Bacillus amyloliquefaciens-9 as an Alternative Approach to Cure Diarrhea in Saanen Kids
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Preparation of GBacillus-9
2.3. Animal Experimental Design
2.4. Serum Collection and Biochemical Analysis
2.5. Fecal Collection and 16S rRNA Gene Sequencing
2.6. Sequencing Data Analysis, Bacterial Richness, and Community Diversity
2.7. Statistical Analysis
3. Results
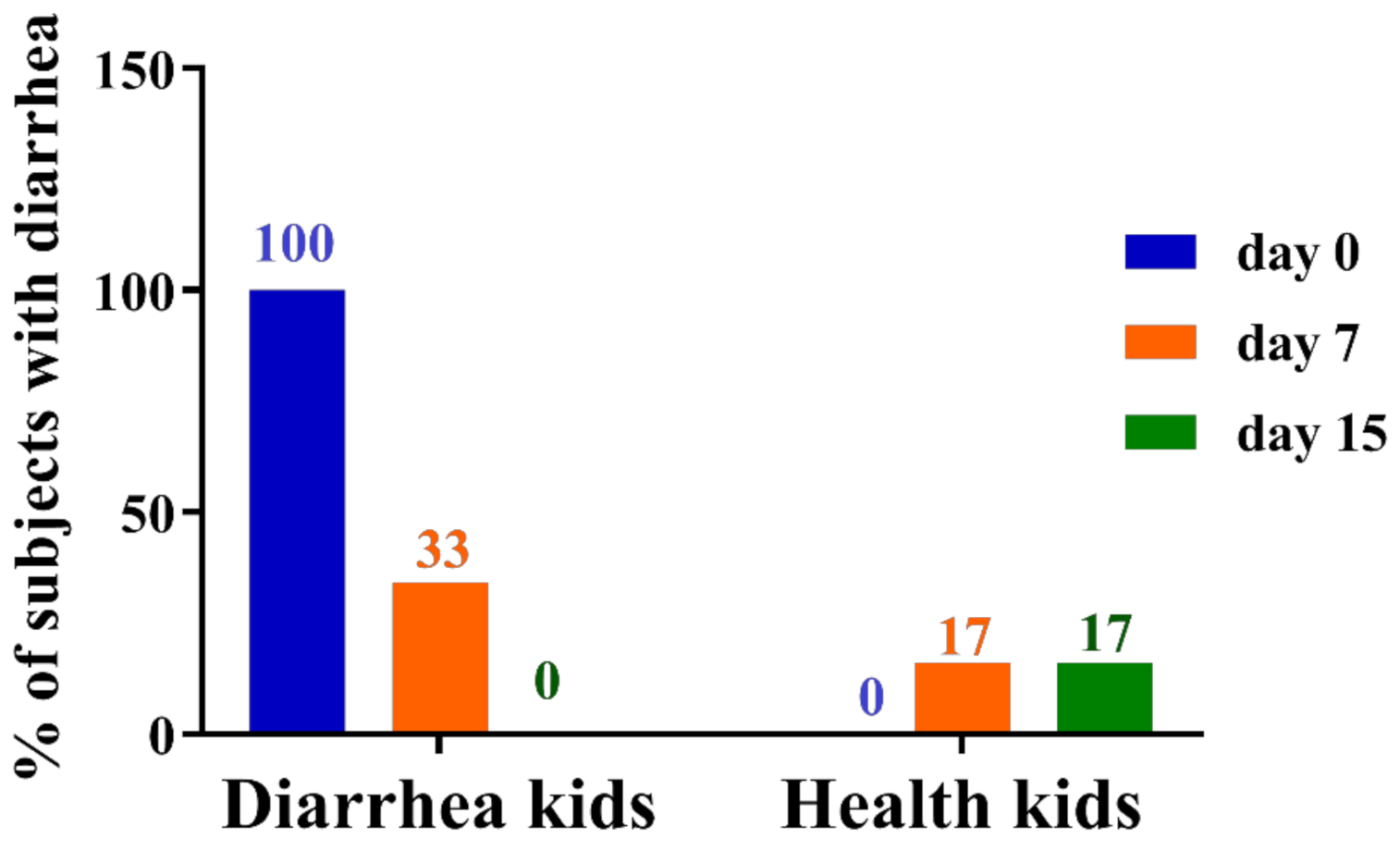
3.1. Role of GBacillus-9 in the Prevention and Cure of Diarrhea in Kids
3.2. Alteration of the Immune and the Biochemical Indices of DL Kids by GBacillus-9
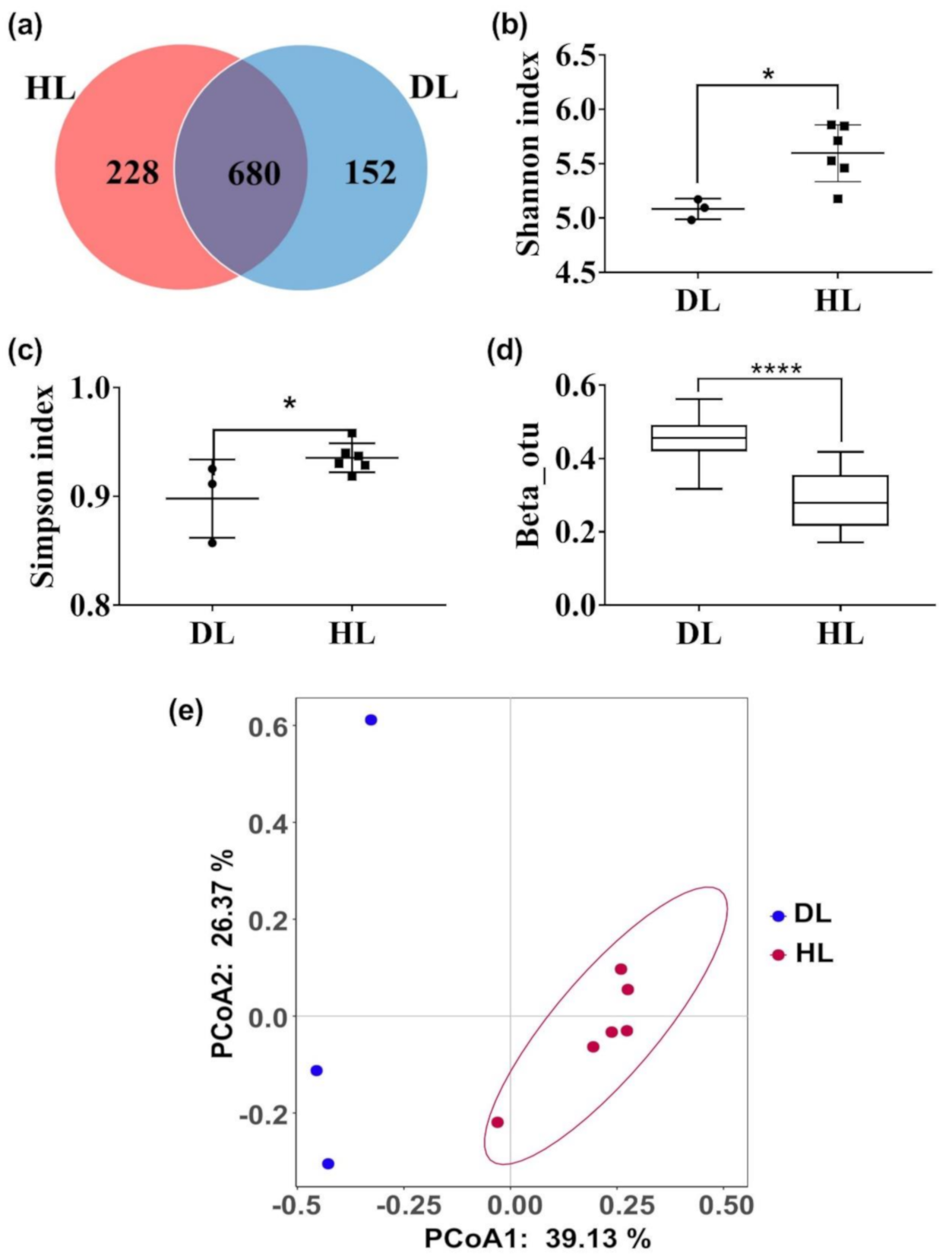
3.3. Comparison of the Fecal Microbiota between HL and DL Kids at Day 0
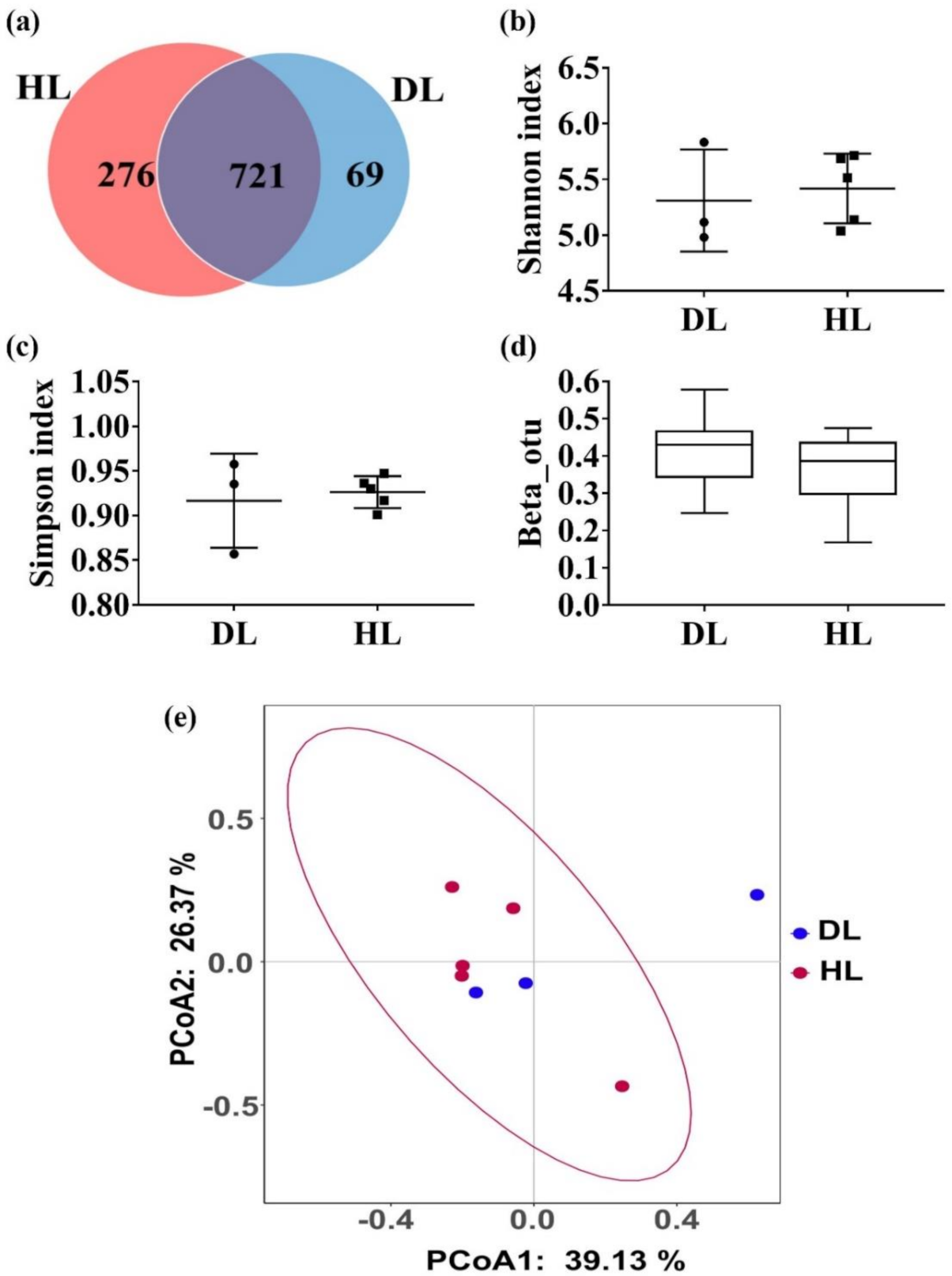
3.4. Changes in the Fecal Microbiota between the HL and the DL Group after the GBacillus-9 Administration
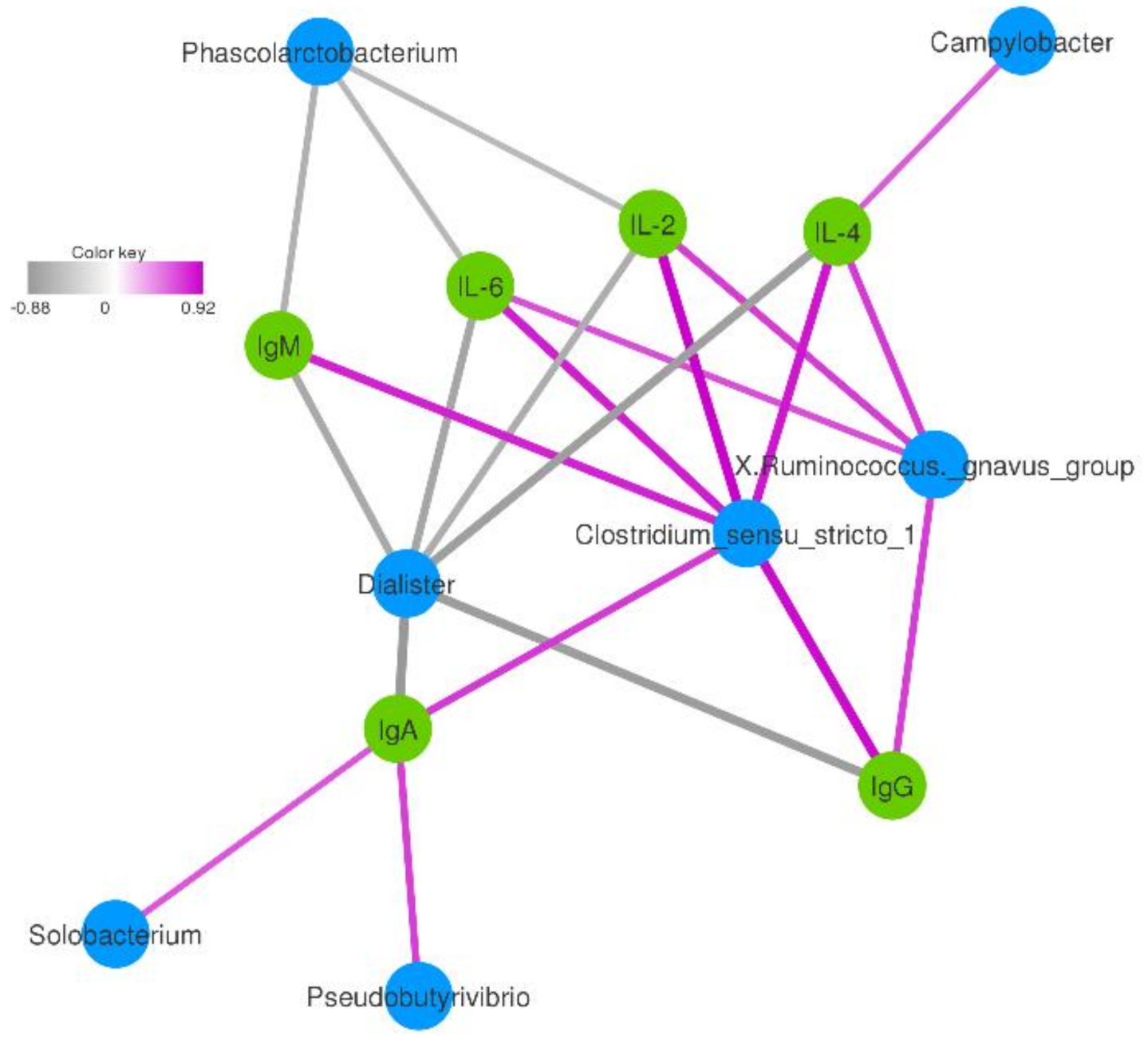
3.5. Correlation between the Immune and the Dominant Genera of Fecal Bacteria
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fortuoso, B.F.; Gebert, R.R.; Griss, L.G.; Glombovisky, P.; Cazarotto, C.J.; Rampazzo, L.; Stefani, L.M.; Ferreira, E.B.; da Silva, A.S. Reduction of stool bacterial counts and prevention of diarrhea using an oral homeopathic product in newborn lambs. Microb. Pathog. 2019, 127, 347–351. [Google Scholar] [CrossRef]
- Lübbert, C. Antimicrobial therapy of acute diarrhoea: A clinical review. Expert Rev. Anti-Infect. Ther. 2016, 14, 193–206. [Google Scholar] [CrossRef]
- Nelson, D.D.; Duprau, J.L.; Wolff, P.L.; Evermann, J.F. Persistent Bovine Viral Diarrhea Virus Infection in Domestic and Wild Small Ruminants and Camelids Including the Mountain Goat (Oreamnos americanus). Front. Microbiol 2015, 6, 1415. [Google Scholar] [CrossRef]
- Trachtman, H.; Christen, E. Pathogenesis, treatment, and therapeutic trials in hemolytic uremic syndrome. Curr. Opin. Pediatr. 1999, 11, 162–168. [Google Scholar] [CrossRef]
- Yang, H.; Sun, Y.; Cai, R.; Chen, Y.; Gu, B. The impact of dietary fiber and probiotics in infectious diseases. Microb. Pathog. 2020, 140, 103931. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Pamer, E.G. Antibiotics, microbiota, and immune defense. Trends Immunol. 2012, 33, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Yadav, H.; Sinha, P.R. Stimulation of innate immunity by oral administration of dahi containing probiotic Lactobacillus casei in mice. J. Med. Food 2008, 11, 652–656. [Google Scholar] [CrossRef]
- Dong, H.; Rowland, I.; Yaqoob, P. Comparative effects of six probiotic strains on immune function in vitro. Br. J. Nutr. 2012, 108, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Mutukumira, A.; Lee, S.J.; Maddox, I.; Shu, Q. Functional properties of free and encapsulated Lactobacillus reuteri DPC16 during and after passage through a simulated gastrointestinal tract. World J. Microbiol. Biotechnol. 2012, 28, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, K.; Meng, D.; Rautava, S.; Lu, L.; Walker, W.A.; Nanthakumar, N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G132–G141. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.; Rosalen, P.L.; Freires, I.A.; Sardi, J.C.O.; Lima, R.F.; Lazarini, J.G.; Costa, T.; Pereira, J.V.; Godoy, G.P.; Costa, E. Anadenanthera Colubrina vell Brenan: Anti-Candida and antibiofilm activities, toxicity and therapeutical action. Braz. Oral Res. 2019, 33, e023. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.I.; Chung, K.C. Production of an antifungal protein for control of Colletotrichum lagenarium by Bacillus amyloliquefaciens MET0908. FEMS Microbiol. Lett. 2004, 234, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Arrebola, E.; Jacobs, R.; Korsten, L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 2010, 108, 386–395. [Google Scholar] [CrossRef]
- Cao, G.T.; Zhan, X.A.; Zhang, L.L.; Zeng, X.F.; Chen, A.G.; Yang, C.M. Modulation of broilers’ caecal microflora and metabolites in response to a potential probiotic Bacillus amyloliquefaciens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 909–917. [Google Scholar] [CrossRef]
- Wu, J.; Xu, G.; Jin, Y.; Sun, C.; Zhou, L.; Lin, G.; Xu, R.; Wei, L.; Fei, H.; Wang, D.; et al. Isolation and characterization of Bacillus sp. GFP-2, a novel Bacillus strain with antimicrobial activities, from Whitespotted bamboo shark intestine. Amb. Express 2018, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Lin, G.D.; Zheng, C.C.; Huang, M.M.; Qian, S.C.; Wu, Z.J.; Sun, C.; Shi, Z.G.; Li, J.Y.; Han, B.N. Effects of Bacillus amyloliquefaciens and Yarrowia lipolytica lipase 2 on immunology and growth performance of Hybrid sturgeon. Fish. Shellfish Immunol. 2018, 82, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, L.; Xu, R.; Lin, G.; Xin, H.; Lv, Z.; Qian, H.; Shi, H. Evaluation of the Antibacterial Material Production in the Fermentation of Bacillus amyloliquefaciens-9 from Whitespotted Bamboo Shark (Chiloscyllium plagiosum). Mar. Drugs 2020, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Infante, D.D.; Segarra, O.O.; Redecillas, S.S.; Alvarez, M.M.; Miserachs, M.M. Modification of stool’s water content in constipated infants: Management with an adapted infant formula. Nutr. J. 2011, 10, 55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sadek, K.; Saleh, E.; Ayoub, M. Selective, reliable blood and milk bio-markers for diagnosing clinical and subclinical bovine mastitis. Trop. Anim. Health Prod. 2017, 49, 431–437. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Wang, D. Effects of body condition on the insulin resistance, lipid metabolism and oxidative stress of lactating dairy cows. Lipids Health Dis. 2020, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, K.; Li, C.; Wang, X.; Chen, Y.; Yang, Y. Characterization and Comparison of Microbiota in the Gastrointestinal Tracts of the Goat (Capra hircus) During Preweaning Development. Front. Microbiol. 2019, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Ali, F.S.; Qiao, W.; Lum, P.; Raju, G.; Shuttlesworth, G.; Stroehlein, J.; et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: Retrospective review at MD Anderson. J. Immunother. Cancer 2018, 6, 37. [Google Scholar] [CrossRef]
- Krones, E.; Högenauer, C. Diarrhea in the immunocompromised patient. Gastroenterol. Clin. N. Am. 2012, 41, 677–701. [Google Scholar] [CrossRef]
- Li, S.; Yang, J.; Zhu, Z.; Zheng, H. Porcine Epidemic Diarrhea Virus and the Host Innate Immune Response. Pathogens 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Piewngam, P.; Zheng, Y.; Nguyen, T.H.; Dickey, S.W.; Joo, H.S.; Villaruz, A.E.; Glose, K.A.; Fisher, E.L.; Hunt, R.L.; Li, B.; et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 2018, 562, 532–537. [Google Scholar] [CrossRef]
- Shu, Q.; Qu, F.; Gill, H.S. Probiotic treatment using Bifidobacterium lactis HN019 reduces weanling diarrhea associated with rotavirus and Escherichia coli infection in a piglet model. J. Pediatric Gastroenterol. Nutr. 2001, 33, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Foditsch, C.; Pereira, R.V.; Ganda, E.K.; Gomez, M.S.; Marques, E.C.; Santin, T.; Bicalho, R.C. Oral Administration of Faecalibacterium prausnitzii Decreased the Incidence of Severe Diarrhea and Related Mortality Rate and Increased Weight Gain in Preweaned Dairy Heifers. PloS ONE 2015, 10, e0145485. [Google Scholar] [CrossRef]
- Soto, L.P.; Astesana, D.M.; Zbrun, M.V.; Blajman, J.E.; Salvetti, N.R.; Berisvil, A.P.; Rosmini, M.R.; Signorini, M.L.; Frizzo, L.S. Probiotic effect on calves infected with Salmonella Dublin: Haematological parameters and serum biochemical profile. Benef. Microbes 2016, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.S.; Munks, M.W.; Marrack, P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity 2007, 27, 687–690. [Google Scholar] [CrossRef]
- Turner, J.L.; Dritz, S.S.; Higgins, J.J.; Minton, J.E. Effects of Ascophyllum nodosum extract on growth performance and immune function of young pigs challenged with Salmonella typhimurium. J. Anim. Sci. 2002, 80, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Mou, C.; Zhang, E.; Wang, Y.; Cao, Y.; Yang, Q. Mucosal immune responses induced by oral administration recombinant Bacillus subtilis expressing the COE antigen of PEDV in newborn piglets. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Renckens, R.; Roelofs, J.J.; Knapp, S.; de Vos, A.F.; Florquin, S.; van der Poll, T. The acute-phase response and serum amyloid A inhibit the inflammatory response to Acinetobacter baumannii Pneumonia. J. Infect. Dis. 2006, 193, 187–195. [Google Scholar] [CrossRef]
- Laskowska, E.; Jarosz, Ł.; Grądzki, Z. Effect of Multi-Microbial Probiotic Formulation Bokashi on Pro- and Anti-Inflammatory Cytokines Profile in the Serum, Colostrum and Milk of Sows, and in a Culture of Polymorphonuclear Cells Isolated from Colostrum. Probiotics Antimicrob. Proteins 2019, 11, 220–232. [Google Scholar] [CrossRef]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef]
- Islam, K.; Ahad, A.; Barua, M.; Islam, A.; Chakma, S.; Dorji, C.; Uddin, M.; Islam, S.; Ahasan, A. Isolation and epidemiology of multidrug resistant Escherichia coli from goats in Cox’s Bazar, Bangladesh. J. Adv. Vet. Anim. Res. 2016, 3, 166. [Google Scholar] [CrossRef]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef]
- Sundset, M.A.; Praesteng, K.E.; Cann, I.K.; Mathiesen, S.D.; Mackie, R.I. Novel rumen bacterial diversity in two geographically separated sub-species of reindeer. Microb. Ecol. 2007, 54, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, A.A.; Evans, P.N.; Wright, A.D.; Al Jassim, R. Molecular diversity of the foregut bacteria community in the dromedary camel (Camelus dromedarius). Environ. Microbiol. 2011, 13, 3024–3035. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.M.; Reda, R.M. Improvement of immunity and disease resistance in the Nile tilapia, Oreochromis niloticus, by dietary supplementation with Bacillus amyloliquefaciens. Fish Shellfish Immunol. 2015, 44, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Dai, Y.; Liu, B.; Wang, L.; Wang, J.; Zhang, J. Diversity analysis of intestinal microflora between healthy and diarrheal neonatal piglets from the same litter in different regions. Anaerobe 2019, 55, 136–141. [Google Scholar] [CrossRef]
- Tito, R.Y.; Cypers, H.; Joossens, M.; Varkas, G.; Van Praet, L.; Glorieus, E.; Van den Bosch, F.; De Vos, M.; Raes, J.; Elewaut, D. Brief Report: Dialister as a Microbial Marker of Disease Activity in Spondyloarthritis. Arthritis Rheumatol. 2017, 69, 114–121. [Google Scholar] [CrossRef] [PubMed]

| Taxonomy | DL (%) | HL (%) | p Value |
|---|---|---|---|
| Firmicutes | 33.24 ± 5.90 | 65.34 ± 9.16 | 0.002 |
| Bacteroidetes | 31.40 ± 12.16 | 18.44 ± 10.55 | 0.19 |
| Actinobacteria | 14.24 ± 18.50 | 9.59 ± 8.55 | 0.66 |
| Verrucomicrobia | 8.36 ± 8.13 | 0.26 ± 0.13 | 0.07 |
| Cyanobacteria | 4.31 ± 5.95 | 0.68 ± 0.66 | 0.23 |
| Proteobacteria | 7.82 ± 2.16 | 3.52 ± 1.18 | 0.01 |
| Euryarchaeota | 0.23 ± 0.11 | 1.74 ± 1.48 | 0.16 |
| Tenericutes | 0.17 ± 0.01 | 0.39 ± 0.48 | 0.50 |
| Fusobacteria | 0.15 ± 0.12 | 0.003 ± 0.003 | 0.028 |
| Saccharibacteria | 0.040 ± 0.037 | 0.037 ± 0.040 | 0.92 |
| Taxonomy | DL (%) | HL (%) | p Value |
|---|---|---|---|
| Firmicutes | 36.54 ± 8.36 | 49.47 ± 19.84 | 0.37 |
| Bacteroidetes | 37.05 ± 15.90 | 20.16 ± 7.28 | 0.095 |
| Actinobacteria | 16.40 ± 16.96 | 9.75 ± 8.59 | 0.51 |
| Proteobacteria | 6.31 ± 2.13 | 10.72 ± 12.77 | 0.62 |
| Verrucomicrobia | 2.17 ± 2.17 | 5.45 ± 7.68 | 0.54 |
| Cyanobacteria | 0.75 ± 0.91 | 2.84 ± 4.45 | 0.50 |
| Euryarchaeota | 0.39 ± 0.27 | 1.15 ± 0.97 | 0.28 |
| Tenericutes | 0.19 ± 0.06 | 0.31 ± 0.27 | 0.54 |
| Saccharibacteria | 0.06 ± 0.04 | 0.06 ± 0.08 | 0.97 |
| Fusobacteria | 0.12 ± 0.08 | 0.05 ± 0.07 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Xin, H.; Jiang, N.; Lv, Z.; Shu, J.; Shi, H. Bacillus amyloliquefaciens-9 as an Alternative Approach to Cure Diarrhea in Saanen Kids. Animals 2021, 11, 592. https://doi.org/10.3390/ani11030592
Zhang W, Xin H, Jiang N, Lv Z, Shu J, Shi H. Bacillus amyloliquefaciens-9 as an Alternative Approach to Cure Diarrhea in Saanen Kids. Animals. 2021; 11(3):592. https://doi.org/10.3390/ani11030592
Chicago/Turabian StyleZhang, Wenying, Huijie Xin, Nannan Jiang, Zhengbing Lv, Jianhong Shu, and Hengbo Shi. 2021. "Bacillus amyloliquefaciens-9 as an Alternative Approach to Cure Diarrhea in Saanen Kids" Animals 11, no. 3: 592. https://doi.org/10.3390/ani11030592
APA StyleZhang, W., Xin, H., Jiang, N., Lv, Z., Shu, J., & Shi, H. (2021). Bacillus amyloliquefaciens-9 as an Alternative Approach to Cure Diarrhea in Saanen Kids. Animals, 11(3), 592. https://doi.org/10.3390/ani11030592






