Canid Reproductive Biology: Norm and Unique Aspects in Strategies and Mechanisms
Abstract
Simple Summary
Abstract
1. Introduction
2. Canid Reproductive Biology
2.1. Reproductive Seasonality
2.2. Gametogenesis and Embryogenesis
3. Assisted Reproductive Technologies
3.1. Genome Rescue
3.2. Cryopreservation
3.3. Estrus Induction
3.4. Artificial Insemination
3.5. In Vitro Oocyte Maturation and Fertilization
3.6. Cloning
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wildt, D.E.; Wemmer, C. Sex and wildlife: The role of reproductive science in conservation. Biodivers. Conserv. 1999, 8, 965–976. [Google Scholar] [CrossRef]
- Wildt, D.; Ellis, S.; Howard, J. Linkage of reproductive sciences: From ’quick fix’ to ’integrated’ conservation. J. Reprod. Fertil. Suppl. 2001, 57, 295–307. [Google Scholar] [PubMed]
- Wildt, D.E.; Comizzoli, P.; Pukazhenthi, B.; Songsasen, N. Lessons from biodiversity—the value of nontraditional species to advance reproductive science, conservation, and human health. Mol. Reprod. Dev. 2010, 77, 397–409. [Google Scholar] [CrossRef]
- Smith, B.P.; Cairns, K.M.; Adams, J.W.; Newsome, T.M.; Fillios, M.; Deaux, E.C.; Parr, W.C.; Letnic, M.; Van Eeden, L.M.; Appleby, R.G. Taxonomic status of the Australian dingo: The case for Canis dingo Meyer, 1793. Zootaxa 2019, 4564, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Lord, K.; Feinstein, M.; Smith, B.; Coppinger, R. Variation in reproductive traits of members of the genus Canis with special attention to the domestic dog (Canis familiaris). Behav. Process. 2013, 92, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P. Endocrinologic control of normal canine ovarian function. Reprod. Domest. Anim. 2009, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.W.; McCann, J.P.; Temple, M. Biology and endocrinology of ovulation, pregnancy and parturition in the dog. J. Reprod. Fertil. Suppl. 1989, 39, 3–25. [Google Scholar]
- Concannon, P.W. Reproductive cycles of the domestic bitch. Anim. Reprod. Sci. 2011, 124, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Songsasen, N.; Rodden, M.; Brown, J.L.; Wildt, D.E. Patterns of fecal gonadal hormone metabolites in the maned wolf (Chrysocyon brachyurus). Theriogenology 2006, 66, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Velloso, A.L.; Wasser, S.K.; Monfort, S.L.; Dietz, J.M. Longitudinal fecal steroid excretion in maned wolves (Chrysocyon brachyurus). Gen. Comp. Endocrinol. 1998, 112, 96–107. [Google Scholar] [CrossRef]
- Souza, N.P.; Furtado, P.V.; da Paz, R.R. Non-invasive monitoring of the estrous cycle in captive crab-eating foxes (Cerdocyon thous). Theriogenology 2012, 77, 233–239. [Google Scholar] [CrossRef]
- Valdespino, C.; Asa, C.S.; Bauman, J.E. Estrous cycles, copulation, and pregnancy in the fennec fox (Vulpes zerda). J. Mammal. 2002, 83, 99–109. [Google Scholar] [CrossRef]
- Van Den Berghe, F.; Paris, D.; Van Soom, A.; Rijsselaere, T.; Van der Weyde, L.; Bertschinger, H.J.; Paris, M. Reproduction in the endangered African wild dog: Basic physiology, reproductive suppression and possible benefits of artificial insemination. Anim. Reprod. Sci. 2012, 133, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asa, C.S.; Valdespino, C. Canid reproductive biology: An integration of proximate mechanisms and ultimate causes. Am. Zool. 1998, 38, 251–259. [Google Scholar] [CrossRef]
- DeMatteo, K.E.; Porton, I.J.; Kleiman, D.G.; Asa, C.S. The effect of the male bush dog (Speothos venaticus) on the female reproductive cycle. J. Mammal. 2006, 87, 723–732. [Google Scholar] [CrossRef]
- Durbin, L.; Venkataraman, A.; Hedges, S.; Duckworth, W. Dhole (Cuon alpinus). In Canids: Foxes, Wolves, Jackals and Dogs; Sillero-Zubiri, C., Hoffmann, M., Macdonald, D.W., Eds.; IUCN/SSC Canid Specialist Group: Gland, Switzerland, 2004; pp. 210–219. [Google Scholar]
- Khonmee, J.; Rojanasthien, S.; Thitaram, C.; Sumretprasong, J.; Aunsusin, A.; Chaisongkram, C.; Songsasen, N. Non-invasive endocrine monitoring indicates seasonal variations in gonadal hormone metabolites in dholes (Cuon alpinus). Conserv. Physiol. 2017, 5, cox001. [Google Scholar] [CrossRef] [PubMed]
- Koler-Matznick, J.; Yates, B.; Bulmer, S.; Brisbin, I.L., Jr. The New Guinea singing dog: Its status and scientific importance. Aust. Mammal. 2007, 29, 47–56. [Google Scholar] [CrossRef]
- Asa, C.S.; Bauman, J.E.; Coonan, T.J.; Gray, M.M. Evidence for induced estrus or ovulation in a canid, the island fox (Urocyon littoralis). J. Mammal. 2007, 88, 436–440. [Google Scholar] [CrossRef]
- Johnson, A.E.M.; Freeman, E.W.; Colgin, M.; McDonough, C.; Songsasen, N. Induction of ovarian activity and ovulation in an induced ovulator, the maned wolf (Chrysocyon brachyurus), using GnRH agonist and recombinant LH. Theriogenology 2014, 82, 71–79. [Google Scholar] [CrossRef]
- Jones, M.K.; Reiter, L.E.; Gilmore, M.P.; Freeman, E.W.; Songsasen, N. Physiological impacts of housing maned wolves (Chrysocyon brachyurus) with female relatives or unrelated males. Gen. Comp. Endocrinol. 2018, 267, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kester, M.E.; Freeman, E.W.; Songsasen, N.; Huff, T.B. Automated headspace solid-phase microextraction of urinary VOCs from eleven maned wolves (Chrysocyon brachyurus): A recursive workflow for GC–MS analysis. In Chemical Signals in Vertebrates 13; Schulete, B.A., Goodwin, T.E., Ferkin, M.H., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 477–498. [Google Scholar] [CrossRef]
- Concannon, P. Research challenges in endocrine aspects of canine ovarian cycles. Reprod. Domest. Anim. 2012, 47, 6–12. [Google Scholar] [CrossRef]
- Mondain-Monval, M.; Bonnin, M.; Canivenc, R.; Scholler, R. Heterologous radioimmunoassay of fox LH: Levels during the reproductive season and the anoestrus of the red fox (Vulpes vulpes L.). Gen. Comp. Endocrinol. 1984, 55, 125–132. [Google Scholar] [CrossRef]
- Farstad, W.; Mondain-Monval, M.; Hyttel, P.; Smith, A.; Markeng, D. Periovulatory endocrinology and oocyte maturation in unmated mature blue fox vixens (Alopex lagopus). Acta Vet. Scand. 1989, 30, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.; Castracane, V.; Temple, M.; Montanez, A. Endocrine control of ovarian function in dogs and other carnivores. Anim. Reprod. 2009, 6, 172–193. [Google Scholar]
- Johannes, J.E. The Basenji annual estrus: Controlled by short-day photoperiod. Basenji 2003, 39, 12–13. [Google Scholar]
- Mech, L.D. Breeding season of wolves, Canis lupus, in relation to latitude. Can. Field-Nat. 2002, 116, 139–140. [Google Scholar]
- Walker, S.; Waddell, W.; Goodrowe, K. Reproductive endocrine patterns in captive female and male red wolves (Canis rufus) assessed by fecal and serum hormone analysis. Zoo Biol. 2002, 21, 321–335. [Google Scholar] [CrossRef]
- Minter, L.; DeLiberto, T. Seasonal variation in serum testosterone, testicular volume, and semen characteristics in the coyote (Canis latrans). Theriogenology 2008, 69, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Mondain-Monval, M.; Dutourne, B.; Bonnin-Laffargue, M.; Canivenc, R.; Scholler, R. Ovarian activity during the anoestrus and the reproductive season of the red fox (Vulpes vulpes L.). J. Steroid Biochem. 1977, 8, 761–769. [Google Scholar] [CrossRef]
- Maia, O.; Jácomo, A.; Bringel, B.; Kashivakura, C.; Oliveira, C.; Teodoro, L.; Silveira, L.; Teixeira da Costa, M.; Malta, M.; Furtado, M. Comparison of serum hormone levels of captive and free-living maned wolves Chrysocyon brachyurus. Braz. J. Med. Biol. Res. 2008, 41, 176–179. [Google Scholar] [CrossRef]
- Faria-Corrêa, M.; Balbueno, R.A.; Vieira, E.M.; de Freitas, T.R. Activity, habitat use, density, and reproductive biology of the crab-eating fox (Cerdocyon thous) and comparison with the pampas fox (Lycalopex gymnocercus) in a Restinga area in the southern Brazilian Atlantic Forest. Mamm. Biol. 2009, 74, 220–229. [Google Scholar] [CrossRef]
- Porton, I.J.; Kleiman, D.G.; Rodden, M. Aseasonality of bush dog reproduction and the influence of social factors on the estrous cycle. J. Mammal. 1987, 68, 867–871. [Google Scholar] [CrossRef]
- Catling, P. Seasonal variation in plasma testosterone and the testis in captive male dingoes, Canis familiaries dingo. Aust. J. Zool. 1979, 27, 939–944. [Google Scholar] [CrossRef]
- Catling, P.; Corbett, L.; Newsome, A. Reproduction in captive and wild dingoes (Canis familiaris dingo) in temperate and arid environments of Australia. Wildl. Res. 1992, 19, 195–209. [Google Scholar] [CrossRef]
- Maia, O.; Gouveia, A. Birth and mortality of maned wolves Chrysocyon brachyurus (Illiger, 1811) in captivity. Braz. J. Biol. 2002, 62, 25–32. [Google Scholar] [CrossRef][Green Version]
- Walton, J.C.; Weil, Z.M.; Nelson, R.J. Influence of photoperiod on hormones, behavior, and immune function. Front. Neuroendocrinol. 2011, 32, 303–319. [Google Scholar] [CrossRef]
- Forsberg, M.; Madej, A. Effects of melatonin implants on plasma concentrations of testosterone, thyroxine and prolactin in the male silver fox (Vulpes vulpes). Reproduction 1990, 89, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Asikainen, J.; Mustonen, A.M.; Hyvärinen, H.; Nieminen, P. Seasonal reproductive endocrine profile of the raccoon dog (Nyctereutes procyonoides)—effects of melatonin and food deprivation. J. Exp. Zool. A Comp. Exp. Biol. 2003, 299, 180–187. [Google Scholar] [CrossRef]
- Nieminen, P.; Pyykönen, T.; Asikainen, J.; Mononen, J.; Mustonen, A.-M. Effects of fasting and exogenous melatonin on annual rhythms in the blue fox (Alopex lagopus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 139, 183–197. [Google Scholar] [CrossRef]
- Forsberg, M.; Fougner, J.; Hofmo, P.; Madej, M.; Einarsson, E. Photoperiodic regulation of reproduction in the male silver fox (Vulpes vulpes). Reproduction 1989, 87, 115–123. [Google Scholar] [CrossRef][Green Version]
- Asa, C.S.; Seal, U.S.; Letellier, M.; Plotka, E.D.; Peterson, E.K. Pinealectomy or superior cervical ganglionectomy do not alter reproduction in the wolf (Canis lupus). Biol. Reprod. 1987, 37, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sillero-Zubiri, C.; Johnson, P.J.; Macdonald, D.W. A hypothesis for breeding synchrony in Ethiopian wolves (Canis simensis). J. Mammal. 1998, 79, 853–858. [Google Scholar] [CrossRef]
- van Kesteren, F.; Paris, M.; Macdonald, D.W.; Millar, R.; Argaw, K.; Johnson, P.J.; Farstad, W.; Sillero-Zubiri, C. The physiology of cooperative breeding in a rare social canid; sex, suppression and pseudopregnancy in female Ethiopian wolves. Physiol. Behav. 2013, 122, 39–45. [Google Scholar] [CrossRef] [PubMed]
- McNutt, J.; Groom, R.; Woodroffe, R. Ambient temperature provides an adaptive explanation for seasonal reproduction in a tropical mammal. J. Zool. 2019, 309, 153–160. [Google Scholar] [CrossRef]
- Comizzoli, P.; Crosier, A.E.; Songsasen, N.; Gunther, M.S.; Howard, J.G.; Wildt, D.E. Advances in reproductive science for wild carnivore conservation. Reprod. Domest. Anim. 2009, 44, 47–52. [Google Scholar] [CrossRef]
- Jewgenow, K.; Songsasen, N. Reproduction and advances in reproductive studies in carnivores. In Reproductive Sciences in Animal Conservation; Wolt, W.V., Ed.; Springer Science+Business: New York, NY, USA, 2014; pp. 205–239. [Google Scholar] [CrossRef]
- Rudert, S.; Brown, J.; Ganslosser, U.; Möbius, G.; Songsasen, N. Activity pattern, reproductive behaviors and gonadal hormones in the raccoon dog (Nyctereutes procyonoides). Zoo Biol. 2011, 30, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Medan, M.S.; Xu, M.; Tsubota, T.; Watanabe, G.; Taya, K. Seasonal changes in immunolocalization of inhibin/activin subunits and testicular activity in wild male raccoon dogs (Nyctereutes procyonoides). J. Reprod. Dev. 2006. [Google Scholar] [CrossRef][Green Version]
- Van den Berghe, F.; Paris, M.C.; Sarnyai, Z.; Briggs, M.B.; Millar, R.P.; Ganswindt, A.; Paris, D.B. Social rank does not affect sperm quality in male African wild dogs (Lycaon pictus). Reprod. Fertil. Dev. 2019, 31, 875–887. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Liu, G.; Yang, X.; Zhang, J. The complete mitochondrial genome of the Tibetan fox (Vulpes ferrilata) and implications for the phylogeny of Canidae. C. R. Biol. 2016, 339, 68–77. [Google Scholar] [CrossRef]
- Sillero-Zubiri, C. Canids: Foxes, Wolves, Jackals and Dogs: Status Survey and Conservation Action Plan; IUCN/SSC Canid Specialist Group: Gland, Switzerland, 2004; Volume 62. [Google Scholar]
- Geffen, E.; Mercure, A.; Girman, D.J.; Macdonald, D.W.; Wayne, R.K. Phylogenetic relationships of the fox-like canids: Mitochondrial DNA restriction fragment, site and cytochrome b sequence analyses. J. Zool. 1992, 228, 27–39. [Google Scholar] [CrossRef]
- Angerbjorn, A.; Hersteinsson, P.; Tannerfeldt, M. Arctic fox (Alopex lagopus). In Canids: Foxes, Wolves, Jackals and Dogs; Sillero-Zubiri, C., Hoffman, M., Mcdonald, D.W., Eds.; IUCN/SSC Canid Specialist Group: Gland, Switzerland, 2004; pp. 117–123. [Google Scholar]
- Geffen, E.; Hefner, R.; Wright, P. Blandford’s fox (Vulpes cana). In Canids: Foxes, Wolves, Jackals and Dogs; Sillero-Zubiri, C., Hoffman, M., Mcdonald, D.W., Eds.; IUCN/SSC Canid Specialist Group: Gland, Switzerland, 2004; pp. 194–200. [Google Scholar]
- Clark, H.O.; Murdoch, J.D.; Newman, D.P.; Sillero-Zubiri, C. Vulpes corsac (Carnivora: Canidae). Mamm. Species 2009, 1–8. [Google Scholar] [CrossRef]
- List, R.; Cypher, B.L. Kit fox (Vulpes macrotis) (Merriuam, 1888). In Canids: Foxes, Wolves, Jackals and Dogs; Sillero-Zubiri, C., Hoffman, M., Mcdonald, D.W., Eds.; IUCN/SSC Canid Specialist Group: Gland, Switzerland, 2004; pp. 105–109. [Google Scholar]
- Parkes, A.S. The reproductive processes of certain mammals. II.—The size of the Graafian follicle at ovulation. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1931, 109, 185–196. [Google Scholar]
- Cuzin, F.; Lenain, D.M. Rüppell’s fox (Vulpes rueppellii). In Canids: Foxes, Wolves, Jackals and Dogs; Sillero-Zubiri, C., Hoffman, M., Mcdonald, D.W., Eds.; IUCN/SSC Canid Specialist Group: Gland, Switzerland, 2004; pp. 201–205. [Google Scholar]
- Moehrenschlager, A.; Sovada, M. Swift fox (Vulpes velox) (Say, 1823). In Canids: Foxes, Wolves, Jackals and Dogs; Sillero-Zubiri, C., Hoffman, M., Mcdonald, D.W., Eds.; IUCN/SSC Canid Specialist Group: Gland, Switzerland, 2004; pp. 117–123. [Google Scholar]
- Clark, H.O., Jr.; Newman, D.P.; Murdoch, J.D.; Tseng, J.; Wang, Z.H.; Harris, R.B. Vulpes ferrilata (Carnivora: Canidae). Mamm. Species 2008, 1–6. [Google Scholar] [CrossRef]
- Bingham, J.; Purchase, G. Reproduction in the jackals Canis adustus Sundevall, 1846, and Canis mesomelas Schreber, 1778 (Carnivora: Canidae), in Zimbabwe. Afr. Zool. 2002, 37, 21–26. [Google Scholar] [CrossRef]
- Venkataraman, A.B. Male-biased adult sex ratios and their significance for cooperative breeding in dhole, Cuon alpinus, packs. Ethology 1998, 104, 671–684. [Google Scholar] [CrossRef]
- Koler-Matznick, J.; Brisbin, I.L., Jr.; Feinstein, M.; Bulmer, S. An updated description of the New Guinea singing dog (Canis hallstromi, Troughton 1957). J. Zool. 2003, 261, 109–118. [Google Scholar] [CrossRef]
- van Kesteren, F. Reproductive Physiology of Ethiopian Wolves (Canis simensis); Oxford University: Oxford, UK, 2011. [Google Scholar]
- Moehlman, P.D.; Hayssen, V. Canis aureus (Carnivore: Canidae). Mamm. Species 2018, 50, 14–25. [Google Scholar] [CrossRef]
- Gonzalez del Solar, R.; Rau, J. Chilla (Pseudalopex griseus). In Canids: Foxes, Wolves, Jackals and Dogs; Sillero-Zubiri, C., Hoffman, M., Mcdonald, D.W., Eds.; IUCN/SSC Canid Specialist Group: Gland, Switzerland, 2004; pp. 56–63. [Google Scholar]
- Jimenez, J.E.; Novard, A.J. Culpeo (Pseudalopex culpaeus). In Canids: Foxes, Wolves, Jackals and Dogs; Sillero-Zubiri, C., Hoffman, M., Mcdonald, D.W., Eds.; IUCN/SSC Canid Specialist Group: Gland, Switzerland, 2004; pp. 44–49. [Google Scholar]
- Candeias, Í.Z.d.; da Motta Lima, C.F.; Lemos, F.G.; Spercoski, K.M.; de Oliveira, C.A.; Songsasen, N.; de Barros Vaz Guimarães, M.A. First assessment of hoary fox (Lycalopex vetulus) seasonal ovarian cyclicity by non-invasive hormonal monitoring technique. Conserv. Physiol. 2020, 8, coaa039. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.W.; Gray, M.M.; Wayne, R.K.; Woodroffe, R.B. Mating tactics and paternity in a socially monogamous canid, the bat-eared fox (Otocyon megalotis). J. Mammal. 2010, 91, 437–446. [Google Scholar] [CrossRef]
- Gompper, M.E.; Vanak, A.T. Vulpes bengalensis . Mamm. Species 2006, 2006, 1–5. [Google Scholar] [CrossRef]
- Sullivan, E.G. Gray fox reproduction, denning, range, and weights in Alabama. J. Mammal. 1956, 37, 346–351. [Google Scholar] [CrossRef]
- Chastant-Maillard, S.; Viaris de Lesegno, C.; Chebrout, M.; Thoumire, S.; Meylheuc, T.; Fontbonne, A.; Chodkiewicz, M.; Saint-Dizier, M.; Reynaud, K. The canine oocyte: Uncommon features of in vivo and in vitro maturation. Reprod. Fertil. Dev. 2011, 23, 391–402. [Google Scholar] [CrossRef]
- Guraya, S.S. A histochemical analysis of lipid yolk deposition in the oocytes of cat and dog. J. Exp. Zool. 1965, 160, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Durrant, B.; Pratt, N.; Russ, K.; Bolamba, D. Isolation and characterization of canine advanced preantral and early antral follicles. Theriogenology 1998, 49, 917–932. [Google Scholar] [CrossRef]
- Tesoriero, J.V. A morphologic, cytochemical, and chromatographic analysis of lipid yolk formation in the oocytes of the dog. Gamete Res. 1982, 6, 267–279. [Google Scholar] [CrossRef]
- Ariu, F.; Strina, A.; Murrone, O.; Falchi, L.; Bebbere, D.; Ledda, S.; Zedda, M.T.; Pau, S.; Bogliolo, L. Lipid droplet distribution of immature canine oocytes in relation to their size and the reproductive stage. Anim. Sci. J. 2016, 87, 147–150. [Google Scholar] [CrossRef]
- Hyttel, P.; Farstad, W.; Mondain-Monval, M.; Lajord, K.B.; Smith, A. Structural aspects of oocyte maturation in the blue fox (Alopex lagopus). Anat. Embryol. 1990, 181, 325–331. [Google Scholar] [CrossRef]
- Xu, B.; Feng, H.L. Ovulation, fertilization and preimplantation embryonic development in raccoon dogs (Nyctereutes procyonoides). Anim. Reprod. Sci. 2017, 176, 78–84. [Google Scholar] [CrossRef]
- Apparicio, M.; Ferreira, C.; Tata, A.; Santos, V.; Alves, A.; Mostachio, G.; Pires-Butler, E.; Motheo, T.; Padilha, L.; Pilau, E. Chemical composition of lipids present in cat and dog oocyte by matrix-assisted desorption ionization mass spectrometry (MALDI-MS). Reprod. Domest. Anim. 2012, 47, 113–117. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Saraiva, S.A.; Catharino, R.R.; Garcia, J.S.; Gozzo, F.C.; Sanvido, G.B.; Santos, L.F.A.; Lo Turco, E.G.; Pontes, J.H.F.; Basso, A.C.; et al. Single embryo and oocyte lipid fingerprinting by mass spectrometry. J. Lipid Res. 2010, 51, 1218–1227. [Google Scholar] [CrossRef]
- Pearson, O.P.; Enders, R.K. Ovulation, maturation and fertilization in the fox. Anat. Rec. 1943, 85, 69–83. [Google Scholar] [CrossRef]
- Reynaud, K.; Fontbonne, A.; Marseloo, N.; Thoumire, S.; Chebrout, M.; de Lesegno, C.V.; Chastant-Maillard, S. In vivo meiotic resumption, fertilization and early embryonic development in the bitch. Reproduction 2005, 130, 193–201. [Google Scholar] [CrossRef]
- Farstad, W. Reproduction in foxes: Current research and future challenges. Anim. Reprod. Sci. 1998, 53, 35–42. [Google Scholar] [CrossRef]
- Tsutsui, T. Gamete physiology and timing of ovulation and fertilization in dogs. J. Reprod. Fertil. Suppl. 1989, 39, 269. [Google Scholar]
- Linde-Forsberg, C. Biology of reproduction of the dog and modern reproductive technology. In The Genetics of the Dog; Ruvinsky, A., Sampson, J., Eds.; CABI Publishing: New York, NY, USA, 2001; pp. 401–432. [Google Scholar]
- Mialot, J.; Guerin, C.; Begon, D. Growth, testicular development and sperm output in the dog from birth to post pubertal period. Andrologia 1985, 17, 450–460. [Google Scholar] [CrossRef]
- Chłopik, A.; Wysokińska, A. Canine spermatozoa—What do we know about their morphology and physiology? An overview. Reprod. Domest. Anim. 2020, 55, 113–126. [Google Scholar] [CrossRef]
- De los Reyes, M.; Anguita, C.; Barros, C.; Palomino, J.; de Lange, J. In vitro sperm penetration through the zona pellucida of immature and in vitro matured oocytes using fresh, chilled and frozen canine semen. Anim. Reprod. Sci. 2009, 110, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, J.B.; Sylvester, S.R.; Nelson, J.L.; Cheong, S.H.; Mukai, C.; Lambo, C.; Flanders, J.A.; Meyers-Wallen, V.N.; Songsasen, N.; Travis, A.J. Live births from domestic dog (Canis familiaris) embryos produced by in vitro fertilization. PLoS ONE 2015, 10, e0143930. [Google Scholar] [CrossRef] [PubMed]
- Farstad, W.; Hyttel, P.; Grondahl, C.; Krogenaes, A.; Mondain-Monval, M.; Hafne, A.L. Fertilization in vitro of oocytes matured in vivo in the blue fox (Alopex lagopus). J. Reprod. Fertil. Suppl. 1993, 47, 219–226. [Google Scholar] [PubMed]
- Abe, Y.; Suwa, Y.; Yanagimoto-Ueta, Y.; Suzuki, H. Preimplantation development of embryos in labrador retrievers. J. Reprod. Dev. 2008, 54, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Mal, G.; Gautam, S.K.; Mukesh, M. Assisted reproduction in dogs. In Advances in Animal Biotechnology; Springer: Cham, Switzerland, 2019; pp. 205–214. [Google Scholar] [CrossRef]
- Christensen, B.W.; Asa, C.S.; Wang, C.; Vansandt, L.; Bauman, K.; Callahan, M.; Jens, J.K.; Ellinwood, N.M. Effect of semen collection method on sperm motility of gray wolves (Canis lupus) and domestic dogs (C. l. familiaris). Theriogenology 2011, 76, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.; Dupuis, J.; Mickelsen, W.; Memon, M.; Platz, C., Jr. Conception by use of postmortem epididymal semen extraction in a dog. J. Am. Vet. Med. Assoc. 1994, 204, 1639–1640. [Google Scholar] [PubMed]
- Kuczmarski, A.H.; de Barros, M.A.; de Lima, L.F.S.; Motheo, T.F.; Bento, H.J.; Iglesias, G.A.; Sônego, D.A.; da Paz, R.C.R. Urethral catheterization after pharmacological induction for semen collection in dog. Theriogenology 2020, 153, 34–38. [Google Scholar] [CrossRef]
- Korochkina, E.; Johannisson, A.; Goodla, L.; Morrell, J.; Axner, E. Effect of prostatic fluid on the quality of fresh and frozen-thawed canine epididymal spermatozoa. Theriogenology 2014, 82, 1206–1211. [Google Scholar] [CrossRef]
- Franklin, A.D.; Waddell, W.T.; Goodrowe, K.L. Red wolf (Canis rufus) sperm quality and quantity is affected by semen collection method, extender components, and post-thaw holding temperature. Theriogenology 2018, 116, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lueders, I.; Luther, I.; Müller, K.; Scheepers, G.; Tordiffe, A.; Horst, G. Semen collection via urethral catheter in exotic feline and canine species: A simple alternative to electroejaculation. In Proceedings of the International Conference on Diseases of Zoo and Wild Animals, Vienna, Austria, 8–11 May 2013; p. 161. [Google Scholar]
- Farstad, W.; Fougner, J.A.; Torres, C.G. The effect of sperm number on fertility in blue fox vixens (Alopex lagopus) artificially inseminated with frozen silver fox (Vulpes vulpes) semen. Theriogenology 1992, 37, 699–711. [Google Scholar] [CrossRef]
- Jarosz, Ł.; Grądzki, Z.; Kalinowski, M.; Laskowska, E. Quality of fresh and chilled-stored raccoon dog semen and its impact on artificial insemination efficiency. BMC Vet. Res. 2016, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.C.; da Silva, F.E.; Rizzoto, G.; Dadalto, C.R.; Rolim, L.S.; Mamprim, M.J.; de Souza, F.F.; Teixeira, C.R.; Kastelic, J.P.; Ferreira, J.C.P. Semen collection, sperm characteristics and ultrasonographic features of reproductive tissues in crab-eating fox (Cerdocyon thous). Theriogenology 2020, 155, 60–69. [Google Scholar] [CrossRef]
- Teodoro, L.; Melo-Junior, A.; Spercoski, K.; Morais, R.; Souza, F. Seasonal aspects of reproductive physiology in captive male maned wolves (Chrysocyon brachyurus, Illiger 1815). Reprod. Domest. Anim. 2012, 47, 250–255. [Google Scholar] [CrossRef]
- Kenagy, G.; Trombulak, S.C. Size and function of mammalian testes in relation to body size. J. Mammal. 1986, 67, 1–22. [Google Scholar] [CrossRef]
- Caldeira, B.C.; Paula, T.A.R.d.; Matta, S.L.P.d.; Balarini, M.K.; Campos, P.K.A. Morphometry of testis and seminiferous tubules of the adult crab-eating fox (Cerdocyon thous, Linnaeus, 1766). Revista Ceres 2010, 57, 569–575. [Google Scholar] [CrossRef]
- Johnston, S.D.; Ward, D.; Lemon, J.; Gunn, I.; MacCallum, C.A.; Keeley, T.; Blyde, D. Studies of male reproduction in captive African wild dogs (Lycaon pictus). Anim. Reprod. Sci. 2007, 100, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, F.; Paris, M.C.J.; Briggs, M.B.; Farstad, W.K.; Paris, D.B.B.P. A two-step dilution tris-egg yolk extender containing Equex STM significantly improves sperm cryopreservation in the African wild dog (Lycaon pictus). Cryobiology 2018, 80, 18–25. [Google Scholar] [CrossRef]
- Bruss, M.; Green, J.; Stellflug, J. Electroejaculation of the coyote. Theriogenology 1983, 20, 53–59. [Google Scholar] [CrossRef]
- Johnson, A.E.M.; Freeman, E.W.; Wildt, D.E.; Songsasen, N. Spermatozoa from the maned wolf (Chrysocyon brachyurus) display typical canid hyper-sensitivity to osmotic and freezing-induced injury, but respond favorably to dimethyl sulfoxide. Cryobiology 2014, 68, 361–370. [Google Scholar] [CrossRef]
- Zindl, C.; Asa, C.; Günzel-Apel, A.-R. Influence of cooling rates and addition of Equex pasta on cooled and frozen-thawed semen of generic gray (Canis lupus) and Mexican gray wolves (C. l. baileyi). Theriogenology 2006, 66, 1797–1802. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Platz, C.; Waddell, W.; Jones, M.; Behrns, S. Semen parameters and electron microscope observations of spermatozoa of the red wolf, Canis rufus. J. Reprod. Fertil. 1998, 114, 95–101. [Google Scholar] [CrossRef]
- Lockyear, K.; MacDonald, S.; Waddell, W.; Goodrowe, K. Investigation of captive red wolf ejaculate characteristics in relation to age and inbreeding. Theriogenology 2016, 86, 1369–1375. [Google Scholar] [CrossRef]
- Woodall, P.; Pavlov, P.; Tolley, L. Comparative dimensions of testes, epididymides and spermatozoa of Australian dingoes (Canis familiaris dingo) and domestic dogs (Canis familiaris familiaris)-Some effects of domestication. Aust. J. Zool. 1993, 41, 133–140. [Google Scholar] [CrossRef]
- Muñoz-Fuentes, V.; Forsberg, C.L.; Vilà, C.; Morrell, J.M. Single-layer centrifugation separates spermatozoa from diploid cells in epididymal samples from gray wolves, Canis lupus (L.). Theriogenology 2014, 82, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Boue, F.; Delhomme, A.; Chaffaux, S. Reproductive management of silver foxes (Vulpes vulpes) in captivity. Theriogenology 2000, 53, 1717–1728. [Google Scholar] [CrossRef]
- Hewitt, D.A.; Leahy, R.; Sheldon, I.M.; England, G.C.W. Cryopreservation of epididymal dog sperm. Anim. Reprod. Sci. 2001, 67, 101–111. [Google Scholar] [CrossRef]
- Andersen, K. Ultrastructural studies of blue fox spermatozoa. Acta Vet. Scand. 1974, 15, 620–630. [Google Scholar] [CrossRef]
- Boutelle, S.; Lenahan, K.; Krisher, R.; Bauman, K.; Asa, C.; Silber, S. Vitrification of oocytes from endangered Mexican gray wolves (Canis lupus baileyi). Theriogenology 2011, 75, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Oh, H.; Park, J.; Hong, S.; Kang, J.; Koo, O.; Kang, S.; Jang, G.; Lee, B. Influence of oocyte donor and embryo recipient conditions on cloning efficiency in dogs. Theriogenology 2010, 74, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Crosier, A.E.; Lamy, J.; Bapodra, P.; Rapp, S.; Maly, M.; Junge, R.; Haefele, H.; Ahistus, J.; Santiestevan, J.; Comizzoli, P. First birth of cheetah cubs from in vitro fertilization and embryo transfer. Animals 2020, 10, 1811. [Google Scholar] [CrossRef]
- Manassero, M.; Viateau, V. Advances in laparoscopic spay techniques for dogs: The past, present and future. Vet. Rec. 2018, 183, 742–744. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef]
- Nagashima, J.B.; Wildt, D.E.; Travis, A.J.; Songsasen, N. Activin promotes growth and antral cavity expansion in the dog ovarian follicle. Theriogenology 2019, 129, 168–177. [Google Scholar] [CrossRef]
- Nagashima, J.; Wildt, D.E.; Travis, A.J.; Songsasen, N. Follicular size and stage and gonadotropin concentration affect alginate-encapsulated in vitro growth and survival of pre- and early antral dog follicles. Reprod. Fertil. Dev. 2015, 262–273. [Google Scholar] [CrossRef]
- Songsasen, N.; Woodruff, T.K.; Wildt, D.E. In vitro growth and steroidogenesis of dog follicles as influenced by the physical and hormonal microenvironment. Reproduction 2011, 142, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, J.; El Assal, R.; Songsasen, N.; Demirci, U. Evaluation of an ovary-on-a-chip in large mammalian models: Species specificity and influence of follicle isolation status. J. Tissue Eng. Regen. Med. 2018, e1926–e1935. [Google Scholar] [CrossRef]
- Fujihara, M.; Comizzoli, P.; Wildt, D.; Songsasen, N. Cat and dog primordial follicles enclosed in ovarian cortex sustain viability after in vitro culture on agarose gel in a protein-free medium. Reprod. Domest. Anim. 2012, 47, 102–108. [Google Scholar] [CrossRef]
- Serafim, M.K.B.; Duarte, A.B.G.; Silva, G.M.; Souza, C.E.A.; Magalhães-Padilha, D.M.; Moura, A.A.A.; Silva, L.D.M.; Campello, C.C.; Figueiredo, J.R. Impact of growth hormone (GH) and follicle stimulating hormone (FSH) on in vitro canine preantral follicle development and estradiol production. Growth Horm. IGF Res. 2015, 25, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Serafim, M.K.; Silva, G.M.; Duarte, A.B.; Araújo, V.; Silva, T.; Lima, A.; Chaves, R.; Campello, C.; Silva, L.; Figueiredo, J. High insulin concentrations promote the in vitro growth and viability of canine preantral follicles. Reprod. Fertil. Dev. 2013, 25, 927–934. [Google Scholar] [CrossRef]
- Serafim, M.K.B.; Araújo, V.R.; Silva, G.M.; Duarte, A.B.G.; Almeida, A.P.; Chaves, R.N.; Campello, C.C.; Lopes, C.A.P.; de Figueiredo, J.R.; da Silva, L.D.M. Canine preantral follicles cultured with various concentrations of follicle-stimulating hormone (FSH). Theriogenology 2010, 74, 749–755. [Google Scholar] [CrossRef]
- Picton, H.M.; Harris, S.E.; Muruvi, W.; Chambers, E.L. The in vitro growth and maturation of follicles. Reproduction 2008, 136, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Songsasen, N.; Fickes, A.; Pukazhenthi, B.S.; Wildt, D.E. Follicular morphology, oocyte diameter and localisation of fibroblast growth factors in the domestic dog ovary. Reprod. Domest. Anim. 2009, 44, 65–70. [Google Scholar] [CrossRef]
- Reynaud, K.; de Lesegno, C.V.; Chebrout, M.; Thoumire, S.; Chastant-Maillard, S. Follicle population, cumulus mucification, and oocyte chromatin configuration during the periovulatory period in the female dog. Theriogenology 2009, 72, 1120–1131. [Google Scholar] [CrossRef]
- Komeya, M.; Kimura, H.; Nakamura, H.; Yokonishi, T.; Sato, T.; Kojima, K.; Hayashi, K.; Katagiri, K.; Yamanaka, H.; Sanjo, H. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Abrishami, M.; Abbasi, S.; Honaramooz, A. The effect of donor age on progression of spermatogenesis in canine testicular tissue after xenografting into immunodeficient mice. Theriogenology 2010, 73, 512–522. [Google Scholar] [CrossRef]
- Johnston, S.D.; Root Kustritz, M.V.; Olson, P.S. The Dog: Semen collection, evaluation and preservation. In Canine and Feline Theriogenology; Kersey, R., LeMelledo, D., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 2001; pp. 287–306. [Google Scholar]
- Watson, P. The roles of lipid and protein in the protection of ram spermatozoa at 5 C by egg-yolk lipoprotein. Reproduction 1981, 62, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Dalmazzo, A.; Losano, J.D.A.; Rocha, C.C.; Tsunoda, R.H.; Angrimani, D.d.S.R.; Mendes, C.M.; Assumpção, M.E.O.D.Á.; Nichi, M.; Barnabe, V.H. Effects of soy lecithin extender on dog sperm cryopreservation. Anim. Biotechnol. 2018, 29, 174–182. [Google Scholar] [CrossRef]
- Sánchez-Calabuig, M.J.; Maillo, V.; Beltrán-Breña, P.; de la Fuente Martínez, J.; Galera-Carrillo, S.; Pérez-Gutiérrez, J.F.; Pérez-Cerezales, S. Cryopreservation of canine sperm using egg yolk and soy bean based extenders. Reprod. Biol. 2017, 17, 233–238. [Google Scholar] [CrossRef]
- Axnér, E.; Lagerson, E. Cryopreservation of dog semen in a tris extender with 1% or 2% soya bean lecithin as a replacement of egg yolk. Reprod. Domest. Anim. 2016, 51, 262–268. [Google Scholar] [CrossRef]
- Nabeel, A.H.T.; Jeon, Y.; Yu, I.J. Use of polyvinyl alcohol as a chemically defined compound in egg yolk-free extender for dog sperm cryopreservation. Reprod. Domest. Anim. 2019, 54, 1449–1458. [Google Scholar] [CrossRef]
- Songsasen, N.; Yu, I.; Murton, S.; Paccamonti, D.L.; Eilts, B.E.; Godke, R.A.; Leibo, S.P. Osmotic sensitivity of canine spermatozoa. Cryobiology 2002, 44, 79–90. [Google Scholar] [CrossRef]
- Hermes, R.; Göritz, F.; Maltzan, J.; Blottner, S.; Proudfoot, J.; Fritsch, G.; Fassbender, M.; Quest, M.; Hildebrandt, T.B. Establishment of assisted reproduction technologies in female and male African wild dogs (Lycaon pictus). J. Reprod. Fertil. Suppl. 2001, 57, 315–321. [Google Scholar] [PubMed]
- Seager, S.; Platz, C.C., Jr.; Hodge, W. Successful pregnancy using frozen semen in the wolf. Int. Zoo Yearb. 1975, 15, 140–143. [Google Scholar] [CrossRef]
- Farstad, W.; Fougner, J.A.; Torres, C.G. The optimum time for single artificial insemination of blue fox vixens (Alopex lagopus) with frozen-thawed semen from silver foxes (Vulpes vulpes). Theriogenology 1992, 38, 853–865. [Google Scholar] [CrossRef]
- Goodrowe, K.; Mastromonaco, G.; Walker, S.; Bateman, H.; Ryckman, D.; Platz, J.C.; Waddell, W. In vitro maintenance, cooling and cryopreservation of red wolf (Canis rufus) spermatozoa. J. Reprod. Fertil. Suppl. 2001, 57, 387–392. [Google Scholar]
- Andrade, G.M.; Bridi, A.; Gimenes, L.U.; Meirelles, F.V.; Perecin, F.; da Silveira, J.C. Extracellular vesicles and its advances in female reproduction. Anim. Reprod. 2019, 16, 31–38. [Google Scholar]
- Nagashima, J.B.; Ferraz, M.A.M.M.; Songsasen, N. Extracellular vesicles and assisted reproductive technology. Clin. Theriogenol. 2020, 12, 89–101. [Google Scholar]
- de Almeida Monteiro Melo Ferraz, M.; Nagashima, J.B.; Noonan, M.J.; Crosier, A.E.; Songsasen, N. Oviductal extracellular vesicles improve post-thaw sperm function in red wolves and cheetahs. Int. J. Mol. Sci. 2020, 21, 3733. [Google Scholar] [CrossRef]
- Abe, Y.; Lee, D.-S.; Kim, S.-K.; Suzuki, H. Vitrification of canine oocytes. J. Mamm. Ova Res. 2008, 25, 32–36. [Google Scholar] [CrossRef]
- Turathum, B.; Saikhun, K.; Sangsuwan, P.; Kitiyanant, Y. Effects of vitrification on nuclear maturation, ultrastructural changes and gene expression of canine oocytes. Reprod. Biol. Endocrinol. 2010, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Suwa, Y.; Asano, T.; Ueta, Y.Y.; Kobayashi, N.; Ohshima, N.; Shirasuna, S.; Abdel-Ghani, M.A.; Oi, M.; Kobayashi, Y.; et al. Cryopreservation of canine embryos. Biol. Reprod. 2010, 84, 363–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hori, T.; Ushijima, H.; Kimura, T.; Kobayashi, M.; Kawakami, E.; Tsutsui, T. Intrauterine embryo transfer with canine embryos cryopreserved by the slow freezing and the Cryotop method. J. Vet. Med. Sci. 2016, 78, 1137–1143. [Google Scholar] [CrossRef]
- Nagashima, J.B.; Kim, Y.; Hollinshead, F.K.; Travis, A.J. IVF in the dog: Recent advancements and future directions. Clin. Theriogenol. 2017, 9, 6. [Google Scholar]
- Nagashima, J.; Travis, A.; Songsasen, N. The domestic dog embryo: In vitro fertilization, culture, and transfer. In Comparative Embryo Culture; Herrick, J., Ed.; Springer: New York, NY, USA, 2019; Volume 2006, pp. 247–267. [Google Scholar]
- Tsutsui, T.; Hori, T.; Endo, S.; Hayama, A.; Kawakami, E. Intrauterine transfer of early canine embryos. Theriogenology 2006, 66, 1703–1705. [Google Scholar] [CrossRef]
- de Freitas Guaitolini, C.R.; Taffarel, M.O.; Teixeira, N.S.; Sudano, M.J.; Freitas, P.M.C.; Lopes, M.D.; da Cruz Landin-Alvarenga, F.; de Oliveira, C.A.; Luz, M.R. Post-thaw viability of in vivo-produced canine blastocysts cryopreserved by slow freezing. Theriogenology 2012, 78, 576–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luz, M.; Holanda, C.; Pereira, J.; Teixeira, N.; Vantini, R.; Salgado, A.; Oliveira, S.; Guaitolini, C.; Santos, M. Survival rate and in vitro development of in vivo-produced and cryopreserved dog embryos. Reprod. Fertil. Dev. 2009, 22, 208–209. [Google Scholar] [CrossRef]
- Farstad, W. Assisted reproductive technology in canid species. Theriogenology 2000, 53, 175–186. [Google Scholar] [CrossRef]
- Santos, R.R.; Amorim, C.; Cecconi, S.; Fassbender, M.; Imhof, M.; Lornage, J.; Paris, M.; Schoenfeldt, V.; Martinez-Madrid, B. Cryopreservation of ovarian tissue: An emerging technology for female germline preservation of endangered species and breeds. Anim. Reprod. Sci. 2010, 122, 151–163. [Google Scholar] [CrossRef]
- Comizzoli, P.; Songsasen, N.; Wildt, D.E. Protecting and extending fertility for females of wild and endangered mammals. In Oncofertility: Ethical, Legal, Social, and Medical Perspectives; Woodruff, T.K., Zoloth, L., Campo-Engelstein, L., Rodriguez, S., Eds.; Springer: Boston, MA, USA, 2010; pp. 87–100. [Google Scholar] [CrossRef]
- Silva, A.M.d.; Pereira, A.F.; Comizzoli, P.; Silva, A.R. Cryopreservation and culture of testicular tissues: An essential tool for biodiversity preservation. Biopreserv. Biobank. 2020, 18, 235–243. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Toner, M. Long-term storage of tissues by cryopreservation: Critical issues. Biomaterials 1996, 17, 243–256. [Google Scholar] [CrossRef]
- Ishijima, T.; Kobayashi, Y.; Lee, D.-S.; Ueta, Y.Y.; Matsui, M.; Lee, J.-Y.; Suwa, Y.; Miyahara, K.; Suzuki, H. Cryopreservation of canine ovaries by vitrification. J. Reprod. Dev. 2005. [Google Scholar] [CrossRef]
- Fujihara, M.; Kaneko, T.; Inoue-Murayama, M. Vitrification of canine ovarian tissues with polyvinylpyrrolidone preserves the survival and developmental capacity of primordial follicles. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, W.Y.; Kim, D.H.; Lee, S.H.; Do, J.T.; Park, C.; Kim, J.H.; Choi, Y.S.; Song, H. Vitrified canine testicular cells allow the formation of spermatogonial stem cells and seminiferous tubules following their xenotransplantation into nude mice. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Andrae, C.; Oliveira, E.; Ferraz, M.; Nagashima, J. Cryopreservation of grey wolf (Canis lupus) testicular tissue. Cryobiology 2021. [Google Scholar] [CrossRef]
- Kutzler, M.A. Induction and synchronization of estrus in dogs. Theriogenology 2005, 64, 766–775. [Google Scholar] [CrossRef]
- Carvalho, A.; Santos, A.; Silva, C. Induction of estrus and methods for control of estrous cycle in bitches. Ciência Anim. 2020, 30, 117–129. [Google Scholar]
- Kutzler, M. Effect of estrus induction on pregnancy rates in domestic bitches and queens. Clin. Theriogenol. 2010, 2, 191–207. [Google Scholar]
- Trigg, T.; Wright, P.; Armour, A.; Williamson, P.; Junaidi, A.; Martin, G.; Doyle, A.; Walsh, J. Use of a GnRH analogue implant to produce reversible long-term suppression of reproductive function in male and female domestic dogs. J. Reprod. Fertil. Suppl. 2001, 57, 255–261. [Google Scholar] [PubMed]
- Concannon, P.; Temple, M.; Montanez, A.; Newton, L. Effects of dose and duration of continuous GnRH-agonist treatment on induction of estrus in beagle dogs: Competing and concurrent up-regulation and down-regulation of LH release. Theriogenology 2006, 66, 1488–1496. [Google Scholar] [CrossRef]
- Volkmann, D.; Kutzler, M.; Wheeler, R.; Krekeler, N. The use of deslorelin implants for the synchronization of estrous in diestrous bitches. Theriogenology 2006, 66, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E.; Mir, F.; Vannier, F.; Gérardin, A.; Albouy, M.; Navarro, C.; Fontbonne, A. Induction of fertile oestrus in the bitch using Deslorelin, a GnRH agonist. Theriogenology 2011, 76, 1561–1566. [Google Scholar] [CrossRef]
- Asa, C.S.; Bauman, K.; Callahan, P.; Bauman, J.; Volkmann, D.H.; Jöchle, W. GnRH-agonist induction of fertile estrus with either natural mating or artificial insemination, followed by birth of pups in gray wolves (Canis lupus). Theriogenology 2006, 66, 1778–1782. [Google Scholar] [CrossRef]
- Mech, L.D.; Christensen, B.W.; Asa, C.S.; Callahan, M.; Young, J.K. Production of hybrids between Western gray wolves and Western coyotes. PLoS ONE 2014, 9, e88861. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Gese, E.M. Influence of exogenous gonadotropin-releasing hormone on seasonal reproductive behavior of the coyote (Canis latrans). Theriogenology 2009, 72, 773–783. [Google Scholar] [CrossRef][Green Version]
- Thomassen, R.; Farstad, W. Artificial insemination in canids: A useful tool in breeding and conservation. Theriogenology 2009, 71, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Songsasen, N.; Wildt, D.E. Size of the donor follicle, but not stage of reproductive cycle or seasonality, influences meiotic competency of selected domestic dog oocytes. Mol. Reprod. Dev. 2005, 72, 113–119. [Google Scholar] [CrossRef]
- Hewitt, D.A.; England, G.C.W. The effect of oocyte size and bitch age upon oocyte nuclear maturation in vitro. Theriogenology 1998, 49, 957–966. [Google Scholar] [CrossRef]
- de Ávila Rodrigues, B.; Rodrigues, J.L. Influence of reproductive status on in vitro oocyte maturation in dogs. Theriogenology 2003, 60, 59–66. [Google Scholar] [CrossRef]
- England, G.C.W.; Verstegen, J.P.; Hewitt, D.A. Pregnancy following in vitro fertilisation of canine oocytes. Vet. Rec. 2001, 148, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Otoi, T.; Shin, T.; Kraemer, D.C.; Westhusin, M.E. Influence of maturation culture period on the development of canine oocytes after in vitro maturation and fertilization. Reprod. Nutr. Dev. 2004, 44, 631–637. [Google Scholar] [CrossRef][Green Version]
- Otoi, T.; Murakami, M.; Fujii, M.; Tanaka, M.; Ooka, A.; Suzuki, T. Development of canine oocytes matured and fertilised in vitro. Vet. Rec. 2000, 146, 52–53. [Google Scholar] [CrossRef]
- Rodrigues, B.d.Á.; Santos, L.C.d.; Rodrigues, J.L. Embryonic development of in vitro matured and in vitro fertilized dog oocytes. Mol. Reprod. Dev. 2004, 67, 215–223. [Google Scholar] [CrossRef]
- Nagashima, J.B.; Ferraz, M.d.A.M.M.; Kamen, S.H.; Songsasen, N. Investigating media that support red wolf (Canis rufus) sperm viability and capacitation in vitro. Reprod. Fertil. 2020, 1, 83–92. [Google Scholar] [CrossRef]
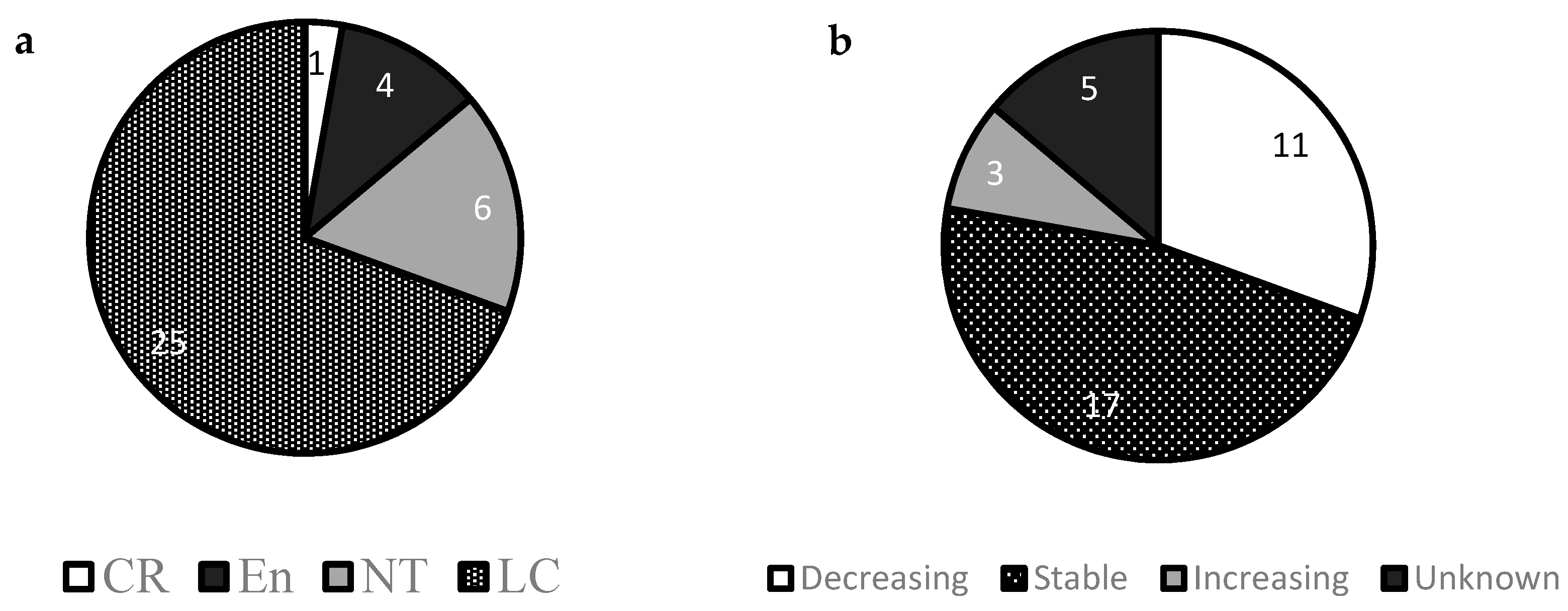
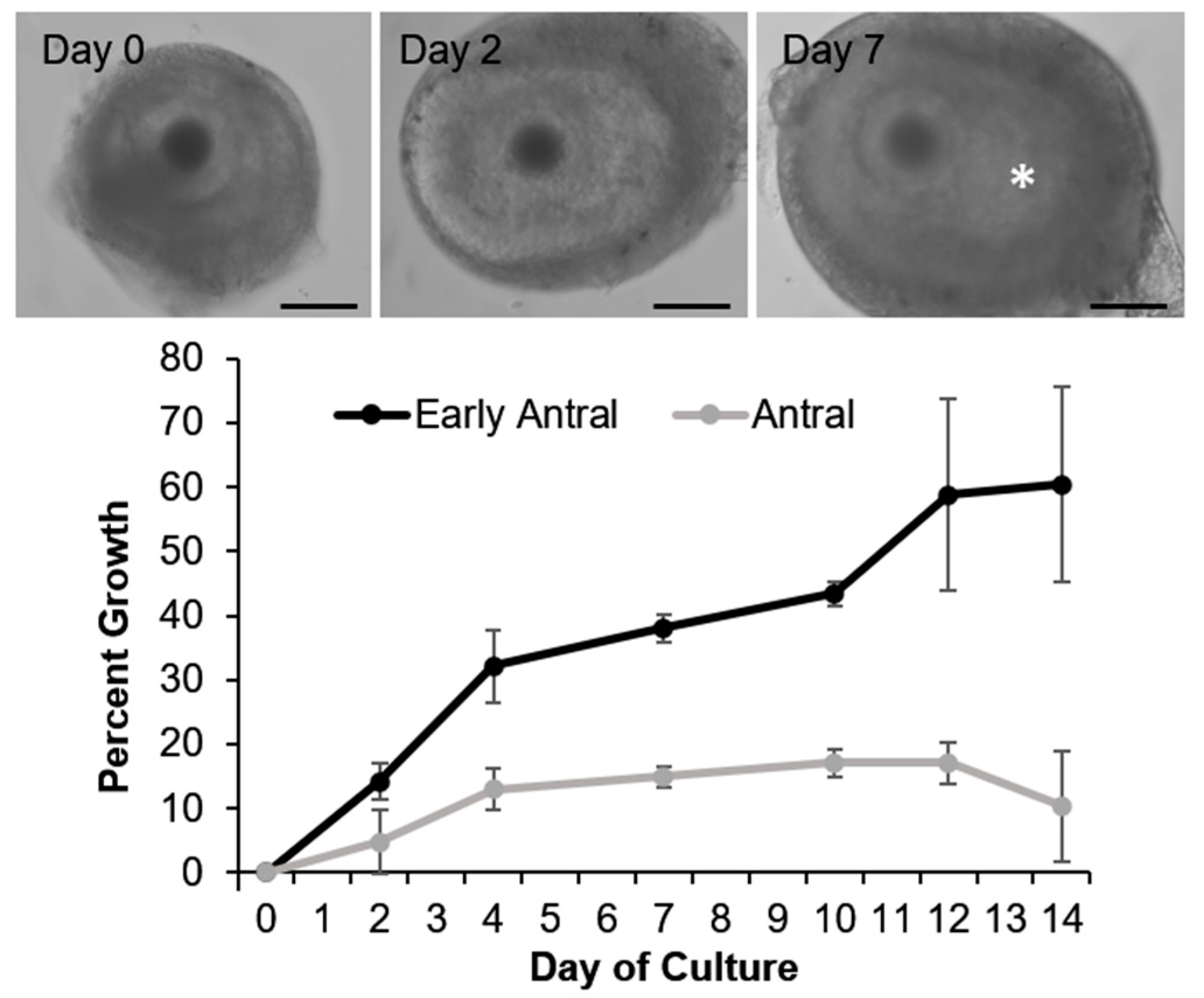
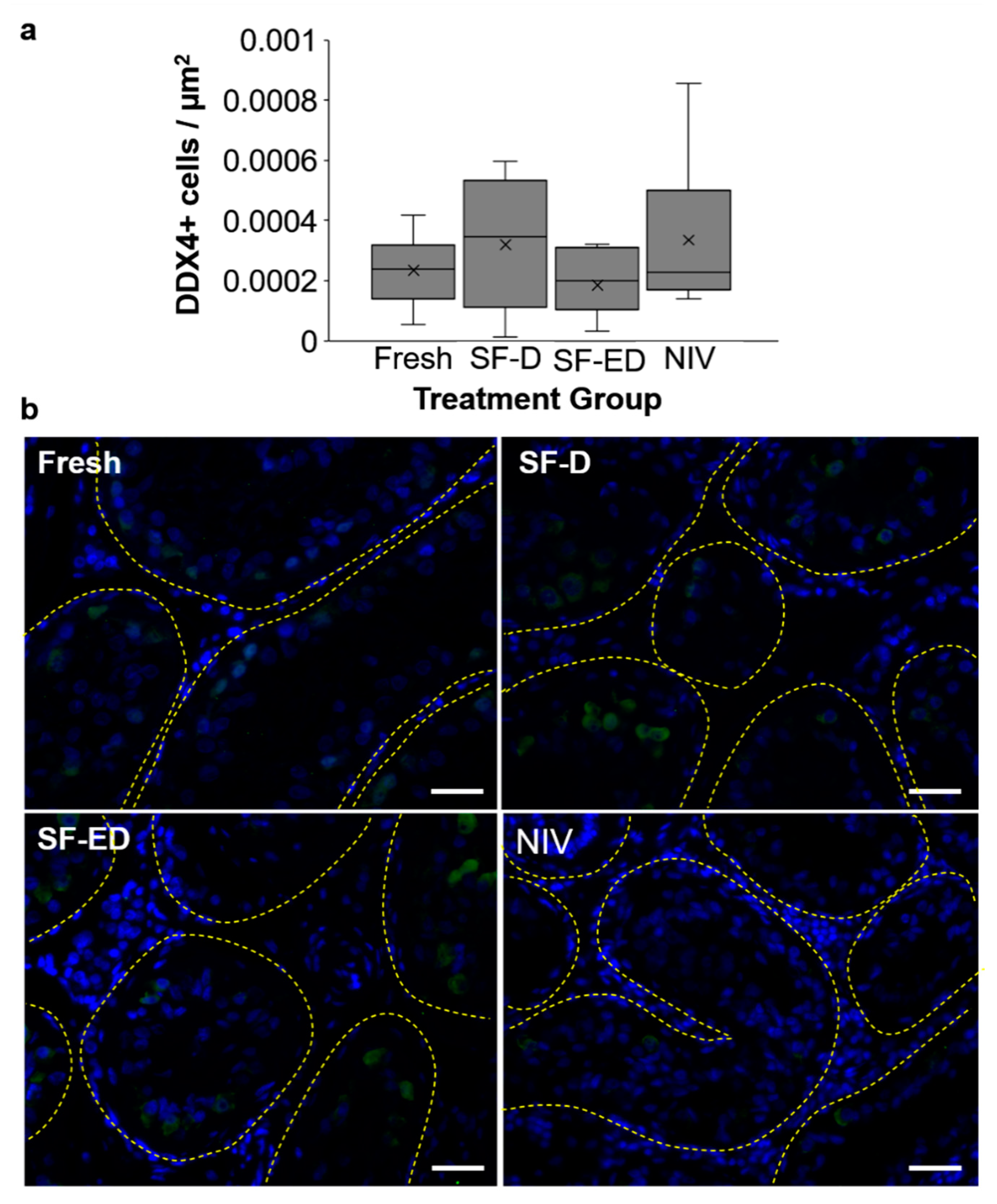
| Taxonomic Group | Species | Scientific Name | Geographical Location | Breeding Seasonality | Time of Breeding Season | Citation |
|---|---|---|---|---|---|---|
| Red fox-like canids | Arctic fox | Vulpes lagopus | Arctic Tundra | Yes | February–May | [55] |
| Blanford’s fox | Vulpes cana | Middle East | Yes | January–February | [56] | |
| Corsac fox | Vulpes corsac | Central Asia | Yes | January–March | [57] | |
| Fennec fox | Vulpes zerda | Africa | Yes | January–July, September | [12] | |
| Kit fox | Vulpes macrotis | North America | Yes | December–January | [58] | |
| Red fox | Vulpes vulpes | Entire Northern hemisphere from Arctic Circle to Asiatic steppes and Australia | Yes | Late January–early March | [31,59] | |
| Ruppell’s fox | Vulpes rueppellii | North Africa and Middle East | Yes | December–February | [60] | |
| Swift fox | Vulpes velox | North America | Yes | December–March | [61] | |
| Tibetan sand fox | Vulpes ferrilata | Steppes and semideserts of the Tibetan plateau | Yes | Late February–March | [62] | |
| Wolf-like canids | African wild dog | Lycaon pictus | Africa | Yes | Southern hemisphere, Feb–May; Northern hemisphere, late August–early October | [13] |
| Black backed jackal | Canis mesomelas | Africa | Yes | June–July | [63] | |
| Coyote | Canis latrans | North America | Yes | December–April | [30] | |
| Dhole | Cuon alpinus | Central and Southeast Asia | Yes | Varies | [16,17,64] | |
| Dog, domestic | Canis familiaris | Global | No | - | [8] | |
| Dog, Dingo | Canis lupus dingo | Australia and Southeast Asia | Varies | April–May | [35,36] | |
| Dog, New Guinea Singing | Canis hallstromi | Papua New Guinea | Yes | August–October | [18,65] | |
| Ethiopian wolf | Canis Simensis | Ethiopia | Yes | August–November | [44,66] | |
| Golden jackal | Canis aureus | Europe, Africa, Middle East, Central and Southeast Asia | Yes | Varies | [67] | |
| Grey wolf | Canis lupus | North America, Europe and North and Central Asia | Yes | Late January– March | [43] | |
| Red wolf | Canis rufus | USA | Yes | January–March | [29] | |
| Side striped jackal | Canis adustus | Africa | Yes | June–July | [63] | |
| South American foxes | Chilla | Pseudalopex griseus | South America | Yes | August–September | [68] |
| Crab eating fox | Cerdocyon thous | South America | Yes | June–September | [11] | |
| Culpeo | Pseudalopex culpaeus | South America | Yes | Male: June–mid-OctoberFemale, August–October | [69] | |
| Hoary fox | Lycalopex vetulus | South America | Yes | July–September | [70] | |
| Other | Bat eared fox | Otocyon megalotis | Africa | Yes | June–July | [71] |
| Bush dog | Speothos venaticus | South America | No | - | [15] | |
| Bengal fox | Vulpes bengalensis | South Asia | Yes | December–January | [72] | |
| Grey fox | Urocyon cinereoargentues | Central and North America | Yes | January–April | [73] | |
| Island fox | Urocyon littoralis | United States | Yes | February–mid March | [19] | |
| Maned wolf | Chrysocyon brachyurus | South America | Yes | Southern hemisphere, March–May, North America, October–January | [32,37] | |
| Raccoon dog | Nyctereutes procyonoides | Northern and Eastern Europe | Yes | February–March | [40,49] |
| Technique | Species | N Animals (samples) | Total Sperm (×106 cells) | Motility (%) |
|---|---|---|---|---|
| Electroejaculation | African wild dog [107] | 7 (7) | ~127.4 ± 52.7 | 69.5 ± 3.3 |
| African wild dog [108] | 17 (17) | 30.5 ± 9.7 | 55.0 ± 6.3 | |
| African wild dog [51] | 20 | 32.3 ± 9.2 | 47.4 ± 6.7 | |
| Coyote [109] | 15 | ~63 ± 12.6 | ~90 | |
| Coyote [30] (peak season/January) | 10 | 917.2 ± 497.2 | 90.4 ± 4.5 | |
| Maned wolf [110] | 14 (25) | 78.1 ± 35.0 | 59.8 ± 4.9 | |
| Mexican grey wolf [111] | 4 (27) | 756.2 ± 153.9 | ~90% † | |
| Grey wolf [111] | 7 (13) | 1597.4 ± 390.4 | n.r. | |
| Grey wolf [95] | 7 (7) | n.r. | ~70 | |
| Red wolf [112] | 15 (31) | 470.0 ± 83.5 | 69.6 ± 3.5 | |
| Red wolf [113] | 15 (37) | 349.4 ± 51.1 | 75.6 ± 2.6 | |
| Red wolf [99] | 39 (38) | 720.0 ± 287.5 | 80.8 ± 16.9 | |
| Epididymal Collection | Dingo dog [114] | 12 | 873.0 ± 229 | n.r. |
| Grey wolf [115] | 9–13 | 69.3 ± 23.3 * | n.r. | |
| Manual/Digital Manipulation | Arctic fox [42] (N:6L:N photoperiod) | 4 (11) | ~ 228.8 ± 25.2 | 86.0 ± 2.0 |
| Crab-eating fox [103] | 2 (13) | 217.4 ± 84.3 | 68.0 ± 6.1 | |
| Maned wolf [104] | 3 (70) | 73.9 ± 10.4 | 76.1 ± 2.9 | |
| Raccoon dog [102] | 20 (20) | ~0.06 | 57.2 | |
| Red (silver) fox [116] | 17 (45) | ~79.8 ± 5.6 | 70.3 ± 2.5 † | |
| Urethral catheterization | African wild dog [100] | 1 (1) | 216.0 | 93.0 |
| Maned wolf [100] | 1 (1) | 1.0 | 40.0 | |
| Maned wolf (unpublished data) | 2 (2) | 1.4 ± 1.3 | 80.0 ± 20.0 | |
| Red wolf [99] | 8 (8) | 30.1 ± 35.1 | 36.3 ± 13.2 |
| Species | ID | Age | Month | Spermic | Total Sperm (×106 cells) | Motility (%) |
|---|---|---|---|---|---|---|
| African Wild Dog | 116047 # | 7 y | September-18 | Yes | 88.0 | 65 |
| 6217 | - | January-19 | Yes | dnc | 0 | |
| 5639 * | 1 y | June-20 | Yes | 2.7 | dnc | |
| 5640 * | 1 y | June-20 | Yes | [Too low] | 50 | |
| 5641 * | 1 y | June-20 | Yes | 17.6 | 55 | |
| 5642 * | 1 y | June-20 | No | - | - | |
| 5643 * | 1 y | June-20 | Yes | 2.4 | 10 | |
| Maned Wolf | 3382 | 5 y | June-18 | No | - | - |
| 3206 | 8 y | December-18 | Yes | 46.0 | 70 | |
| 3230 †# | 8 y | August-19 | Yes | 23.5 | 60–65 | |
| 2954 | 12 y | October-19 | No | - | - | |
| 3153 | 10 y | June-20 | Yes | [Too low] | Few | |
| 3176 | 10 y | December-20 | Yes | 4.8 | 50 | |
| Red Wolf | 1460 | 12 y | July-17 | No | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagashima, J.B.; Songsasen, N. Canid Reproductive Biology: Norm and Unique Aspects in Strategies and Mechanisms. Animals 2021, 11, 653. https://doi.org/10.3390/ani11030653
Nagashima JB, Songsasen N. Canid Reproductive Biology: Norm and Unique Aspects in Strategies and Mechanisms. Animals. 2021; 11(3):653. https://doi.org/10.3390/ani11030653
Chicago/Turabian StyleNagashima, Jennifer B., and Nucharin Songsasen. 2021. "Canid Reproductive Biology: Norm and Unique Aspects in Strategies and Mechanisms" Animals 11, no. 3: 653. https://doi.org/10.3390/ani11030653
APA StyleNagashima, J. B., & Songsasen, N. (2021). Canid Reproductive Biology: Norm and Unique Aspects in Strategies and Mechanisms. Animals, 11(3), 653. https://doi.org/10.3390/ani11030653






