Carcass Traits and Meat Quality of Fat-Tailed Lambs Fed Rosemary Residues as a Part of Concentrate
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Design, Feeds and Animals
2.2. Animals and Feeding
2.3. Slaughter Procedure and Measurements
2.4. Carcass Cutting and Dissection
2.5. Meat Physical Properties Measurement
2.6. Meat Vitamin E and Total Phenolic Content (TPC) Analyses
2.7. Calculation and Statistical Analysis
3. Results
3.1. Feed Intake and Lamb’s Growth
3.2. Carcass Weights and Dressing Percentage
3.3. Non-Carcass Components
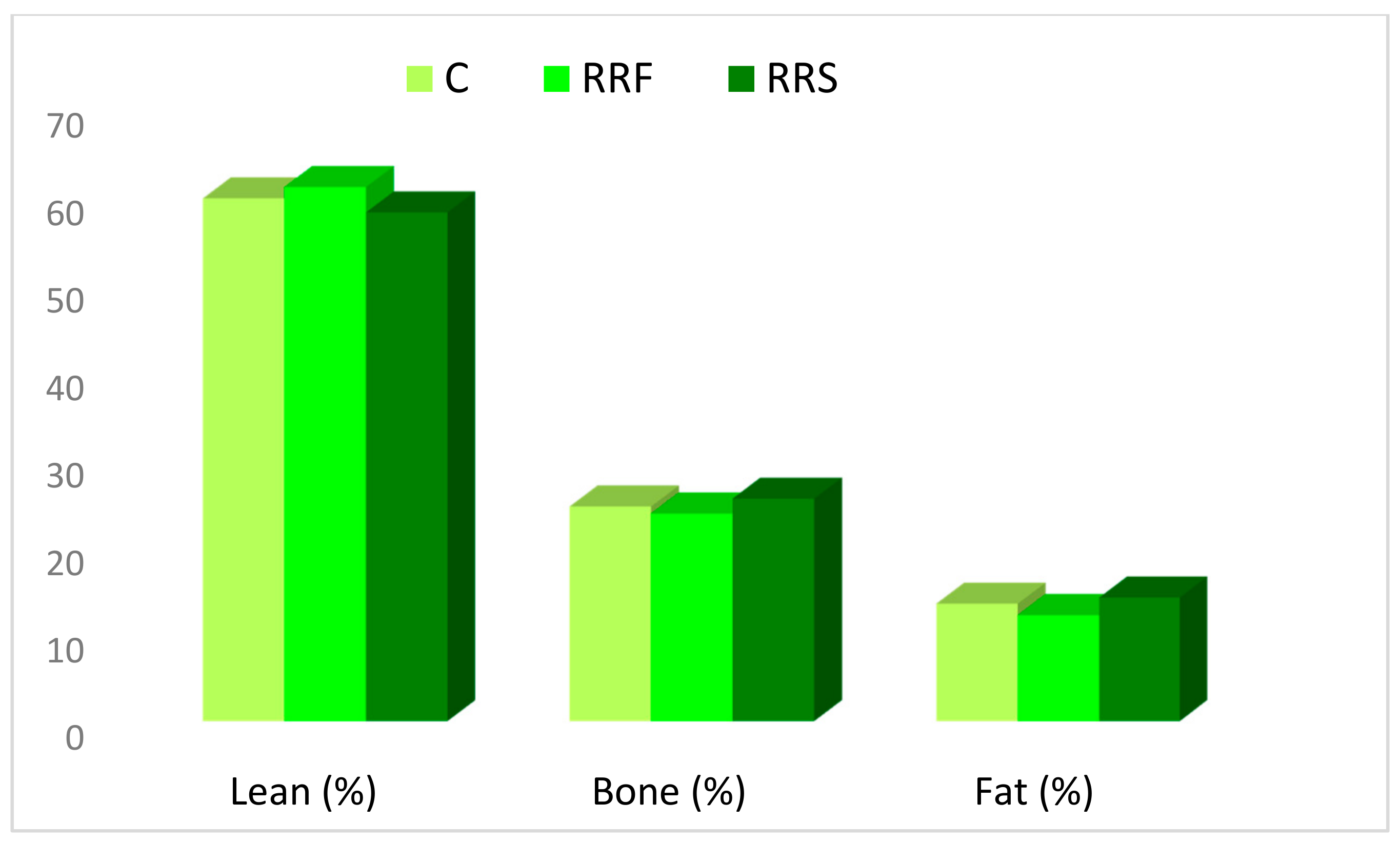
3.4. Carcass Sectional and Tissular Composition
3.5. Meat Quality
4. Discussion
4.1. Feed Intake and Growth Performance
4.2. Carcass Weights, Dressing Percentage and Non-Carcass Components
4.3. Carcass Sectional and Tissular Composition
4.4. Meat Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wreford, A.; Topp, C.F.E. Impacts of climate change on livestock and possible adaptations: A case study of the United Kingdom. Agric. Syst. 2020, 178, 102737. [Google Scholar]
- Mahouachi, M.; Atti, N.; Hajji, H. Use of spineless cactus (Opuntia ficus indica f. inermis) for dairy goats and growing kids: Impacts on milk production, kid’s growth and meat quality. Sci. World J. 2012, 4, 321567. [Google Scholar] [CrossRef]
- Obeidat, B.S.; Mahmoud, K.Z.; Maswadeh, J.A.; Bsoul, E.Y. Effects of feeding Atriplexhalimus L. on growth performance and carcass characteristics of fattening Awassi lambs. Small Rum. Res. 2016, 137, 65–70. [Google Scholar]
- Yagoubi, Y.; Hajji, H.; Smeti, S.; Mahouachi, M.; Kamoun, M.; Atti, N. Growth performance, carcass and non-carcass traits and meat quality of Barbarine lambs fed rosemary distillation residues. Animal 2018, 12, 2407–2414. [Google Scholar]
- Yagoubi, Y.; Joy, M.; Ripoll, G.; Mahouachi, M.; Bertolin, J.R.; Atti, N. Rosemary distillation residues reduce lipid oxidation, increase alpha-tocopherol content and improve fatty acid profile of lamb meat. Meat Sci. 2018, 136, 23–29. [Google Scholar]
- Tibaoui, S.; Smeti, S.; Essid, I.; Bertolin, J.R.; Joy, M.; Atti, N. Physicochemical characteristics, fatty acid profile, Alpha-Tocopherol content and lipid oxidation of meat from ewes fed different levels of distilled myrtle residues. Molecules 2020, 25, 4975. [Google Scholar] [CrossRef]
- Haak, L.; Raes, K.; Van Dyck, S.; De Smet, S. Effect of dietary rosemary and alpha-tocopherol acetate on the oxidative stability of raw and cooked pork following oxidized linseed oil administration. Meat Sci. 2008, 78, 239–247. [Google Scholar]
- Smeti, S.; Hajji, H.; Mekki, I.; Mahouachi, M.; Atti, N. Effects of dose and administration form of rosemary essential oils on meat quality and fatty acid profile of lamb. Small Rum. Res. 2018, 158, 62–68. [Google Scholar]
- Ito, N.; Fukushinma, S.; Hasegawa, A.; Shibata, M.; Ogiso, T. Carcinogenicity of butylated hydroxy anisole in F344 rats. J. Natl. Cancer Inst. 1983, 70, 343–347. [Google Scholar]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar]
- APIA. Aperçu Sur le Secteur Des Plantes Aromatiques et Médicinales “P.A.M”; Agro-Services: Tunis, Tunisia, 2003; 122p. [Google Scholar]
- Smeti, S.; Hajji, H.; Bouzid, K.; Abdelmoula, J.; Munoz, F.; Mahouachi, M.; Atti, N. Effects of Rosmarinus officinalis L. as essential oils or in form of leaves supplementation on goat’s production and metabolic statute. Trop. Anim. Health Prod. 2015, 47, 451–457. [Google Scholar]
- Ben Abdelmalek, Y.; Essid, I.; Smeti, S.; Atti, N. The anti-oxidant and antimicrobial effect of Rosmarinus officinalis L. distillation residues’ intake on cooked sausages from ewes fed linseed. Small Rumin. Res. 2018, 168, 87–93. [Google Scholar]
- Asadollahi, S.; Sari, M.; Erafanimajd, N.; Kiani, A. Supplementation of sugar beet pulp and roasted canola seed in a concentrate diet altered carcass traits, muscle (Longissimus Dorsi) composition and meat sensory properties of Arabian fattening lambs. Small Rumin. Res. 2017, 153, 95–102. [Google Scholar]
- Bonanno, A.; Tornambè, G.; Di Grigoli, A.; Genna, V.; Bellina, V.; Di Miceli, G.; Giambalvo, D. Effect of legume grains as a source of dietary protein on the quality of organic lamb meat. J. Sci. Food Agric. 2012, 92, 2870–2875. [Google Scholar]
- Atti, N.; Khaldi, G. Effects of slaughter weight of Barbary and noir of Thibar lambs on their carcass composition and meat qualities. Ann. INRAT 1988, 61, 24. [Google Scholar]
- Chauveau-Duriot, B.; Doreau, M.; Nozière, P.; Graulet, B. Simultaneous quantification of carotenoids, retinol and tocopherols in forages, bovine plasma and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 2010, 397, 777–790. [Google Scholar] [PubMed]
- Vázquez, C.V.; Rojas, M.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Martínez, R.A.F.; Montemayor, H.M.A. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin–Ciocalteu method. Food Chem. 2015, 176, 480–486. [Google Scholar]
- SAS. Statistical Analysis System, User’s Guide. Statistical, Version 7th ed.; SAS. Inst. Inc.: Cary, NC, USA, 2004. [Google Scholar]
- Atti, N.; Mahouachi, M. Effects of rearing system and nitrogen source on lamb growth, meat characteristics and fatty acid composition. Meat Sci. 2009, 81, 344–348. [Google Scholar]
- Polidori, P.; Quagliarini, C.; Vincenzetti, S. Use of faba bean as a replacer of soybean meal in diet of Fabrianese lambs. J. Food Sci. Technol. 2018, 3, 350–360. [Google Scholar]
- Sents, A.E.; Walters, L.E.; Whiterman, J.V. Performance and carcass characteristics of ram lambs slaughtered at different weights. J. Anim. Sci. 1982, 55, 1360–1369. [Google Scholar]
- Atti, N.; Ben Salem, H.; Priolo, A. Effects of polyethylene glycol in concentrate or feed blocks on carcass composition and offal weight of Barbarine lambs fed Acacia cyanophylla Lindl Foliage. Anim. Res. 2003, 52, 363–375. [Google Scholar] [CrossRef][Green Version]
- Atti, N.; Mahouachi, M. The effects of diet, slaughter weight, and docking on growth, carcass composition and meat quality of fat-tailed Barbarine lambs: A review. Trop. Anim. Health Prod. 2011, 43, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Hajji, H.; Smeti, S.; Ben Hammouda, M.; Atti, N. Effect of protein level on growth performance, non-carcass components and carcass characteristics of young sheep from three breeds. Anim. Prod. Sci. 2016, 6, 2115–2121. [Google Scholar] [CrossRef]
- Tibaoui, S.; Hajji, H.; Smeti, S.; Mekki, I.; Essid, I.; Atti, N. Effects of distillated myrtle (Myrtus communis L.) leaves’ intake on cull ewes’ body weight gain, carcass composition and meat quality. Span. J. Agric. Res. 2020, 18. [Google Scholar] [CrossRef]
- Papi, N.; Mostafa-Tehrani, A.; Amanlou, H.; Memarian, M. Effects of dietary forage-to-concentrate ratios on performance and carcass characteristics of growing fat-tailed lambs. Anim. Feed. Sci. Technol. 2011, 163, 93–98. [Google Scholar] [CrossRef]
- Smeti, S.; Atti, N.; Mahouachi, M. Effects of finishing lambs in rich aromatic plant pasture or in feedlot on lamb growth and meat quality. J. Appl. Anim. Res. 2014, 42, 297–303. [Google Scholar] [CrossRef]
- Kamalzadeh, A.; Koops, W.J.; van Bruchem Bangma, G.A.; Tamminga, S.; Zwart, D. Feed quality restriction and compensatory growth in growing sheep: Development of body organs. Small Rum. Res. 1998, 29, 71–82. [Google Scholar] [CrossRef]
- Mahouachi, M.; Atti, N. Effects of restricted feeding and re-feeding of Barbarine lambs: Intake, growth and non-carcass components. Anim. Sci. 2005, 81, 305–312. [Google Scholar] [CrossRef]
- Prud’hon, M. La croissance globale de l’agneau: Ses caractéristiques et ses lois. In Deuxième Journées de la Recherché Ovine et Caprine: Croissance, Engraissement et Qualité des Carcasses D’agneaux et de Chevreaux, INRA-ITOVIC; INRA: Paris, France, 1976; pp. 6–20. [Google Scholar]
- Ben Abdelmalek, Y.; Smeti, S.; Mekki, I.; Hajji, H.; Essid, I.; Atti, N. Rehabilitation of Barbarine cull ewes using rosemary residues and linseed: Effect on weight gain, carcass and non-carcass characteristics and meat quality. Animal Int. J. Anim. Biosci. 2019, 13, 879–887. [Google Scholar] [CrossRef]
- Reynolds, C.K. Quantitative aspects of liver metabolism in ruminants. In Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction; Engelhardt, W.V., Leonhard-mark, S., Breves, G., Giesecke, D., Eds.; Ferdinand Enke Verlag: Stuttgart, Germany, 1995; pp. 351–371. [Google Scholar]
- Kabbali, A.; Johnson, D.W.; Goodrich, R.D.; Allen, C.E. Effects of undernutrition and refeeding on weights of body parts and chemical components of growing Moroccan lambs. J. Anim. Sci. 1992, 70, 2859–2865. [Google Scholar] [CrossRef]
- Boccard, R.; Dumont, B.L. Etude de la production de la viande chez les ovins. Variation de l’importance relative de différentes régions corporelles de l’agneau de boucherie. Ann. Zootech. 1960, 9, 385–398. [Google Scholar] [CrossRef][Green Version]
- Joy, M.; Ripoll, G.; Delfa, R. Effects of feeding system on carcass and non-carcass composition of Churra Tensina light lambs. Small Rum. Res. 2008, 78, 123–133. [Google Scholar] [CrossRef]
- Murphy, T.A.; Loerch, S.C.; McClure, K.E.; Solomon, M.B. Effects of restricted feeding on growth performance and carcass composition of lambs. J. Anim. Sci. 1994, 72, 3131–3137. [Google Scholar] [CrossRef] [PubMed]
- Simitzis, P.E.; Deligeorgis, S.G.; Bizelis, J.A.; Dardamani, A.; Theodosiou, I.; Fegeros, K. Effect of dietary oregano oil supplementation on lamb meat characteristics. Meat Sci. 2008, 79, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Smeti, S.; Tibaoui, S.; Bertolin, J.R.; Yagoubi, Y.; Mekki, I.; Joy, M.; Atti, N. Effects of myrtle (Myrtus communis L.) essential oils as dietary antioxidant supplementation on carcass and meat quality of goat meat. J. Anim. Physiol. Anim. Nutr. 2020, in press. [Google Scholar] [CrossRef]
- Khliji, S.; Van de Ven, R.; Lamb, T.A.; Lanza, M.; Hopkins, D.L. Relationship between consumer ranking of lamb colour and objective measures of colour. Meat Sci. 2010, 85, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Simitzis, P.E.; Symeon, G.K.; Charismiadou, M.A.; Bizelis, J.A.; Deligeorgis, S.G. The effects of dietary oregano oil supplementation on pigment characteristics. Meat Sci. 2010, 84, 670–676. [Google Scholar] [CrossRef]
- Stinnett, J.D. Nutrition and the Immune Response; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Cook-Mills, J.M. Vitamin E Isoform-Specific Functions in Allergic Inflammation and Asthma. In Nutrition and Functional Foods for Healthy Aging; Elsevier Inc: Cambridge, MA, USA, 2017; Chapter 17; pp. 167–188. [Google Scholar]
- Traber, M.G. Vitamin E: Metabolism and Requirements. In Encyclopedia of Human Nutrition; Elsevier: Cambridge, MA, USA, 2013; pp. 383–389. [Google Scholar]
- Gomez-Fernandez, J.C.; Villalain, J.; Aranda, F.J.; Ortiz, A.; Micol, V.; Coutinho, A.; Berberan-Santos, M.N.; Prieto, M.J. Localization of alpha-tocopherol in membranes. Ann. N. Y. Acad. Sci. 1989, 570, 109–120. [Google Scholar] [CrossRef]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Bellés, M.; Del Mar Campo, M.; Roncalés, P.; Beltran, J.A. Supranutritional doses of vitamin E to improve lamb meat quality. Meat Sci. 2019, 149, 14–23. [Google Scholar] [CrossRef]
- Weiss, W.P.; Smith, K.L.; Hogan, J.S.; Steiner, T.E. Effect of forage to concentrate ratio on disappearance of vitamins A and E during in vitro ruminal fermentation. J. Dairy Sci. 1995, 78, 1837–1842. [Google Scholar] [CrossRef]
- Hymøller, L.; Jensen, S.K. Stability in the rumen and effect on plasma status of single oral doses of vitamin D and vitamin E in high-yielding dairy cows. J. Dairy Sci. 2010, 93, 5748–5757. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C.; Jensen, S.K. α-tocopherol incorporation in mitochondria and microsomes upon supranutritional vitamin E supplementation. Genes Nutr. 2012, 7, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, A. Absortion, transport and tissue delivery of vitamin E. Mol. Asp. Med. 2007, 28, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Ponnampalam, E.N.; Burnett, V.F.; Norng, S.; Warmer, R.D.; Jacobs, J.L. Vitamin E and fatty acid content of lamb meat from perennial or annual pasture systems with supplements. Anim. Prod. Sci. 2012, 52, 255–262. [Google Scholar] [CrossRef]
- Hopkins, D.L.; Lamb, T.A.; Kerr, M.J.; van de Ven, R.J.; Ponnampalam, E.N. Examination of the effect of ageing and temperature at rigor on colour stability of lamb meat. Meat Sci. 2013, 95, 311–316. [Google Scholar] [CrossRef]
- Monino, M.I.; Martínez, C.; Sotomayor, J.A.; Lafuente, A.; Jordán, M.J. Polyphenolic transmission to segureño lamb meat from ewes dietary supplemented with the distillate from rosemary (Rosmarinus officinalis) leaves. J. Agric. Food Chem. 2008, 56, 3363–3367. [Google Scholar] [CrossRef]

| Item | Oat Hay | RR | Standard Concentrate | RRF | RRS |
|---|---|---|---|---|---|
| Dry matter (%) | 91.95 | 84.89 | 88.69 | 92.35 | 90.67 |
| Crude Protein (%DM) | 5.47 | 7.31 | 16.33 | 17.30 | 17.38 |
| Organic Matter (%DM) | 93.04 | 92.62 | 80.65 | 89.56 | 91.22 |
| Neutral detergent fiber (%DM) | 69.15 | 38.53 | 20.31 | 29.47 | 34.01 |
| α-Tocopherol (µg/g DM) | 4.42 | 217.20 | 0.45 | 52.71 | 62.98 |
| γ-Tocopherol (µg/g DM) | 3.97 | 3.78 | 0.78 | 11.85 | 7.38 |
| Item | C | RRF | RRS | SEM | p |
|---|---|---|---|---|---|
| Slaughter body weight (kg) | 25.75 | 24.25 | 25.12 | 1.91 | 0.67 |
| Empty body weight(kg) | 20.37 | 19.62 | 20.12 | 0.80 | 0.80 |
| Hot carcass weight (kg) | 11.28 | 10.61 | 11.01 | 0.54 | 0.71 |
| Cold carcass weight (kg) | 10.98 | 10.40 | 10.91 | 0.46 | 0.68 |
| Commercial dressing percentage (%) | 43.95 | 43.84 | 44.26 | 0.38 | 0.90 |
| Real dressing percentage (%) | 54.23 | 53.33 | 54.39 | 0.39 | 0.51 |
| Organs | C | RRF | RRS | SEM | p |
|---|---|---|---|---|---|
| Skin (kg) | 2.90 | 2.66 | 2.77 | 0.14 | 0.51 |
| Skin (%) | 14.38 | 13.65 | 13.78 | 0.24 | 0.44 |
| Head (kg) | 1.49 | 1.39 | 1.41 | 0.06 | 0.43 |
| Head (%) | 7.39 | 7.15 | 7.07 | 0.10 | 0.45 |
| Gut (kg) | 5.48 | 4.71 | 4.85 | 0.51 | 0.52 |
| Gut (%) | 26.76 | 24.33 | 24.07 | 1.18 | 0.59 |
| Red organs (g) | 874.00 | 749.89 | 719.49 | 0.03 | 0.60 |
| Red organs (%) | 4.34 | 3.92 | 3.73 | 0.34 | 0.76 |
| Liver (g) | 384.00 | 369.63 | 403.25 | 0.02 | 0.52 |
| Liver (%) | 1.90 | 1.90 | 2.02 | 0.05 | 0.63 |
| Testis (g) | 73.06 | 48.63 | 62.21 | 0.02 | 0.54 |
| Testis (%) | 0.34 | 0.24 | 0.30 | 0.03 | 0.56 |
| Meat Physical Parameters | C | RRF | RRS | SEM | p |
|---|---|---|---|---|---|
| Initial pH | 6.33 a | 6.18 ab | 5.96 b | 0.05 | 0.03 |
| Ultimate pH | 5.86 a | 5.59 b | 5.51 b | 0.03 | 0.01 |
| dpH | −0.46 | −0.59 | −0.44 | 0.04 | 0.39 |
| Water cooking loss | 21.96 | 23.21 | 20.29 | 0.91 | 0.43 |
| Lightness (L*) | 43.94 | 42.65 | 43.33 | 0.81 | 0.81 |
| Redness (a*) | 17.05 | 16.92 | 16.81 | 0.32 | 0.95 |
| Yellowness (b*) | 4.05 | 4.39 | 3.04 | 0.27 | 0.13 |
| Chroma (C*) | 17.64 | 17.50 | 17.11 | 0.33 | 0.83 |
| Hue angle (H*) | 13.07 ab | 14.41 a | 10.09 b | 0.74 | 0.07 |
| Item | C | RRF | RRS | SEM | p-Value |
|---|---|---|---|---|---|
| α-tocopherol (μg/g DM) | 3.36 b | 6.48 a | 6.32 a | 0.12 | 0.001 |
| Total phenolic content | 51.33 b | 60.34 a | 60.29 a | 2.11 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagoubi, Y.; Smeti, S.; Ben Saïd, S.; Srihi, H.; Mekki, I.; Mahouachi, M.; Atti, N. Carcass Traits and Meat Quality of Fat-Tailed Lambs Fed Rosemary Residues as a Part of Concentrate. Animals 2021, 11, 655. https://doi.org/10.3390/ani11030655
Yagoubi Y, Smeti S, Ben Saïd S, Srihi H, Mekki I, Mahouachi M, Atti N. Carcass Traits and Meat Quality of Fat-Tailed Lambs Fed Rosemary Residues as a Part of Concentrate. Animals. 2021; 11(3):655. https://doi.org/10.3390/ani11030655
Chicago/Turabian StyleYagoubi, Yathreb, Samir Smeti, Samia Ben Saïd, Houssem Srihi, Ilyes Mekki, Mokhtar Mahouachi, and Naziha Atti. 2021. "Carcass Traits and Meat Quality of Fat-Tailed Lambs Fed Rosemary Residues as a Part of Concentrate" Animals 11, no. 3: 655. https://doi.org/10.3390/ani11030655
APA StyleYagoubi, Y., Smeti, S., Ben Saïd, S., Srihi, H., Mekki, I., Mahouachi, M., & Atti, N. (2021). Carcass Traits and Meat Quality of Fat-Tailed Lambs Fed Rosemary Residues as a Part of Concentrate. Animals, 11(3), 655. https://doi.org/10.3390/ani11030655





