Histological and Ultrastructural Description of Benign Adipocytic Tumors in Farmed Striped Sea Bream (Lythognathus mormyrus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Histology
2.3. Transmission Electron Microscopy
3. Results
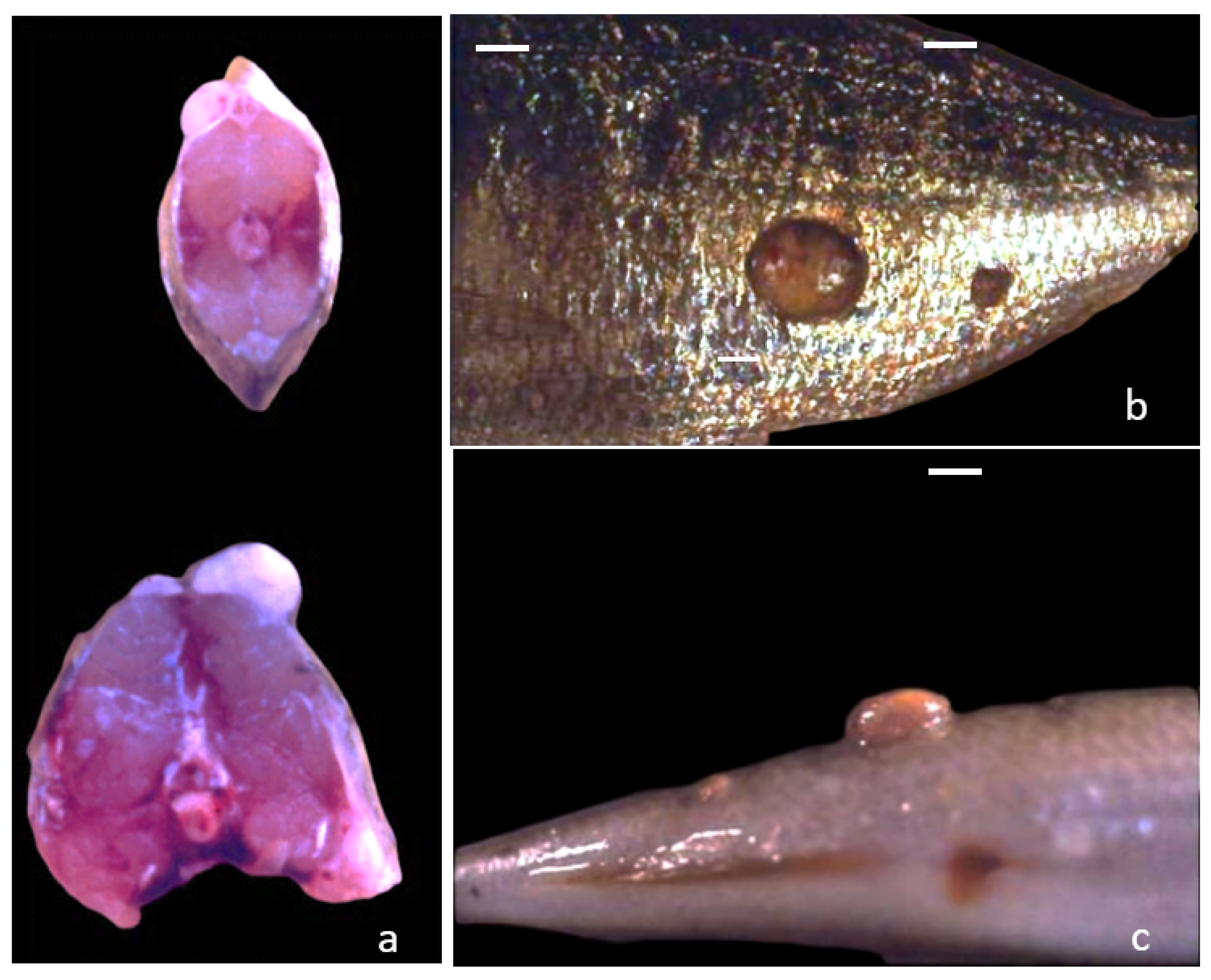
3.1. Gross Morphology
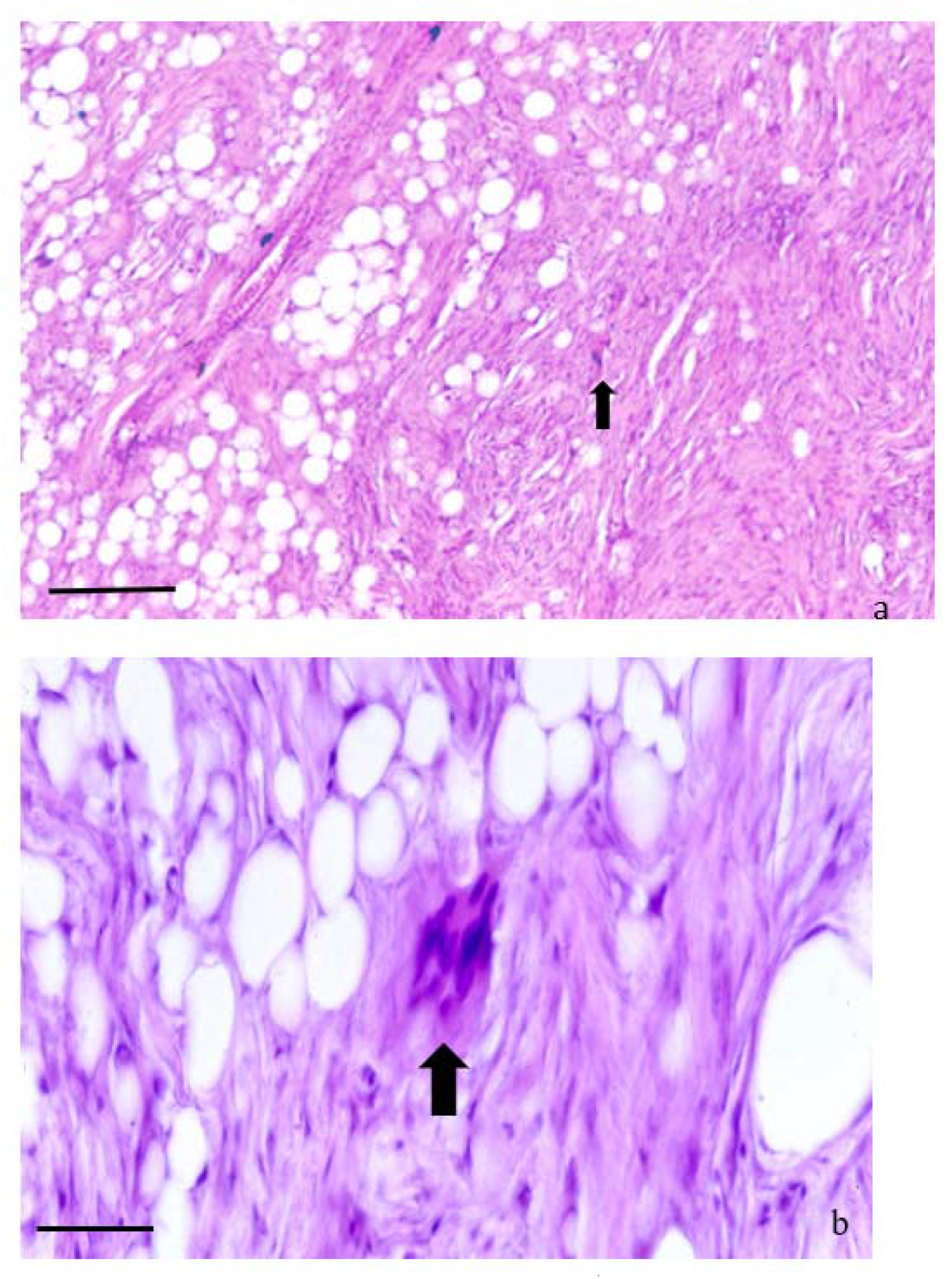
3.2. Histological Features
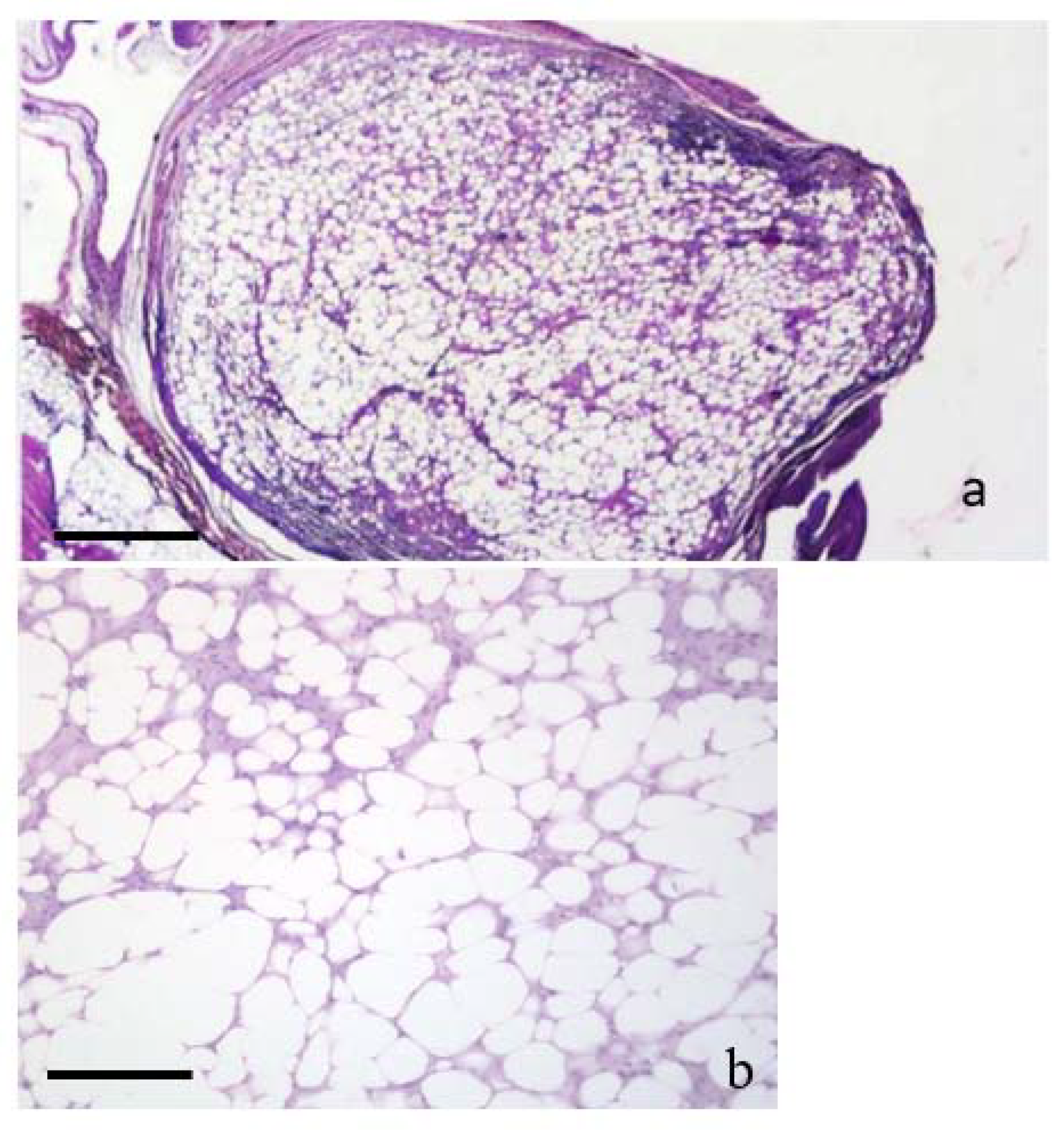
3.3. Neoplasms n. 1 to 3: Lipoma
3.4. Neoplasms n. 4 to 8 Fibrolipoma
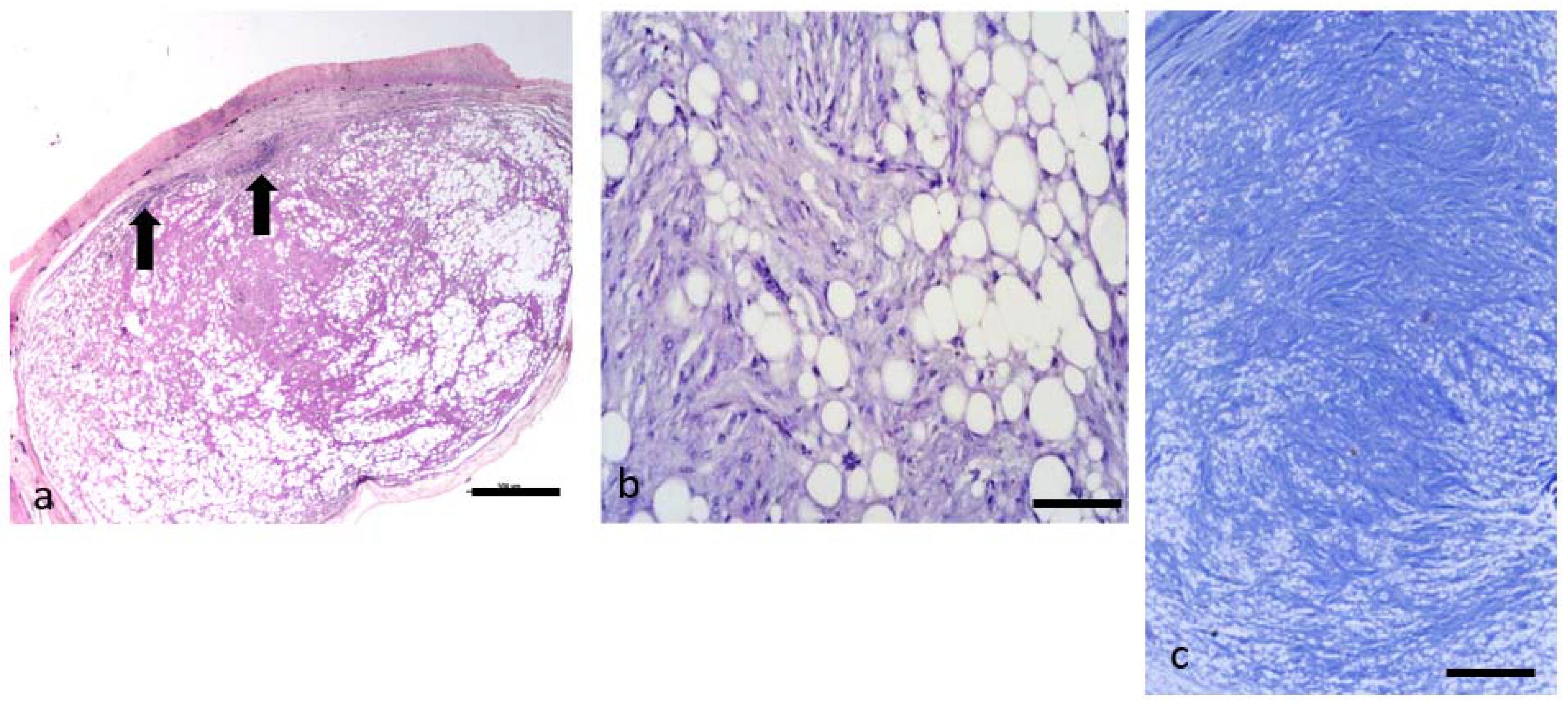
3.5. Neoplasms from n. 9 to 11: Spindle Cell Lipoma
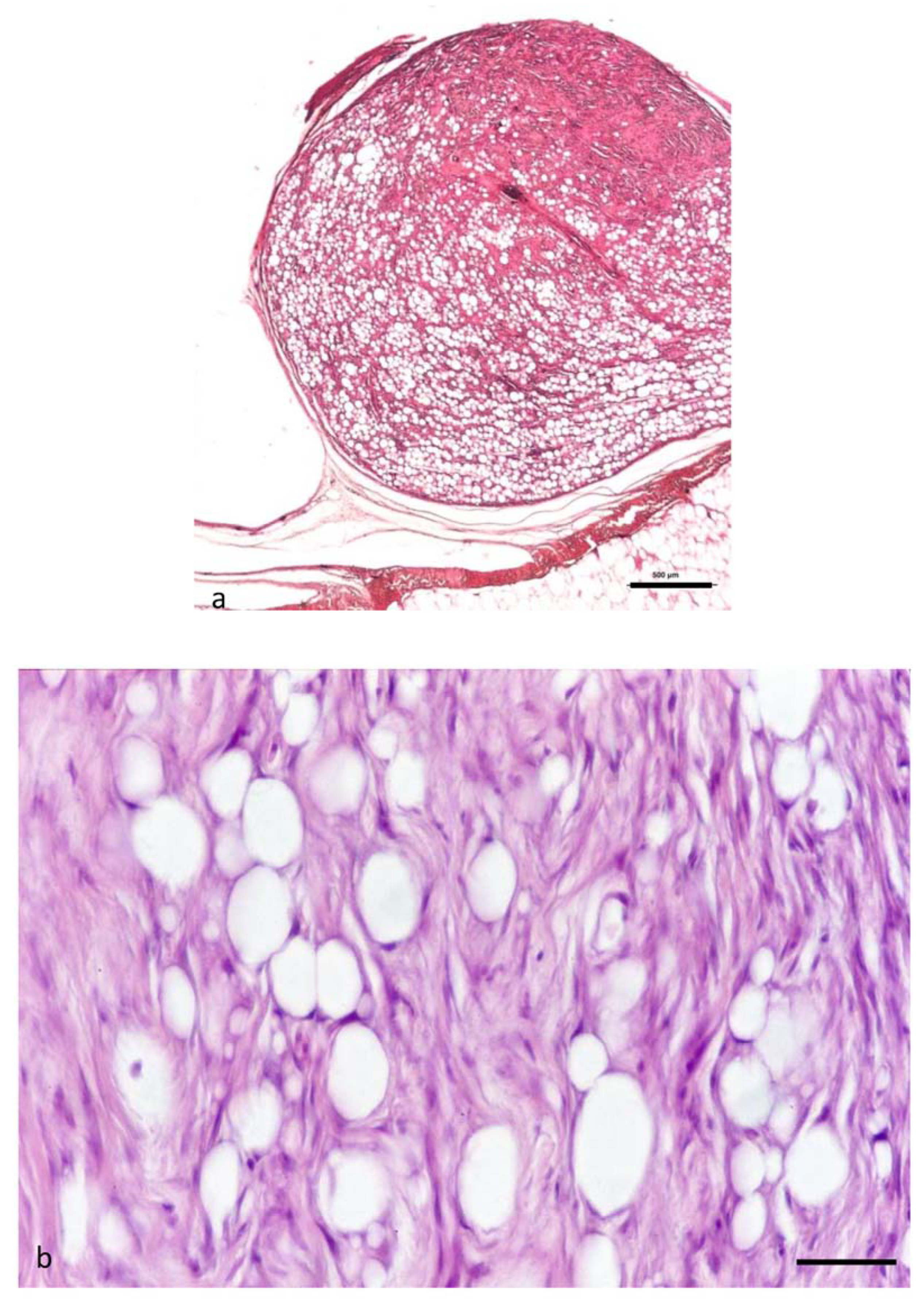
3.6. Neoplasms from n. 12 to 16: Atypical Spindle Cell-like Lipoma (ASCL)
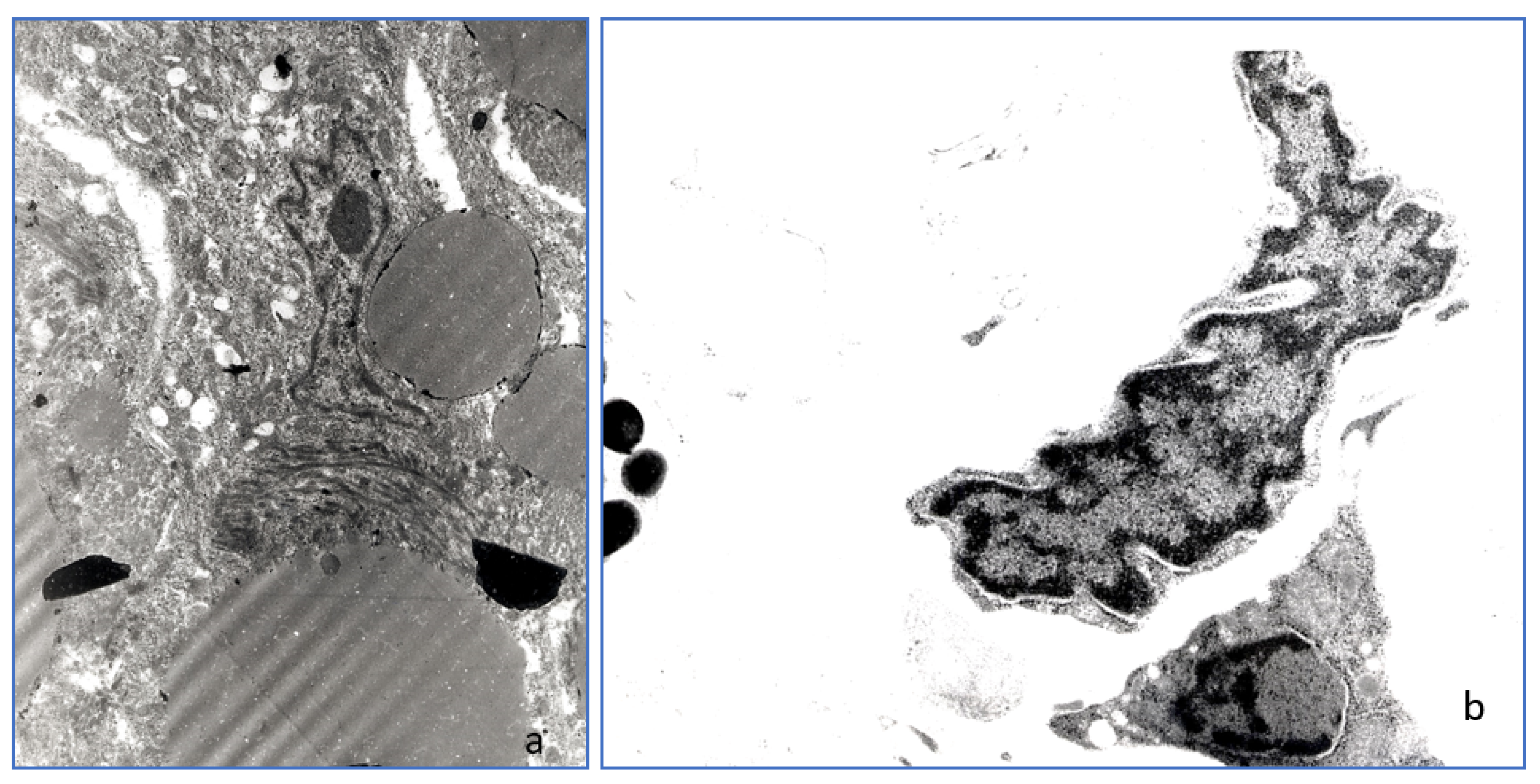
3.7. Transmission Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, R.J. (Ed.) Neoplasia of teleosts. In Fish Pathology, 4th ed.; WB Saunders Co: London, UK, 2012; pp. 167–185. [Google Scholar]
- Vergneau-Grosset, C.; Nadeau, M.E.; Groff, J.M. Fish Oncology: Diseases, diagnostics and therapeutics. Vet. Clin. N. Am. Exot. Anim. Pract. 2017, 20, 21–56. [Google Scholar] [CrossRef]
- Romano, L.A.; Pedrosa, V.F. Neoplasias in Fish: Review of the Last 20 Years. A Look from the Pathology. Annu. Res. Rev. Biol. 2020, 35, 134–153. [Google Scholar] [CrossRef]
- Hendrick, M.J.; Mahaffey, E.A.; Moore, F.M.; Vos, J.H.; Walder, E.J. Histological Classification of Mesenchymal Tumors of Skin and Soft Tissues of Domestic Animals; 2nd Series; Armed Forces Institute of Pathology: Washington, DC, USA, 1998; Volume 2. [Google Scholar]
- Hendrick, M.J. Mesenchymal Tumors of the Skin and Soft Tissues. In Tumors in Domestic Animals, 5th ed.; Meuten, D.J., Ed.; Iowa State Press: Ames, IA, USA, 2016; pp. 142–165. [Google Scholar]
- Harshbarger, J.C. Registry of tumors in lower animals. Natl. Cancer Inst. Monogr. 1981, 31, XI–XVI. [Google Scholar]
- Harshbarger, J.C. Neoplasia and developmental anomalies. In BSAVA Manual of Ornamental Fish, 2nd ed.; Wildgoose, W.H., Ed.; British Small Animal Veterinary Association: Gloucester, UK, 2001; pp. 219–224. [Google Scholar]
- Roccabianca, P.; Schulman, F.Y.; Avallone, G.; Foster, R.A.; Scruggs, J.L.; Dittmer, K.; Kiupel, M. Surgical Pathology of Tumors of Domestic Animals, 1st ed.; Kiupel, M., Ed.; Davis-Thompson Foundation: Gurnee, IL, USA, 2020; ISBN 978-1-7337491-2-1. Available online: https://davisthompsonfoundation.org/bookstore/surgical-pathology-of-tumors-of-domestic-animals-vol-3-tumors-of-soft-tissue-2/ (accessed on 12 November 2021).
- Mawdseley-Thomas, L.E.; Bucke, D. A lipoma in a bream (Abramis brama L.). Vet. Rec. 1968, 82, 673–674. [Google Scholar]
- Mawdesley-Thomas, L.E. Some tumours of fish. In Proceedings of the A Symposium Organized Jointly by the Fisheries Society of the British Isles and the Zoological Society of London, London, UK, 20–21 May 1971; pp. 191–283. [Google Scholar]
- McCoy, C.P.; Bowser, P.R.; Steeby, J.; Bleau, M.; Schwedler, T.E. Lipoma in channel catfish (Ictalurus punctatus Rafinesque). J. Wildl. Dis. 1985, 21, 74–76. [Google Scholar] [CrossRef][Green Version]
- Easa, M.E.-S.; Faisal, M.; Harshbarger, J.C.; Hetrick, F.M. A pseudocystic lipoma in the European eel (Anguilla anguilla). J. Appl. Ichthyol. 1989, 5, 85–87. [Google Scholar] [CrossRef]
- Easa, M.E.; Harshbarger, J.C.; Hetrick, F.M. Hypodermal lipoma in a striped (grey) mullet (Mugil cephalus). Dis. Aquat. Org. 1989, 6, 157–160. [Google Scholar] [CrossRef]
- Bruno, D.W.; Mc Vicar, A.H.; Fraser, C.O. Multiple lipoma in the common dab (Limanda limanda L.). Appl. Ichtyol. 1991, 7, 238–243. [Google Scholar] [CrossRef]
- Lester, R.J.G.; Kelly, W.R. Tumour-like growths from southern Australian marine fish. Tasman. Fish Res. 1983, 25, 27–32. [Google Scholar]
- Marino, F.; Monaco, S.; Salvaggio, A.; Macrì, B. Lipoma in a farmed northern bluefin tuna, Thunnus thynnus (L.). J. Fish Dis. 2006, 29, 697–699. [Google Scholar] [CrossRef]
- Johnston, C.J.; Deveney, M.R.; Bayly, T.; Nowak, B.F. Gross and histopathological characteristics of two lipomas and a neurofibrosarcoma detected in aquacultured southern bluefin tuna, Thunnus maccoyii (Castelnau), in South Australia. J. Fish Dis. 2008, 31, 241–247. [Google Scholar] [CrossRef]
- Gómez, S. Multiple dermal lipomas in farmed striped seabream Lithognathus mormyrus on the Spanish Mediterranean coast. Dis. Aquat. Org. 2009, 85, 77–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marino, F.; Chiofalo, B.; Mazzullo, G.; Panebianco, A. Multicentric infiltrative lipoma in a farmed Mediterranean seabass Dicentrarchus labrax: A pathological and biochemical case study. Dis. Aquat. Org. 2011, 96, 259–264. [Google Scholar] [CrossRef][Green Version]
- De Stefano, C.; Bonfiglio, R.; Montalbano, G.; Giorgianni, P.; Lanteri, G. Multicentric lipoma in a molly (Poecilia velifera). Bull. Eur. Assoc. Fish Pathol. 2012, 32, 220–224. [Google Scholar]
- Kehoe, S.P.; Divers, S.J.; Mayer, J.; Comolli, J.R.; Armwood, A.R.; Clarke, L.L.; Ruby, J.L.; Sharma, A. Computed tomographic and ultrasonographic diagnosis with successful excision of a lipoma in a shusui koi (Cyprinus carpio). J. Am. Vet. Med. Assoc. 2020, 256, 1379–1385. [Google Scholar] [CrossRef]
- Rahmati-Holasoo, H.; Alishahi, M.; Shokrpoor, S.; Jangarannejad, A.; Mohammadian, B. Invasion of melanoma to angiolipoma in a male Siamese fighting fish, Betta splendens, Regan. J. Fish Dis. 2014, 39, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.; Blake, I. Neoplasms in fish: A report of six cases with a summary of the literature. J. Pathol. 1993, 36, 41–47. [Google Scholar] [CrossRef]
- Bambir, S.; Helgason, S.; Marino, F.; Macrì, B. Some interesting tumours in fish. In Proceedings of the 11th Ljudevit Jurak International Symposium on Comparative Pathology, Zagreb, Croatia, 9–10 June 2000; p. 64. [Google Scholar]
- Sharon, G.; Benharroch, D.; Kachko, L. Liposarcoma in clownfish, Amphiprion ocellaris Cuvier, produced in indoor aquaculture. J. Fish Dis. 2015, 38, 575–580. [Google Scholar] [CrossRef]
- Rahmati-Holasoo, H.; Shokrpoor, S.; Tavakkoli, A. Liposarcoma or invasive lipomatosis in flower horn fish, hybrid cichlid: Clinical, radiological, ultrasonographical and histopathological study. J. Fish Dis. 2016, 39, 309–315. [Google Scholar] [CrossRef]
- Dei Tos, A.P. Liposarcoma: New entities and evolving concepts. Ann. Diagn. Pathol. 2000, 4, 252–266. [Google Scholar] [CrossRef]
- Rocha, A.F.L.; Miotto, L.N.; Morandin Ferrisse, T.; Silveira, H.A.; Yamamoto Almeida, L.; Bufalino, A.; León, J.E. Low-Fat and Fat-Free spindle cell lipomas in the oral cavity: Immunohistochemical analysis and review of the literature. J. Cutan. Pathol. 2019, 46, 778–783. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; He, S.; Wang, W.; Zhao, R.; Li, L.; Cui, Z.; Zhang, R. Spindle cell lipoma: Clinicopathologic characterization of 40 cases. Int. J. Clin. Exp. Pathol. 2019, 12, 2613–2621. [Google Scholar]
- Enzinger, F.M.; Weiss, S.W. Soft Tissue Tumours, 7th ed.; Mosby: St Louis, MO, USA, 2019; pp. 393–451. [Google Scholar]
- Iaria, C.; Ieni, A.; Corti, I.; Puleio, R.; Brachelente, C.; Mazzullo, G.; Lanteri, G. Immunohistochemical study of four fish tumors. J. Aquat. Anim. Health 2019, 31, 97–106. [Google Scholar] [CrossRef]
- Marino, F.; Macrì, D.; Lanteri, G.; Manganaro, M.; Monaco, S.; Germanà, A. Neurofibroma in a striped mullet: Histochemical and immunohistochemical study. J. Aquat. Anim. Health 2010, 22, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Licata, L.; Albano, M.; Ieni, A.; Di Caro, G.; Macrì, B. Angioleiomyoma in a conger (Conger conger). Dis. Aquat. Org. 2016, 119, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Pan, I.J.; Tu, W.J.; Lin, W.H.; Hong, C.C.; Brittelli, M.R. Neoplastic Response in Japanese medaka and channel catfish exposed to N-Methyl-N′-NitroN-Nitrosoguanidine. Environ. Toxicol. Pathol. 1996, 24, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Anders, K.; Yoshimizu, M. Role of viruses in the induction of skin tumours and tumour-like proliferations of fish. Dis. Aquat. Org. 1994, 19, 215–232. [Google Scholar] [CrossRef]

| Number | Histological Classification | Histological Features |
|---|---|---|
| 1–3 | Lipoma |
|
| 4–8 | Fibrolipoma |
|
| 9–11 | Spindle cell lipoma (SCL) |
|
| 12–16 | Atypical spindle cell-like lipoma (ASCL) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orioles, M.; Galeotti, M.; Patarnello, P.; Pizzolitto, S.; Volpatti, D. Histological and Ultrastructural Description of Benign Adipocytic Tumors in Farmed Striped Sea Bream (Lythognathus mormyrus). Animals 2021, 11, 3413. https://doi.org/10.3390/ani11123413
Orioles M, Galeotti M, Patarnello P, Pizzolitto S, Volpatti D. Histological and Ultrastructural Description of Benign Adipocytic Tumors in Farmed Striped Sea Bream (Lythognathus mormyrus). Animals. 2021; 11(12):3413. https://doi.org/10.3390/ani11123413
Chicago/Turabian StyleOrioles, Massimo, Marco Galeotti, Pierpaolo Patarnello, Stefano Pizzolitto, and Donatella Volpatti. 2021. "Histological and Ultrastructural Description of Benign Adipocytic Tumors in Farmed Striped Sea Bream (Lythognathus mormyrus)" Animals 11, no. 12: 3413. https://doi.org/10.3390/ani11123413
APA StyleOrioles, M., Galeotti, M., Patarnello, P., Pizzolitto, S., & Volpatti, D. (2021). Histological and Ultrastructural Description of Benign Adipocytic Tumors in Farmed Striped Sea Bream (Lythognathus mormyrus). Animals, 11(12), 3413. https://doi.org/10.3390/ani11123413






