The Improvement of the Adaptation Process of Tocopherol and Acetylsalicylic Acid in Offspring of Mothers Exposed to TCDD
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
- −
- control: not treated with any chemicals;
- −
- TCDD: a single dose of TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin; Greyhound Chromatography and Allied Chemicals, Birkenhead, UK) at a concentration of 5 mg/mL dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, Poznań, Poland) was administered IM (intra-muscular) at a dose of 5 µg/kg BW;
- −
- TCDD and tocopherol (TCDD + E): TCDD in a single dose of 5 µg/kg BW was administered IM and a solution of α-tocopherol acetate (oil solution of the drug prepared individually by Hasco-Lek S.A., Wroclaw, Poland) was administered subdermal once a day for 3 weeks at a dose of 30 mg/kg BW.
- −
- TCDD and acetylsalicylic acid (TCDD + ASA): TCDD at a single dose of 5 µg/kg BW was administered IM and a suspension of ASA in a starch solution (acetylsalicylic acid, Bayer, Berlin, Germany) was administered P.O. (per os) once a day for 3 weeks at a dose of 30 mg/kg BW.
2.2. Histopathological Examination
2.3. Biochemical Assays
2.4. Statistical Analysis
3. Results
3.1. Histopathological Examination
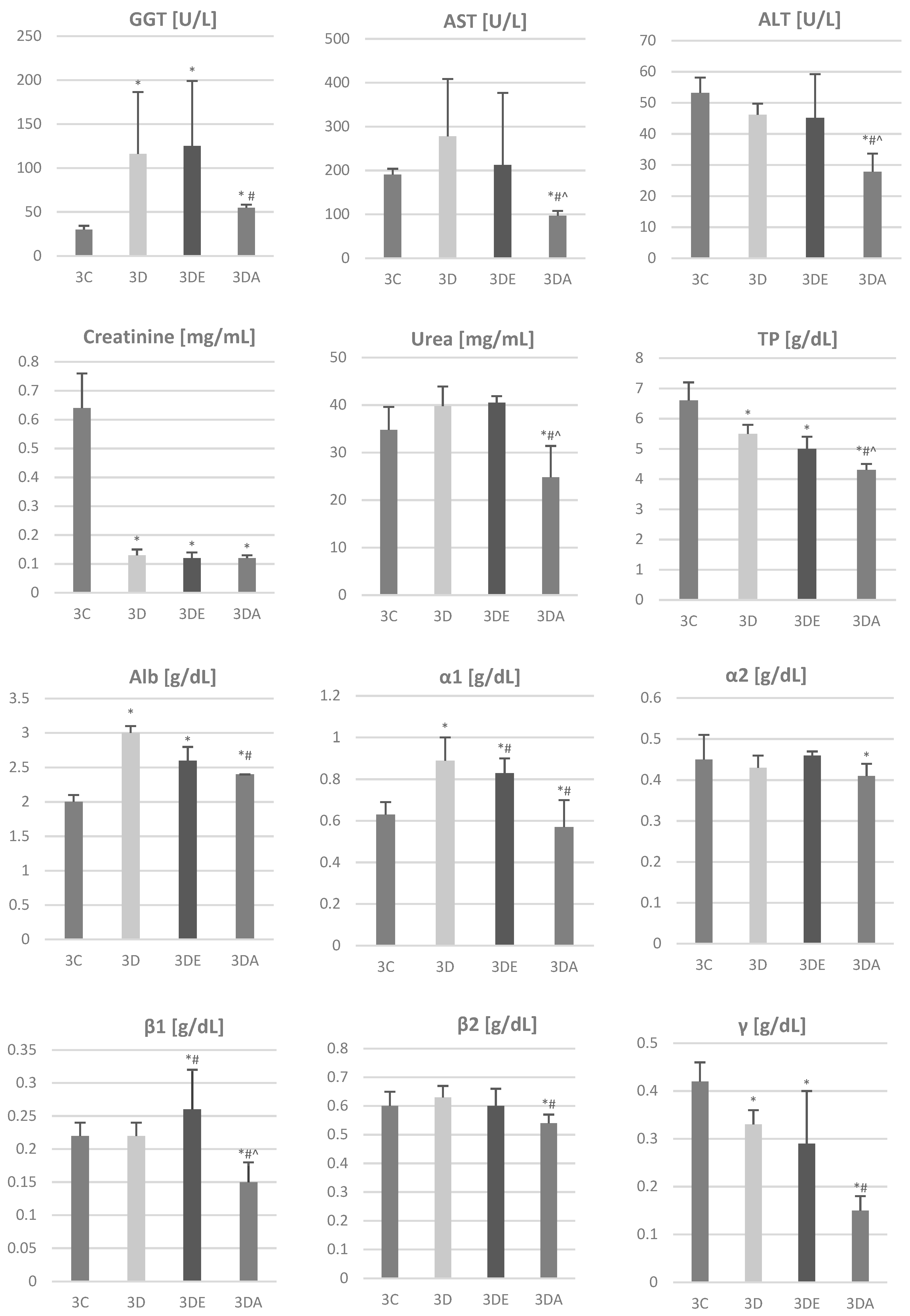
3.2. Biochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Całkosiński, I.; Dobrzyński, M.; Całkosińska, M.; Seweryn, E.; Bronowicka-Szydełko, A.; Dzierzba, K. Characterization of an Inflammatory Response. Postępy Hig. Med. Dośw. 2009, 63, 395–408. [Google Scholar]
- Dobrzyński, M.; Kuropka, P.; Leśków, A.; Herman, K.; Tarnowska, M.; Wiglusz, R.J. Co-Expression of the Aryl Hydrocarbon Receptor and Estrogen Receptor in the Developing Teeth of Rat Offspring after Rat Mothers’ Exposure to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin and the Protective Action of α-Tocopherol and Acetylsalicylic Acid. Adv. Clin. Exp. Med. 2019, 28. [Google Scholar] [CrossRef] [PubMed]
- Całkosiński, I.; Majda, J.; Terlecki, G.; Gostomska-Pampuch, K.; Małolepsza-Jarmołowska, K.; Sobolewska, S.; Całkosińska, A.; Kumala, A.; Gamian, A. Dynamic Analysis of Changes of Protein Levels and Selected Biochemical Indices in Rat Serum in the Course of Experimental Pleurisy. Inflammation 2016, 39, 1076–1089. [Google Scholar] [CrossRef][Green Version]
- Całkosiński, I.; Stańda, M.; Borodulin-Nadzieja, L.; Wasilewska, U.; Majda, J.; Cegielski, M.; Dzięgiel, P.; Woźniak, W. The Influence of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) on Changes of Parenchymal Organs Structure and Oestradiol and Cholesterol Concentration in Female Rats. Adv. Clin. Exp. Med. 2005, 14, 211–215. [Google Scholar]
- Całkosiński, I.; Rosińczuk-Tonderys, J.; Dzierzba, K.; Bronowicka-Szydełko, A.; Seweryn, E.; Majda, J.; Całkosińska, M.; Gamian, A. Estimation of the Action of Three Different Mechlorethamine Doses on Biochemical Parameters during Experimentally Induced Pleuritis in Rats. Pharmacol. Rep. 2011, 63, 501–517. [Google Scholar] [CrossRef]
- Całkosiński, I.; Rosińczuk-Tonderys, J.; Bazan, J.; Dzierzba, K.; Całkosińska, M.; Majda, J.; Dobrzyński, M.; Bronowicka-Szydełko, A. The Influence of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) on Hematological Parameters During Experimentally Induced Pleuritis in Rats. Inflammation 2013, 36, 387–404. [Google Scholar] [CrossRef][Green Version]
- Kędzior, M.; Seredyński, R.; Godzik, U.; Tomczyk, D.; Gutowicz, J.; Terlecka, E.; Całkosiński, I.; Terlecki, G. Inhibition of Cathepsin B Activity by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Environ. Sci. Pollut. Res. 2015, 22, 733–737. [Google Scholar] [CrossRef]
- Calkosinski, I.; Rosinczuk-Tonderys, J.; Dobrzynski, M.; Palka, L.; Bazan, J. Occurrence of Disseminated Intravascular Coagulation in 2,3,7,8-Tetrachlorodibenzo-p-Dioxin-Induced Pneumonia in the Rat. Adv. Exp. Med. Biol. 2013, 788, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Całkosiński, I.; Rosińczuk-Tonderys, J.; Szopa, M.; Dobrzyński, M.; Gamian, A. High Doses of Tocopherol in the Prevention and Potentiation of Dioxin in Experimental Inflammation—Potential Application. Postepy Hig. Med. Dosw. 2011, 65, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Całkosiński, I.; Rosińczuk-Tonderys, J.; Bronowicka-Szydełko, A.; Dzierzba, K.; Bazan, J.; Dobrzyński, M.; Majda, J.; Gamian, A.; Dobrzyński, M.; Majda, J.; et al. Effect of Tocopherol on Biochemical Blood Parameters in Pleuritis-Induced Rats Treated with 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Toxicol. Ind. Health 2015, 31, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, M.; Bazan, J.; Gamian, A.; Rosińczuk-Tonderys, J.; Parulska, O.; Całkosiński, I. The Use of Acetylsalicylic Acid and Tocopherol in Protecting Bone against the Effects of Dioxin Contamination. Rocz. Ochr. Środowiska 2014, 16, 300–322. [Google Scholar]
- Vondráček, J.; Krčmář, P.; Procházková, J.; Trilecová, L.; Gavelová, M.; Skálová, L.; Szotáková, B.; Bunček, M.; Radilová, H.; Kozubík, A.; et al. The Role of Aryl Hydrocarbon Receptor in Regulation of Enzymes Involved in Metabolic Activation of Polycyclic Aromatic Hydrocarbons in a Model of Rat Liver Progenitor Cells. Chem.-Biol. Interact. 2009, 180, 226–237. [Google Scholar] [CrossRef]
- Lim, J.; DeWitt, J.C.; Sanders, R.A.; Watkins, J.B.; Henshel, D.S. Suppression of Endogenous Antioxidant Enzymes by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin–Induced Oxidative Stress in Chicken Liver During Development. Arch. Environ. Contam. Toxicol. 2007, 52, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Genter, M.B.; Clay, C.D.; Dalton, T.P.; Dong, H.; Nebert, D.W.; Shertzer, H.G. Comparison of Mouse Hepatic Mitochondrial versus Microsomal Cytochromes P450 Following TCDD Treatment. Biochem. Biophys. Res. Commun. 2006, 342, 1375–1381. [Google Scholar] [CrossRef]
- Całkosiński, I.; Rosińczuk-Tonderys, J.; Bazan, J.; Dobrzyński, M.; Bronowicka-Szydełko, A.; Dzierzba, K. The Influence of Dioxin Intoxication on the Human System and Possibilities of Limiting Its Negative Effects on the Environment and Living Organisms. Ann. Agric. Environ. Med. 2014, 21, 518–524. [Google Scholar] [CrossRef]
- Różewicz, M.; Bombik, E.; Janocha, A.; Łagowska, K.; Bednarczyk, M. Dioxins—Their Influence on Human Health and the Contamination of Products of Animal Origin. Folia Pomeranae Univ. Technol. Stetin. 2016, 328, 189–202. [Google Scholar] [CrossRef]
- Kloser, E.; Böhmdorfer, S.; Brecker, L.; Kählig, H.; Netscher, T.; Mereiter, K.; Rosenau, T. Synthesis of 5-(Fluorophenyl)Tocopherols as Novel Dioxin Receptor Antagonists. Eur. J. Org. Chem. 2011, 2450–2457. [Google Scholar] [CrossRef]
- Dobrzyński, M.; Korczyński, M.; Herman, K.; Całkosiński, I. Evaluation of the Protective Effect of Different Doses of Alpha-Tocopherol on Calcium and Magnesium Content in Bone Tissue of Rats Treated with 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Przemysł Chem. 2016, 1, 155–158. [Google Scholar] [CrossRef]
- Kuropka, P.; Dobrzynski, M.; Tarnowska, M.; Styczynska, M.; Dudek, K.; Leskow, A.; Wiglusz, R.J. The Influence of High Doses of A-Tocopherol on the Content of Selected Trace Elements in the Liver of Developing Chicken Embryos in Experimentally Induced 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Intoxication. Acta Biochim. Pol. 2019, 66. [Google Scholar] [CrossRef]
- MacDonald, C.J.; Cheng, R.Y.S.; Roberts, D.D.; Wink, D.A.; Yeh, G.C. Modulation of Carcinogen Metabolism by Nitric Oxide-Aspirin 2 Is Associated with Suppression of DNA Damage and DNA Adduct Formation. J. Biol. Chem. 2009, 284, 22099–22107. [Google Scholar] [CrossRef]
- Leśków, A.; Tarnowska, M.; Rosińczuk, J.; Dobrzyński, M.; Kaliszewski, K.; Majda, J.; Żybura-Wszol̷a, K.; Sobolewska, S.; Diakowska, D. Xanthohumol Effect on 2,3,7,8-Tetrachlorodibenzo-p-Dioxin-Treated Japanese Quails in Terms of Serum Lipids, Liver Enzymes, Estradiol, and Thyroid Hormones. ACS Omega 2020, 5, 24445–24452. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, J.; Uno, S.; Ogura, M.; Choi, M.; Maeda, T.; Sakurai, K.; Matsuo, S.; Amano, S.; Nebert, D.W.; Makishima, M. Aryl Hydrocarbon Receptor Ligand 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Enhances Liver Damage in Bile Duct-Ligated Mice. Toxicology 2011, 280, 10–17. [Google Scholar] [CrossRef] [PubMed]
- González-Correa, J.A.; Arrebola, M.M.; Guerrero, A.; Cañada, M.J.; Muñoz Marín, J.; Sánchez De La Cuesta, F.; De La Cruz, J.P. Antioxidant and Antiplatelet Effects of the Alpha-Tocopherol–Aspirin Combination in Type 1-like Diabetic Rats. Life Sci. 2006, 79, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Inui, H.; Itoh, T.; Yamamoto, K.; Ikushiro, S.I.; Sakaki, T. Mammalian Cytochrome P450-Dependent Metabolism of Polychlorinated Dibenzo-p-Dioxins and Coplanar Polychlorinated Biphenyls. Int. J. Mol. Sci. 2014, 15, 14044–14057. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, M.; Kaczmarek, U.; Kuropka, P.; Reichert, P.; Grzech-Leśniak, K.; Całkosiński, I. Tooth Development Disorders in Infants of Rat Dams Exposed to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin and Protective Role of Tocopherol and Acetyl Salicylic Acid. Pol. J. Vet. Sci. 2017, 20, 769–778. [Google Scholar] [CrossRef]
- Marinković, N.; Pašalić, D.; Ferenčak, G.; Gršković, B.; Rukavina, A.S. Dioxins and Human Toxicity. Arhiv Za Hig. Rada I Toksikol. 2010, 61, 445–453. [Google Scholar] [CrossRef]
- White, S.S.; Birnbaum, L.S. An Overview of the Effects of Dioxins and Dioxin-Like Compounds on Vertebrates, as Documented in Human and Ecological Epidemiology. J. Environ. Sci. Health Part C 2009, 27, 197–211. [Google Scholar] [CrossRef]
- Gagliano-Jucá, T.; Tang, E.R.; Bhasin, S.; Pencina, K.M.; Anderson, S.; Jara, H.; Li, Z.; Melamud, K.; Coleman, S.L.; Aakil, A.; et al. Effects of Testosterone Administration (and Its 5-Alpha-Reduction) on Parenchymal Organ Volumes in Healthy Young Men: Findings from a Dose-Response Trial. Andrology 2017, 5, 889–897. [Google Scholar] [CrossRef]
- Ae, R.; Nakamura, Y.; Tada, H.; Kono, Y.; Matsui, E.; Itabashi, K.; Ogawa, M.; Sasahara, T.; Matsubara, Y.; Kojo, T.; et al. An 18-Year Follow-up Survey of Dioxin Levels in Human Milk in Japan. J. Epidemiol. 2018, 28, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, G.M.; Lakind, J.S.; Davis, M.H.; Hines, E.P.; Marchitti, S.A.; Alcala, C.; Lorber, M. Environmental Chemicals in Breast Milk and Formula: Exposure and Risk Assessment Implications. Environ. Health Perspect. 2018, 126. [Google Scholar] [CrossRef]
- Fürst, P. Dioxins, Polychlorinated Biphenyls and Other Organohalogen Compounds in Human Milk. Mol. Nutr. Food Res. 2006, 50, 922–933. [Google Scholar] [CrossRef]
- Klopfleisch, R. Multiparametric and Semiquantitative Scoring Systems for the Evaluation of Mouse Model Histopathology—A Systematic Review. BMC Vet. Res. 2013, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Skeggs, L.T.; Hochstrasser, H. Multiple automatic sequential analysis. Clin. Chem. 1964, 10, 918–936. [Google Scholar] [CrossRef] [PubMed]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin Standards and the Measurement of Serum Albumin with Bromcresol Green. Clin. Chim. Acta 1997, 258, 21–30. [Google Scholar] [CrossRef]
- Talke, H.; Schubert, G.E. Enzymatic Urea Determination in The Blood and Serum i The Warburg Optical Test. Klin. Wochenschr. 1965, 43, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Peake, M.; Whiting, M. Measurement of Serum Creatinine—Current Status and Future Goals. Clin. Biochem. Rev. 2006, 27, 173. [Google Scholar]
- Bergmeyer, H.U.; Fyrder, M. IFCC Methods for the Measurement of Catalytic Concentration of Enzymes. Clin. Chem. Lab. Med. 1980, 18, 521–541. [Google Scholar] [CrossRef]
- Takeda, T.; Fujii, M.; Izumoto, W.; Hattori, Y.; Matsushita, T.; Yamada, H.; Ishii, Y. Gestational Dioxin Exposure Suppresses Prolactin-Stimulated Nursing in Lactating Dam Rats to Impair Development of Postnatal Offspring. Biochem. Pharmacol. 2020, 178. [Google Scholar] [CrossRef]
- Kennedy, G.D.; Nukaya, M.; Moran, S.M.; Glover, E.; Weinberg, S.; Balbo, S.; Hecht, S.S.; Pitot, H.C.; Drinkwater, N.R.; Bradfield, C.A. Liver Tumor Promotion by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Is Dependent on the Aryl Hydrocarbon Receptor and TNF/IL-1 Receptors. Toxicol. Sci. 2014, 140, 135–143. [Google Scholar] [CrossRef]
- Duval, C.; Teixeira-Clerc, F.; Leblanc, A.F.; Touch, S.; Emond, C.; Guerre-Millo, M.; Lotersztajn, S.; Barouki, R.; Aggerbeck, M.; Coumoul, X. Chronic Exposure to Low Doses of Dioxin Promotes Liver Fibrosis Development in the C57BL/6J Diet-Induced Obesity Mouse Model. Environ. Health Perspect. 2017, 125, 428–436. [Google Scholar] [CrossRef]
- Yoshioka, W.; Higashiyama, W.; Tohyama, C. Involvement of MicroRNAs in Dioxin-Induced Liver Damage in the Mouse. Toxicol. Sci. 2011, 122, 457–465. [Google Scholar] [CrossRef]
- MacDonald, C.J.; Ciolino, H.P.; Yeh, G.C. The Drug Salicylamide Is an Antagonist of the Aryl Hydrocarbon Receptor That Inhibits Signal Transduction Induced by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Cancer Res. 2004, 64, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyński, M.; Całkosiński, I.; Przywitowska, I.; Kobierska−Brzoza, J.; Czajczyńska−Waszkiewicz, A.; Sołtan, E.; Parulska, O. The Effects of Dioxins in Environmental Pollution on Development of Teeth Disorders. Pol. J. Environ. Stud. 2009, 18, 319–323. [Google Scholar]
- Fowler, B.A.; Lucier, G.W.; Brown, H.W.; McDaniel, O.S. Ultrastructural Changes in Rat Liver Cells Following a Single Oral Dose of TCDD. Environ. Health Perspect. 1973, 5, 141–148. [Google Scholar] [CrossRef]
- Kakizuka, S.; Takeda, T.; Komiya, Y.; Koba, A.; Uchi, H.; Yamamoto, M.; Furue, M.; Ishii, Y.; Yamada, H. Dioxin-Produced Alteration in the Profiles of Fecal and Urinary Metabolomes: A Change in Bile Acids and Its Relevance to Toxicity. Biol. Pharm. Bull. 2015, 38, 1484–1495. [Google Scholar] [CrossRef]
- Tomita, S.; Jiang, H.-B.; Ueno, T.; Takagi, S.; Tohi, K.; Maekawa, S.-I.; Miyatake, A.; Furukawa, Y.T.; Gonzalez, F.J.; Takeda, J.; et al. T Cell-Specific Disruption of Arylhydrocarbon Receptor Nuclear Translocator (Arnt) Gene Causes Resistance to 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Induced Thymic Involution. J. Immunol. 2003, 171, 4113–4120. [Google Scholar] [CrossRef]
- Kido, T.; Van Dao, T.; Ho, M.D.; Dang, N.D.; Pham, N.T.; Okamoto, R.; Pham, T.T.; Maruzeni, S.; Nishijo, M.; Nakagawa, H.; et al. High Cortisol and Cortisone Levels Are Associated with Breast Milk Dioxin Concentrations in Vietnamese Women. Eur. J. Endocrinol. 2014, 170, 131–139. [Google Scholar] [CrossRef]
- Hilscherova, K.; Blankenship, A.L.; Nie, M.; Coady, K.K.; Upham, B.L.; Trosko, J.E.; Giesy, J.P. Oxidative Stress in Liver and Brain of the Hatchling Chicken (Gallus domesticus) Following in Ovo Injection with TCDD. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 29–45. [Google Scholar] [CrossRef]
- Całkosiński, I.; Dobrzyński, M.; Cegielski, M.; Sieja, A.; Całkosińska, M. The Multifaceted Effect of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) in Organisms, Especially Dentition Changes. Postepy Hig. I Med. Dosw. 2006, 60, 237–240. [Google Scholar]
- Gostomska-Pampuch, K.; Ostrowska, A.; Kuropka, P.; Dobrzyński, M.; Ziółkowski, P.; Kowalczyk, A.; Łukaszewicz, E.; Gamian, A.; Całkosiński, I. Protective Effects of Levamisole, Acetylsalicylic Acid, and α-Tocopherol against Dioxin Toxicity Measured as the Expression of AhR and COX-2 in a Chicken Embryo Model. Histochem. Cell Biol. 2017, 147, 523–536. [Google Scholar] [CrossRef]
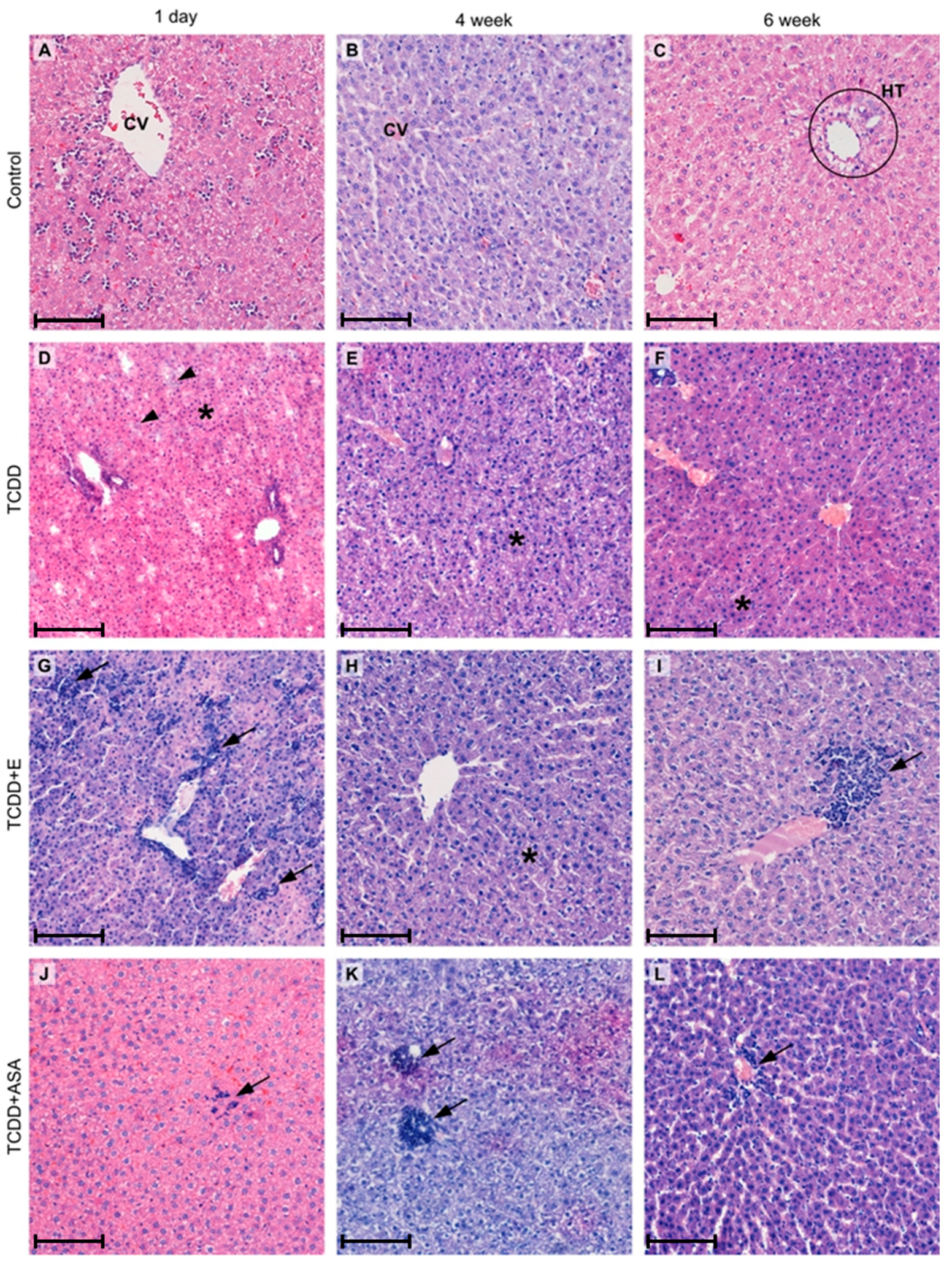


| Control | TCDD | TCDD + E | TCDD + ASA | |
|---|---|---|---|---|
| 1st day after birth | 1C | 1D | 1DE | 1DA |
| 4th week after birth | 2C | 2D | 2DE | 2DA |
| 6th week after birth | 3C | 3D | 3DE | 3DA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyński, M.; Madej, J.P.; Leśków, A.; Tarnowska, M.; Majda, J.; Szopa, M.; Gamian, A.; Kuropka, P. The Improvement of the Adaptation Process of Tocopherol and Acetylsalicylic Acid in Offspring of Mothers Exposed to TCDD. Animals 2021, 11, 3430. https://doi.org/10.3390/ani11123430
Dobrzyński M, Madej JP, Leśków A, Tarnowska M, Majda J, Szopa M, Gamian A, Kuropka P. The Improvement of the Adaptation Process of Tocopherol and Acetylsalicylic Acid in Offspring of Mothers Exposed to TCDD. Animals. 2021; 11(12):3430. https://doi.org/10.3390/ani11123430
Chicago/Turabian StyleDobrzyński, Maciej, Jan P. Madej, Anna Leśków, Małgorzata Tarnowska, Jacek Majda, Monika Szopa, Andrzej Gamian, and Piotr Kuropka. 2021. "The Improvement of the Adaptation Process of Tocopherol and Acetylsalicylic Acid in Offspring of Mothers Exposed to TCDD" Animals 11, no. 12: 3430. https://doi.org/10.3390/ani11123430
APA StyleDobrzyński, M., Madej, J. P., Leśków, A., Tarnowska, M., Majda, J., Szopa, M., Gamian, A., & Kuropka, P. (2021). The Improvement of the Adaptation Process of Tocopherol and Acetylsalicylic Acid in Offspring of Mothers Exposed to TCDD. Animals, 11(12), 3430. https://doi.org/10.3390/ani11123430







