/father |
| Offspring/parent per life | Average number of offspring per parent in its future lifetime per year | Per year | Males/Females, Mean, standard deviation, maximum |
| Litter size | Number of litters of a certain size per year | Per year | Litter size 1, 2, etc. |
| Age fathers | # of young born with fathers of a certain age | Per year | 1, 2, 3 etc. years old |
| Age mothers | # of young born with mothers of a certain age | Per year | 1, 2, 3 etc. years old |
| Generation interval | Average age of parents at time of birth | Per year | Males, females, parents |
| Pedigree depth | Generations of ancestors known | Per year | Generation equivalent, % with 0, 1, 2, 3, 4, >4 generations of ancestors completely known |
| Top-sires | Contribution of top sires to total number of offspring | Per year | # of fathers, contribution of 1st, 2nd,..., 10th most popular sire |
| Varieties | Number of young born | per year | Variety1, 2 etc. |
| Inbreeding and kinship | Average coefficients of young | per year | Inbreeding, kinship including and excluding self-kinships, kinship future parents, fathers, mothers |
| Inbreeding and kinship rates | Delta F | Entire period, + per 5 year | Year based, generation based, effective population size if deltaF > 0 |
| Sub-population numbers | Number of pups born | Per year | Subpopulation 1, 2 etc. |
| Descent of Sub-populations | Origin and number of parents for pups born in each subpopulation | Entire period | Subpopulation 1, 2 etc. |
| Sub-populations relatedness | Average relatedness between pups | Per year | For each combination of subpopulations |
| Summary for Pointer software | Summary of results to be used as input in pointer software | Last 6 years | #litter, breeding males and females per year, contribution top 4 sires, liter size and a parental age distribution |
| Parameters | Example | Description |
|---|---|---|
| number of years number of runs | 100, 25 | Less years if population goes extinct |
| Random seed | −123,456,789 | Starting value for pseudo random number generator |
| number of breeding animals | 10 males, 50 females | Will stay constant, unless not enough animals are born or genetic management or genetic defects limits numbers |
| number of litters/year | 25 | Will stay constant unless not enough parents available |
| Litter size distribution | 0.20 0.70 0.10 for litter size 1,2 and 3 | Will stay constant, but see under number of breeding animals |
| Age distribution | 0.75, 0.20 0.05 for Ages 1, 2 and 3 | |
| Number of top sires plus their contribution | 4, 0.50 | |
| number of subpoulations and size | 2 with sizes 2, 8 (males) and 20, 30 (females) | |
| Genome data: number of Loci, number of chromosomes, map length, mutation frequency | 10, 2, 1 Morgan 1 × 10−6 | Up to 32,768 Loci can be specified |
| Loci data: starting frequency, mortality, mortality heterozygote, first year when effective | 0.50, 100%, 0%, 0 | Can be specified for all loci or individual loci. Effect can be on fertility or survival, selection against carriers of alleles possible |
| Genetic management: restrictions on number of offspring, relatedness, inbreeding, Mean kinships or use of optimal contributions | 5 liters per sire per year | (Combinations of) options can be set on or off |
| Fertilization across subpopulations | 1.0 0.0 0.1 0.9 | On diagonal probability (or number) of litters sired by males from own subpopulation, off diagonal by males from other subpopulation. These can be varied between years. Example specifies that females of subpopulation 2 have 10% chance being fertilized by a male from 1st subpopulation |
| Migration between sub-populations | * 0 5 * | Off diagonal number (or prababilty) of animals migrating between subpopulations. * on diagonal indicates all other animals remain in their own subpopulation. Migration can be restricted to ages or sexes, can be varied over years. Example specifies that each year 5 animals migrate from subpopulation 1 to 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Windig, J.J.; Hulsegge, I. Retriever and Pointer: Software to Evaluate Inbreeding and Genetic Management in Captive Populations. Animals 2021, 11, 1332. https://doi.org/10.3390/ani11051332
Windig JJ, Hulsegge I. Retriever and Pointer: Software to Evaluate Inbreeding and Genetic Management in Captive Populations. Animals. 2021; 11(5):1332. https://doi.org/10.3390/ani11051332
Chicago/Turabian StyleWindig, Jack J., and Ina Hulsegge. 2021. "Retriever and Pointer: Software to Evaluate Inbreeding and Genetic Management in Captive Populations" Animals 11, no. 5: 1332. https://doi.org/10.3390/ani11051332
APA StyleWindig, J. J., & Hulsegge, I. (2021). Retriever and Pointer: Software to Evaluate Inbreeding and Genetic Management in Captive Populations. Animals, 11(5), 1332. https://doi.org/10.3390/ani11051332






