Responses of Vaginal Microbiota to Dietary Supplementation with Lysozyme and its Relationship with Rectal Microbiota and Sow Performance from Late Gestation to Early Lactation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Serum Analyses
2.4. Bacterial Community Analysis
2.5. Statistical Analysis
3. Results
3.1. Effect of Lysozyme Diet Supplementation on Serum Cytokines of Sow
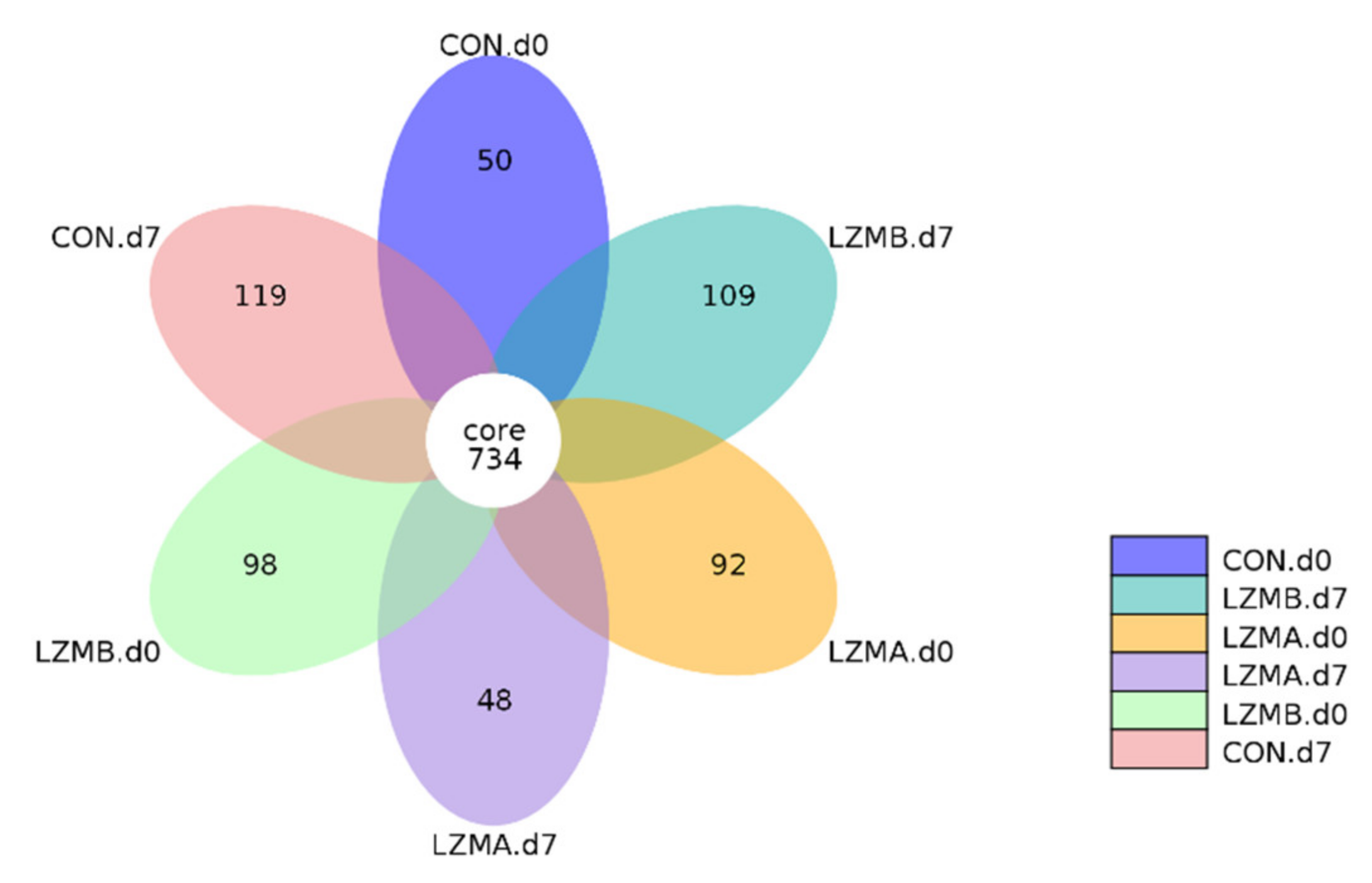
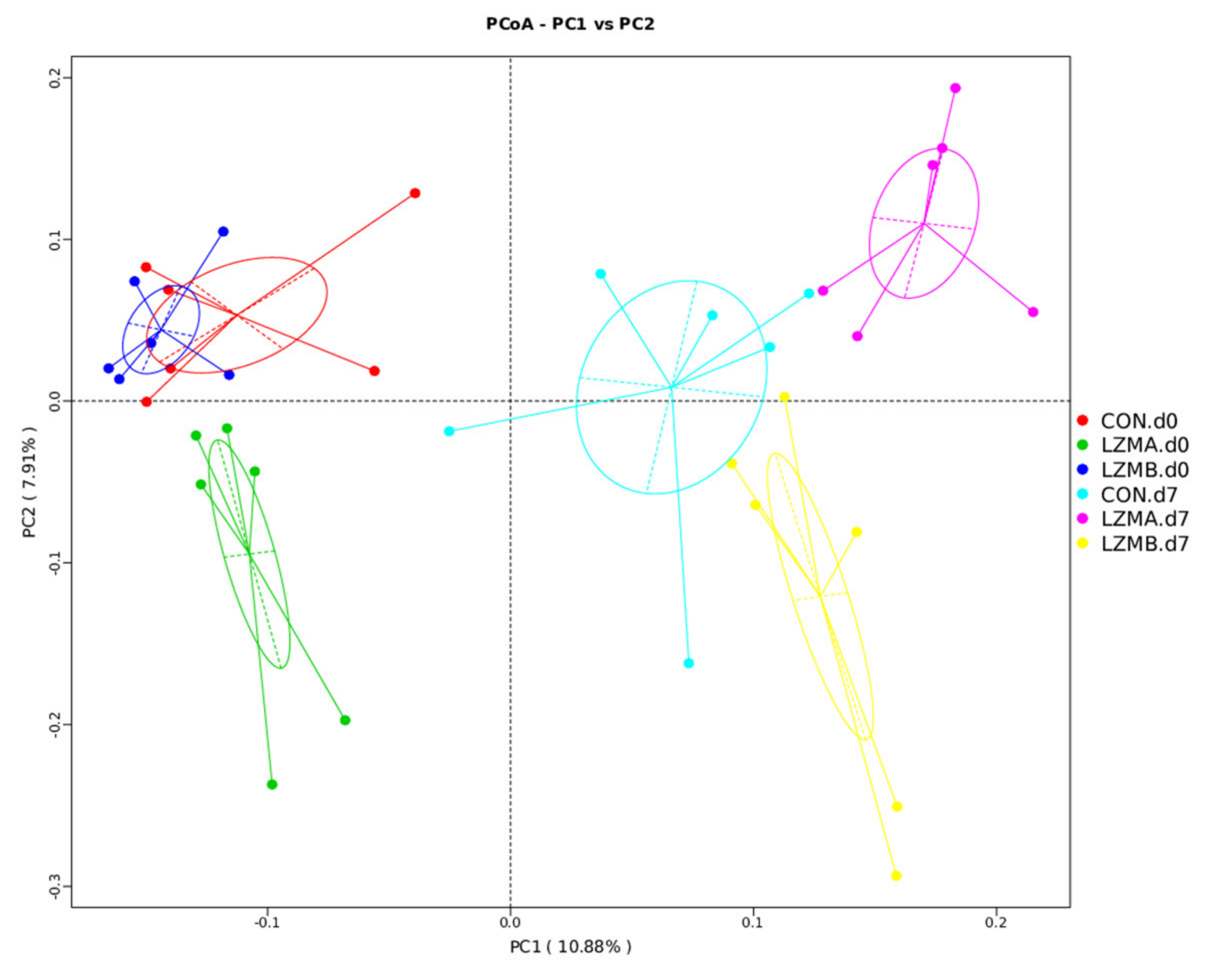
3.2. Effect of Lysozyme Diet Supplementation on Sows’ Vagina Microbial Diversity
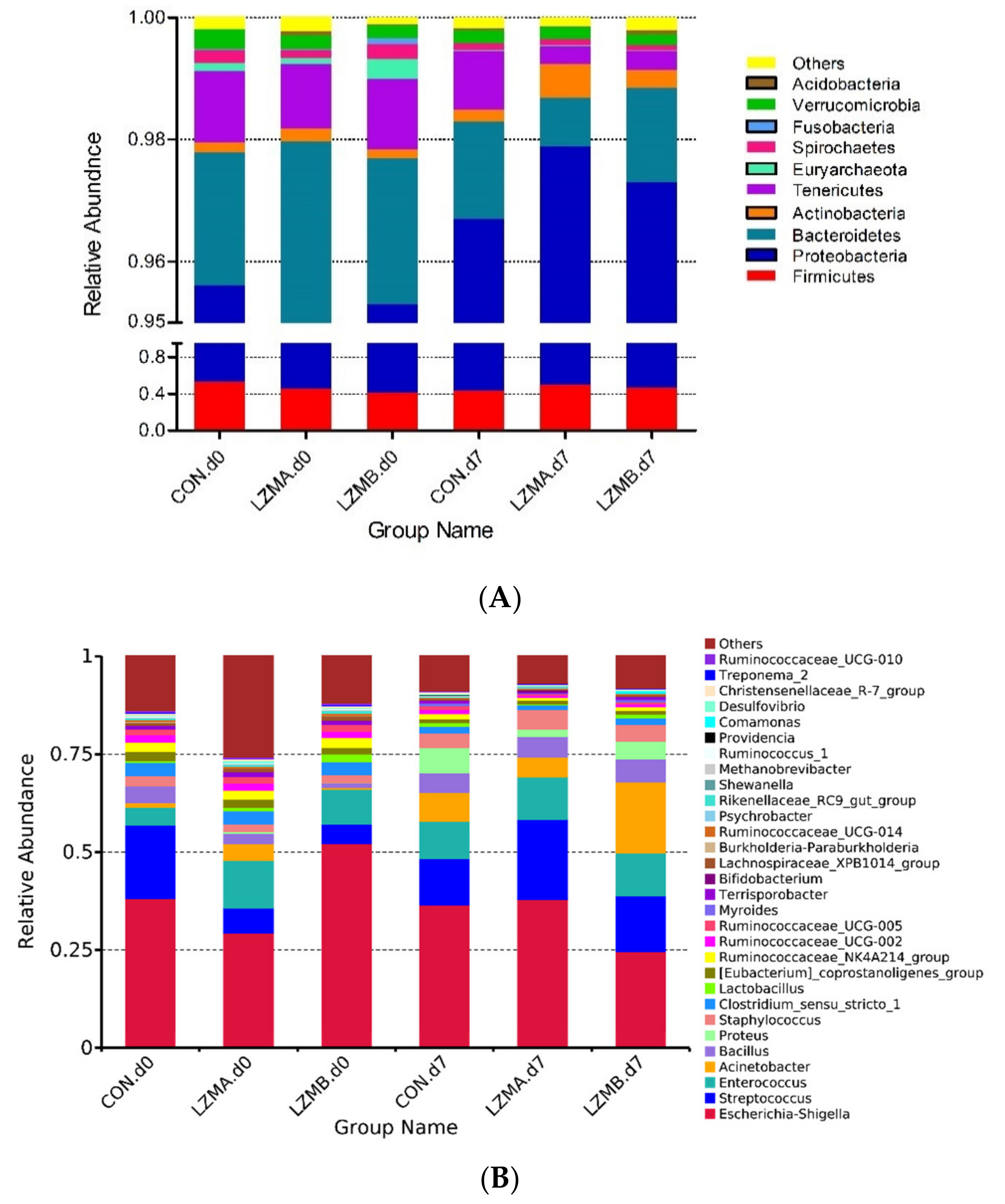
3.3. Changes of Vaginal Microbiota Composition by Lysozyme Supplementation in Sow
3.4. Correlations between the Vaginal Microbiota and Cytokines in Sow
3.5. Relationship between the Vaginal or Rectum Microbiota and the Sow Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Coppa, G.V.; Zampini, L.; Galeazzi, T.; Gabrielli, O. Prebiotics in human milk: A review. Dig. Liver Dis. 2006, 38, S291–S294. [Google Scholar] [CrossRef]
- Masschalck, B.; Michiels, C.W. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 2003, 29, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Nyachoti, C.M.; Kiarie, E.; Bhandari, S.K.; Zhang, G.; Krause, D.O. Weaned pig responses to Escherichia coli K88 oral challenge when receiving a lysozyme supplement. J. Anim. Sci. 2012, 90, 252–260. [Google Scholar] [CrossRef]
- Callewaert, L.; Michiels, C.W. Lysozymes in the animal kingdom. J. Biosci. 2010, 35, 127–160. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef]
- Cooper, C.A.; Garas Klobas, L.C.; Maga, E.A.; Murray, J.D. Consuming transgenic goats’ milk containing the antimicrobial protein lysozyme helps resolve diarrhea in young pigs. PLoS ONE 2013, 8, e58409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zou, L.; Xiong, X.; Liu, H.; Zhou, J.; Liu, Y.; Yin, Y. Effects of dietary lysozyme levels on growth performance, intestinal morphology, immunity response and microbiota community of growing pigs. J. Sci. Food. Agric. 2019, 99, 1643–1650. [Google Scholar] [CrossRef]
- Humphrey, B.D.; Huang, N.; Klasing, K.C. Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. J. Nutr. 2002, 132, 1214–1218. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Y.; Wang, Z.; Yuan, J. Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian. Pathol. 2010, 39, 17–24. [Google Scholar] [CrossRef]
- Long, Y.; Lin, S.; Zhu, J.; Pang, X.; Fang, Z.; Lin, Y.; Che, L.; Xu, S.; Li, J.; Huang, Y.; et al. Effects of dietary lysozyme levels on growth performance, intestinal morphology, non-specific immunity and mRNA expression in weanling piglets. Anim. Sci. J. 2016, 87, 411–418. [Google Scholar] [CrossRef]
- Oliver, W.T.; Wells, J.E. Lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology in nursery pigs. J. Anim. Sci. 2013, 91, 3129–3136. [Google Scholar] [CrossRef]
- Akira, S.; Hirano, T.; Taga, T.; Kishimoto, T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990, 4, 2860–2867. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.T.; Wells, J.E.; Maxwell, C.V. Lysozyme as an alternative to antibiotics improves performance in nursery pigs during an indirect immune challenge. J. Anim. Sci. 2014, 92, 4927–4934. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Agric. Food Chem. 2009, 57, 2233–2240. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Harrison, O.J. Homeostatic immunity and the microbiota. Immunity. 2017, 46, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhao, Y.; Zhang, P.; Li, Y.; Gui, T.; Wang, J.; Jin, C.; Che, L.; Li, J.; Lin, Y.; et al. Microbial mechanistic insight into the role of inulin in improving maternal health in a pregnant sow model. Front Microbiol. 2017, 8, 2242. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shi, J.; Dong, Y.; Li, Z.; Wu, X.; Lin, Y.; Che, L.; Li, J.; Feng, B.; Fang, Z.; et al. Fecal bacteria and metabolite responses to dietary lysozyme in a sow model from late gestation until lactation. Sci. Rep. 2020, 10, 3210. [Google Scholar] [CrossRef]
- Xu, S.; Shi, J.; Shi, X.; Dong, Y.; Wu, X.; Li, Z.; Fang, Z.; Lin, Y.; Che, L.; Li, J.; et al. Effects of dietary supplementation with lysozyme during late gestation and lactation stage on the performance of sows and their offspring. J. Anim. Sci. 2018, 96, 4768–4779. [Google Scholar]
- Lamont, R.F.; Sobel, J.D.; Akins, R.A.; Hassan, S.S.; Chaiworapongsa, T.; Kusanovic, J.P.; Romero, R. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG 2011, 118, 533–549. [Google Scholar] [CrossRef]
- Oakley, B.B.; Fiedler, T.L.; Marrazzo, J.M.; Fredricks, D.N. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl. Environ. Microbiol. 2008, 74, 4898–4909. [Google Scholar] [CrossRef]
- Niederwerder, M.C. Role of the microbiome in swine respiratory disease. Vet. Microbiol. 2017, 209, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Molgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of intestinal microbiota during early life: A longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Feng, B.; Xuan, Y.; Che, L.; Fang, Z.; Lin, Y.; Xu, S.; Li, J.; Feng, B.; Wu, D. Inclusion of purified dietary fiber during gestation improved the reproductive performance of sows. J. Anim. Sci. Biotechnol. 2020, 11, 47. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Nesengani, L.T.; Gong, Y.; Zhang, S.; Lu, W. Characterization of vaginal microbiota of endometritis and healthy sows using high-throughput pyrosequencing of 16S rRNA gene. Microb. Pathog. 2017, 111, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Kwawukume, A. The Gut and Vaginal Microbiome Profiles of Pregnant Sows and Their Contribution to Neonatal Piglet Gut Microbiome Development. Master’s Thesis, Department of Animal Science, University of Manitoba Winnipeg, Winnipeg, MB, Canada, 2017. [Google Scholar]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; van Teijlingen, N.H.; Geijtenbeek, T.B.; Wastling, J.M.; van de Wijgert, J.H. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal. Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J. Educ. Behav. Stat. 2000, 25, 60–83. [Google Scholar] [CrossRef]
- Cheng, C.; Wei, H.; Yu, H.; Xu, C.; Jiang, S.; Peng, J. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front. Microbiol. 2018, 9, 1989. [Google Scholar] [CrossRef]
- Jeon, S.J.; Vieira-Neto, A.; Gobikrushanth, M.; Daetz, R.; Mingoti, R.D.; Parize, A.C.; de Freitas, S.L.; da Costa, A.N.; Bicalho, R.C.; Lima, S.; et al. Uterine microbiota progression from calving until establishment of metritis in dairy cows. Appl. Environ. Microbiol. 2015, 81, 6324–6332. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Santin, T.; Rodrigues, M.X.; Marques, C.E.; Lima, S.F.; Bicalho, R.C. Dynamics of the microbiota found in the vaginas of dairy cows during the transition period: Associations with uterine diseases and reproductive outcome. J. Dairy Sci. 2017, 100, 3043–3058. [Google Scholar] [CrossRef]
- Elad, D.; Friedgut, O.; Alpert, N.; Stram, Y.; Lahav, D.; Tiomkin, D.; Avramson, M.; Grinberg, K.; Bernstein, M. Bovine necrotic vulvovaginitis associated with Porphyromonas levii. Emerg. Infect. Dis. 2004, 10, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Matamoros, S.E.B.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio. 2014, 5, e1011–e1014. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.; Ingerslev, H.C.; Sturek, M.; Alloosh, M.; Cirera, S.; Christoffersen, B.Ø.; Moesgaard, S.G.; Larsen, N.; Boye, M. Characterisation of gut microbiota in Ossabaw and Göttingen minipigs as models of obesity and metabolic syndrome. PLoS ONE 2013, 8, e56612. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect. Dis. 2002, 2, 171–179. [Google Scholar] [CrossRef]
- Muller, C.A.; Autenrieth, I.B.; Peschel, A. Innate defenses of the intestinal epithelial barrier. Cell Mol. Life Sci. 2005, 62, 1297–1307. [Google Scholar] [CrossRef]
- Bara, M.R.; McGowan, M.R.; O’Boyle, D.; Cameron, R.D. A study of the microbial flora of the anterior vagina of normal sows during different stages of the reproductive cycle. Aust. Vet. J. 1993, 70, 256–259. [Google Scholar] [CrossRef]
- Wlodarska, M.; Willing, B.P.; Bravo, D.M.; Finlay, B.B. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci. Rep. 2015, 5, 9253. [Google Scholar] [CrossRef]
- Adhikari, B.; Tellez-Isaias, G.; Jiang, T.; Wooming, B.; Kwon, Y.M. Comprehensive survey of the litter bacterial communities in commercial turkey farms. Front. Vet. Sci. 2020, 7, 596933. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Jacobson, M.; Andersen, P.H.; Bækbo, P.; Cerón, J.J.; Dahl, J.; Escribano, D.; Jacobsen, S. Inflammatory markers before and after farrowing in healthy sows and in sows affected with postpartum dysgalactia syndrome. BMC Vet. Res. 2018, 14, 83. [Google Scholar]
- Böhmer, B.M.; Kramer, W.; Roth-Maier, D.A. Dietary probiotic supplementation and resulting effects on performance, health status, and microbial characteristics of primiparous sows. J. Anim. Physiol. Anim. Nutr. 2006, 90, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Swartz, J.D.; Lachman, M.; Westveer, K.; O’Neill, T.; Geary, T.; Kott, R.W.; Berardinelli, J.G.; Hatfield, P.G.; Thomson, J.M.; Roberts, A.; et al. Characterization of the vaginal microbiota of ewes and cows reveals a unique microbiota with low levels of Lactobacilli and near-neutral pH. Front. Vet. Sci. 2014, 1, 19. [Google Scholar] [CrossRef]
- Laguardia-Nascimento, M.; Branco, K.M.; Gasparini, M.R.; Giannattasio-Ferraz, S.; Leite, L.R.; Araujo, F.M.; Salim, A.C.; Nicoli, J.R.; de Oliveira, G.C.; Barbosa-Stancioli, E.F. Vaginal microbiome characterization of Nellore cattle using metagenomic analysis. PLoS ONE 2015, 10, e0143294. [Google Scholar] [CrossRef] [PubMed]
- Aroutcheva, A.; Gariti, D.; Simon, M.; Shott, S.; Faro, J.; Simoes, J.A.; Gurguis, A.; Faro, S. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 2001, 185, 375–379. [Google Scholar] [CrossRef]
- Hickey, R.J.; Zhou, X.; Pierson, J.D.; Ravel, J.; Forney, L.J. Understanding vaginal microbiome complexity from an ecological perspective. Transl. Res. 2012, 160, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, C.; Cheng, C.; Cui, J.; Ji, Y.; Hao, X.; Li, Q.; Ren, W.; Deng, B.; Yin, Y.; et al. Unraveling the association of fecal microbiota and oxidative stress with stillbirth rate of sows. Theriogenology 2019, 136, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, C.; Georgoulakis, I.E.; Tzivara, A.; Kritas, S.K.; Siochu, A.; Kyriakis, S.C. Field evaluation of the efficacy of a probiotic containing Bacillus licheniformis and Bacillus subtilis spores, on the health status and performance of sows and their litters. J. Anim. Physiol. Anim. Nutr. 2004, 88, 381–392. [Google Scholar] [CrossRef]
- Kritas, S.K.; Marubashi, T.; Filioussis, G.; Petridou, E.; Christodoulopoulos, G.; Burriel, A.R.; Tzivara, A.; Theodoridis, A.; Pískoriková, M. Reproductive performance of sows was improved by administration of a sporing bacillary probiotic (Bacillus subtilis C-3102). J. Anim. Sci. 2015, 93, 405–413. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Song, D.; Peng, Q.; Chen, Y.; Zhou, X.; Zhang, F.; Li, A.; Huang, D.; Wu, Q.; Ye, Y.; He, H.; et al. Altered gut microbiota profiles in sows and neonatal piglets associated with porcine epidemic diarrhea virus infection. Sci. Rep. 2017, 12, 17439. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K.; Stackebrandt, E.; Hamilton, W.A. Energy metabolism and phylogenetic diversity of sulphate-reducing bacteria. In Sulphate-Reducing Bacteria Environmental and Engineered, Systems; Barton, L.L., Hamilton, W.A., Eds.; Cambridge University Press: New York, NY, USA, 2007; pp. 1–37. [Google Scholar]
- Coleman, M.L.; Hedrick, D.B.; Lovley, D.R.; White, D.C.; Pye, K. Reduction of Fe(III) in sediments by sulphate-reducing bacteria. Nature 1993, 361, 436–438. [Google Scholar] [CrossRef]
- Michel, C.; Brugna, M.; Aubert, C.; Bernadac, A.; Bruschi, M. Enzymatic reduction of chromate: Comparative studies using sulfate-reducing bacteria. Key role of polyheme cytochromes c and hydrogenases. Appl. Microbiol. Biotechnol. 2001, 55, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Kuehl, J.V.; Price, M.N.; Ray, J.; Deutschbauer, A.M.; Arkin, A.P.; Stahl, D.A. The energy-conserving electron transfer system used by Desulfovibrio alaskensis strain G20 during pyruvate fermentation involves reduction of endogenously formed fumarate and cytoplasmic and membrane-bound complexes, Hdr-Flox and Rnf. Environ. Microbiol. 2014, 16, 3463–3486. [Google Scholar] [CrossRef] [PubMed]


| Item | Time | Treatment | p-Value | FDR | ||
|---|---|---|---|---|---|---|
| CON. | LZMA | LZMB | ||||
| IL-6 (ng/l) | d 0 | 58.40 ± 7.12 | 56.48 ± 9.86 | 56.08 ± 9.93 | 0.90 | 0.93 |
| d 7 | 67.09 ± 3.41 | 63.88 ± 3.12 | 63.58 ± 3.58 | 0.23 | 0.35 | |
| IL-10 (ng/l) | d 0 | 250.78 ± 20.39 | 280.4 ± 14.75 | 274.06 ± 20.17 | 0.08 | 0.09 |
| d 7 | 266.67 ± 10.60 b | 287.36 ± 8.48 a | 284.09 ± 12.27 a | 0.02 | 0.04 | |
| TNF-α (ng/l) | d 0 | 122.68 ± 10.32 | 119.54 ± 9.21 | 121.1 ± 10.67 | 0.86 | 0.97 |
| d 7 | 123.38 ± 15.29 | 121.65 ± 18.77 | 122.54 ± 14.38 | 0.98 | 0.99 |
| Phyla | Genera | Treatment | p-Value | FDR | ||
|---|---|---|---|---|---|---|
| CON. | LZMA | LZMB | ||||
| d 0 | d 0 | |||||
| Firmicutes | 52.77 ± 9.08 | 45.10 ± 6.94 | 41.20 ± 6.56 | 0.56 | 0.69 | |
| Streptococcus | 18.65 ± 7.15 a | 6.37 ± 1.35 b | 5.05 ± 1.13 b | 0.01 | 0.02 | |
| Enterococcus | 4.67 ± 1.16 b | 12.19 ± 4.67 a | 8.88 ± 2.32 a | 0.05 | 0.06 | |
| Bacillus | 4.22 ± 2.29 a | 2.66 ± 0.27 b | 1.19 ± 0.19 b | 0.03 | 0.04 | |
| Lachnospiraceae_XPB1014_group | 0.59 ± 0.10 b | 0.81 ± 0.15 ab* | 1.17 ± 0.25 a | 0.04 | 0.05 | |
| Lactobacillus | 0.46 ± 0.20 b | 0.89 ± 0.25 ab* | 2.11 ± 0.76 a* | 0.04 | 0.05 | |
| Oscillospira | 0.34 ± 0.04 a* | 0.23 ± 0.02 b* | 0.22 ± 0.03 b* | 0.03 | 0.04 | |
| Family_XIII_AD3011_group | 0.31 ± 0.04 a* | 0.17 ± 0.02 b* | 0.24 ± 0.03 b* | 0.04 | 0.05 | |
| Lachnospiraceae_AC2044_group | 0.28 ± 0.04 b | 0.23 ± 0.02 b | 0.39 ± 0.05 a | 0.03 | 0.04 | |
| Proteobacteria | 42.88 ± 0.09 | 49.87 ± 6.7 | 54.19 ± 6.85 | 0.59 | 0.71 | |
| Acinetobacter | 1.09 ± 0.51 b | 4.28 ± 0.81 a | 4.53 ± 0.05 a | <0.05 | <0.05 | |
| Burkholderia-Paraburkholderia | 0.31 ± 0.27 a | 0.04 ± 0.01 b | 0.24 ± 0.09 a | 0.04 | 0.04 | |
| Bacteroidetes | 2.12 ± 0.11 b | 3.06 ± 0.43 a* | 2.36 ± 0.37 ab* | 0.03 | 0.04 | |
| Tenericutes | 1.16 ± 0.08 | 1.04 ± 0.10 * | 1.14 ± 0.11 * | 0.87 | 0.95 | |
| Verrucomicrobia | 0.32 ± 0.06 | 0.24 ± 0.01 | 0.19 ± 0.01 | 0.61 | 0.76 | |
| Spirochaetes | 0.21 ± 0.04 * | 0.13 ± 0.001 | 0.23 ± 0.06 * | 0.58 | 0.68 | |
| Actinobacteria | 0.17 ± 0.001 | 0.21 ± 0.001 | 0.14 ± 0.01 | 0.69 | 0.81 | |
| Euryarchaeota | 0.14 ± 0.001 * | 0.10 ± 0.001 * | 0.33 ± 0.11 * | 0.64 | 0.79 | |
| d 7 | ||||||
| Firmicutes | 43.81 ± 2.08 | 50.31 ± 6.84 | 46.58 ± 3.49 | 0.61 | 0.75 | |
| Streptococcus | 11.84 ± 2.21 b | 20.32 ± 4. 41 a* | 14.20 ± 1.22 ab* | 0.04 | 0.05 | |
| Ruminococcaceae_NK4A214_group | 1.37 ± 0.24 a | 0.85 ± 0.21 b | 0.98 ± 0.27 ab | 0.04 | 0.05 | |
| Ruminococcaceae_UCG-002 | 1.13 ± 0.26 a | 0.53 ± 0.08 b | 0.62 ± 0.10 b | 0.03 | 0.04 | |
| Ruminococcaceae_UCG-005 | 0.90 ± 0.12 a | 0.29 ± 0.05 b | 0.55 ± 0.10 b | <0.05 | <0.05 | |
| Terrisporobacter | 0.63 ± 0.18 a | 0.39 ± 0.18 b | 0.74 ± 0.05 a | 0.04 | 0.04 | |
| Lachnospiraceae_XPB1014_group | 0.55 ± 0.10 | 0.22 ± 0.04 | 0.54 ± 0.16 | 0.05 | 0.05 | |
| Christensenellaceae_R-7_group | 0.37 ± 0.03 | 0.24 ± 0.04 | 0.29 ± 0.05 | 0.05 | 0.06 | |
| Lactobacillus | 0.94 ± 0.46 a | 0.31 ± 0.21 b | 1.07 ± 0.33 a | 0.04 | 0.05 | |
| Family_XIII_AD3011_group | 0.22 ± 0.02 a | 0.09 ± 0.02 b | 0.13 ± 0.02 b | <0.05 | <0.05 | |
| Proteobacteria | 52.96 ± 2.14 | 47.59 ± 7.14 | 50.77 ± 3.39 | 0.73 | 0.86 | |
| Escherichia-Shigella | 36.58 ± 6.16 a | 37.98 ± 8.14 a | 24.60 ± 3.34 b | 0.03 | 0.04 | |
| Acinetobacter | 7.31 ± 2.28 b* | 5.03 ± 1.62 b | 18.15 ± 2.31 a* | <0.05 | <0.05 | |
| Bacteroidetes | 1.58 ± 0.45 | 0.79 ± 0.12 | 1.52 ± 0.22 | 0.15 | 0.38 | |
| Tenericutes | 0.96 ± 0.16 a | 0.28 ± 0.06 b | 0.30 ± 0.05 b | <0.05 | <0.05 | |
| Verrucomicrobia | 0.20 ± 0.003 | 0.20 ± 0.001 | 0.17 ± 0.001 | 0.69 | 0.79 | |
| Spirochaetes | 0.11 ± 0.006 | 0.10 ± 0.001 | 0.09 ± 0.001 | 0.85 | 0.91 | |
| Actinobacteria | 0.20 ± 0.01 | 0.37 ± 0.02 * | 0.29 ± 0.05 * | 0.07 | 0.08 | |
| Euryarchaeota | 0.01 ± 0.01 | 0.01 ± 0.02 | 0.01 ± 0.001 | 0.87 | 0.94 | |
| Item | Time | Treatment | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON. | LZMA | LZMB | Diet | Time | Diet*Time | ||
| Observed species | d 0 | 821.50 ± 35.67 * | 931.33 ± 38.94 * | 792.50 ± 32.59 * | 0.89 | <0.01 | <0.01 |
| d 7 | 734.00 ± 55.27 a | 586.67 ± 34.27 b | 651.83 ± 43.07 b | ||||
| Chao 1 | d 0 | 919.63 ± 42.72 | 1009.63 ± 43.39 * | 890.06 ± 37.91 * | 0.81 | <0.01 | <0.01 |
| d 7 | 841.68 ± 62.32 a | 663.14 ± 40.74 b | 779.90 ± 51.05 b | ||||
| Shannon | d 0 | 4.14 ± 0.42 | 4.29 ± 0.20 | 3.84 ± 0.37 | 0.37 | 0.77 | 0.15 |
| d 7 | 4.01 ± 0.22 | 3.45 ± 0.28 | 4.17 ± 0.14 | ||||
| Escherichia.Shigella | Streptococcus | Clostridium_sensu_stricto_1 | Lactobacillus | Ruminococcaceae_UCG.014 | Terrisporobacter | Desulfovibrio | Bacillus | |
| Correlations between the vagina microbiota and sow performance | ||||||||
| Total born | −0.083 | −0.004 | 0.140 | 0.173 | 0.003 | 0.081 | 0.273 | −0.107 |
| Born alive | −0.069 | −0.169 | 0.254 | 0.147 | −0.136 | 0.159 | 0.309 * | −0.073 |
| Stillborn | 0.078 | 0.214 | −0.131 | −0.116 | 0.165 | −0.154 | −0.011 | −0.001 |
| Neonatal weight | 0.236 | −0.162 | 0.116 | 0.152 | −0.098 | 0.146 | 0.181 | −0.141 |
| Correlations between the rectum microbiota and sow performance | ||||||||
| Total born | −0.131 | −0.091 | 0.177 | 0.089 | −0.177 | 0.218 | 0.357 ** | −0.072 |
| Born alive | −0.070 | −0.255 | −0.355 ** | 0.064 | −0.376 ** | 0.375 ** | 0.446 ** | 0.069 |
| Stillborn | −0.078 | 0.214 | −0.208 | 0.007 | 0.284 | −0.281 | −0.166 | −0.235 |
| Neonatal weight | −0.090 | 0.148 | −0.134 | 0.096 | −0.201 | −0.017 | −0.143 | 0.368 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Dong, Y.; Shi, J.; Li, Z.; Che, L.; Lin, Y.; Li, J.; Feng, B.; Fang, Z.; Yong, Z.; et al. Responses of Vaginal Microbiota to Dietary Supplementation with Lysozyme and its Relationship with Rectal Microbiota and Sow Performance from Late Gestation to Early Lactation. Animals 2021, 11, 593. https://doi.org/10.3390/ani11030593
Xu S, Dong Y, Shi J, Li Z, Che L, Lin Y, Li J, Feng B, Fang Z, Yong Z, et al. Responses of Vaginal Microbiota to Dietary Supplementation with Lysozyme and its Relationship with Rectal Microbiota and Sow Performance from Late Gestation to Early Lactation. Animals. 2021; 11(3):593. https://doi.org/10.3390/ani11030593
Chicago/Turabian StyleXu, Shengyu, Yanpeng Dong, Jiankai Shi, Zimei Li, Lianqiang Che, Yan Lin, Jian Li, Bin Feng, Zhengfeng Fang, Zhuo Yong, and et al. 2021. "Responses of Vaginal Microbiota to Dietary Supplementation with Lysozyme and its Relationship with Rectal Microbiota and Sow Performance from Late Gestation to Early Lactation" Animals 11, no. 3: 593. https://doi.org/10.3390/ani11030593
APA StyleXu, S., Dong, Y., Shi, J., Li, Z., Che, L., Lin, Y., Li, J., Feng, B., Fang, Z., Yong, Z., Wang, J., & Wu, D. (2021). Responses of Vaginal Microbiota to Dietary Supplementation with Lysozyme and its Relationship with Rectal Microbiota and Sow Performance from Late Gestation to Early Lactation. Animals, 11(3), 593. https://doi.org/10.3390/ani11030593







