Peripheral Nerve Sheath Tumors Resembling Human Atypical Neurofibroma in Goldfish (Carassius auratus, Linnaeus, 1758)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Findings, Anesthesia, Surgery, and Sample Collection
2.2. Cytological and Histopathological Sample Processing and Evaluation
2.3. Histochemical and Immunohistochemical Stains
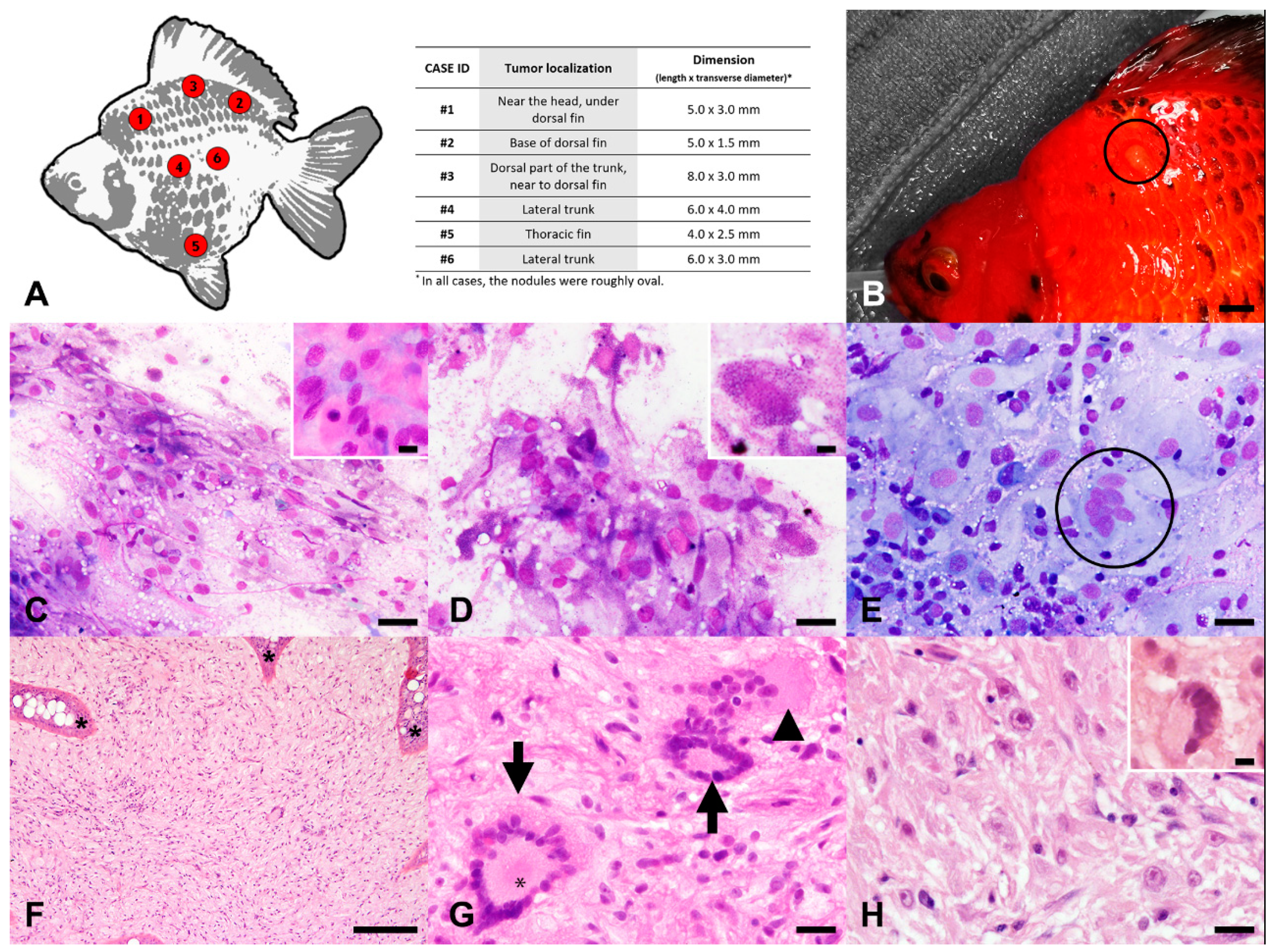
3. Results
3.1. Macroscopical and Cytological Evaluation
3.2. Histopathological Evaluation
3.3. Histochemical Stains
3.4. Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meuten, D.J. Tumors in Domestic Animals, 5th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2017; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119181200 (accessed on 5 September 2021).
- Quesenberry, K.; Carpenter, J. Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9781416066217. Available online: https://www.elsevier.com/books/ferrets-rabbits-and-rodents/quesenberry/978-0-323-48435-0 (accessed on 5 September 2021).
- Terio, K.; McAloose, D.; Leger, J. Pathology of Wildlife and Zoo Animals, 1st ed.; Academic Press: Waltham, MA, USA, 2018; Available online: https://www.elsevier.com/books/pathology-of-wildlife-and-zoo-animals/terio/978-0-12-805306-5 (accessed on 5 September 2021).
- Groff, J.M. Neoplasia in fishes. Vet. Clin. N. Am. Exot. Anim. Pract. 2004, 7, 705–756. [Google Scholar] [CrossRef] [PubMed]
- Vergneau-Grosset, C.; Nadeau, M.E.; Groff, J.M. Fish Oncology: Diseases, diagnostics, and therapeutics. Vet. Clin. N. Am. Exot. Anim. Pract. 2017, 20, 21–56. [Google Scholar] [CrossRef] [PubMed]
- Reavill, D.; Roberts, H. Diagnostic cytology of fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2007, 10, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J. Fish Pathology, 4th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2012; Available online: https://download.e-bookshelf.de/download/0000/5940/51/L-G-0000594051-0002363767.pdf (accessed on 5 September 2021).
- Edwards, P.; Zhang, W.; Belton, B.; Little, D.C. Misunderstandings, myths and mantras in aquaculture: Its contribution to world food supplies has been systematically over reported. Mar. Policy 2019, 106, 103547. [Google Scholar] [CrossRef]
- Bambino, K.; Chu, J. Zebrafish in toxicology and environmental health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar]
- Broussard, G.W.; Norris, M.B.; Schwindt, A.R.; Fournie, J.W.; Winn, R.N.; Kent, M.L.; Ennis, D.G. Chronic Mycobacterium marinum infection acts as a tumor promoter in Japanese Medaka (Oryzias latipes). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 152–160. [Google Scholar] [CrossRef]
- Dale, O.B.; Tørud, B.; Kvellestad, A.; Koppang, H.S.; Koppang, E.O. From chronic feed-induced intestinal inflammation to adenocarcinoma with metastases in salmonid fish. Cancer Res. 2009, 69, 4355–4362. [Google Scholar] [CrossRef]
- Marino, F.; Lanteri, G.; Rapisarda, G.; Perillo, A.; Macrì, B. Spontaneous schwannoma in zebrafish, Danio rerio (Hamilton). J. Fish Dis. 2012, 35, 239–242. [Google Scholar] [CrossRef]
- Schmale, M.C.; Hensley, G.T.; Udey, L.R. Neurofibromatosis in the bicolor damselfish (Pomacentrus partitus) as a model of von Recklinghausen neurofibromatosis. Ann. N. Y. Acad. Sci. 1986, 486, 386–402. [Google Scholar] [CrossRef]
- Roberts, H.E. Fundamentals of Ornamental Fish Health, 1st ed.; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780813808130. Available online: https://openlibrary.org/books/OL29013221M/Fundamentals_of_Ornamental_Fish_Health (accessed on 5 September 2021).
- Iaria, C.; Capparucci, F.; De Benedetto, G.; Natale, S.; Panebianco, R.; Puleio, R.; Lanteri, G. Gastric leiomyoma in a sea bass Dicentrarchus labrax broodfish. Dis. Aquat. Organ. 2020, 137, 211–216. [Google Scholar] [CrossRef]
- Marino, F.; Germanà, A.; Panebianco, A. A case of schwannoma in farmed seabream Sparus aurata. Dis. Aquat. Organ. 2008, 82, 249–252. [Google Scholar] [CrossRef]
- Marino, F.; Licata, L.; Albano, M.; Ieni, A.; Di Caro, G.; Macrì, B. Angioleiomyoma in a conger (Conger conger). Dis. Aquat. Organ. 2016, 119, 85–89. [Google Scholar] [CrossRef]
- Natale, S.; Capparucci, F.; Abbate, J.M.; Panebianco, R.; Puleio, R.; Iaria, C. Testicular leiomyoma and spermatogenic failure syndrome in a seabass from broodstock. J. Fish Dis. 2020, 43, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, H.G. Tumors characteristic for certain animal apecies A review. Cancer Res. 1957, 17, 823–832. [Google Scholar]
- Coffee, L.L.; Casey, J.W.; Bowser, P.R. Pathology of tumors in fish associated with retroviruses: A review. Vet. Pathol. 2013, 50, 390–403. [Google Scholar] [CrossRef]
- Kent, M.L.; Bishop-Stewart, J.K.; Matthews, J.L.; Spitsbergen, J.M. Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. Comp. Med. 2002, 52, 354–358. [Google Scholar] [PubMed]
- Boorman, G.; Crabbs, T.A.; Kolenda-Roberts, H.; Latimer, K.; Miller, A.D.; Muravnick, K.B.; Nyska, A.; Ochoa, R.; Pardo, I.D.; Ramot, Y.; et al. Proceedings of the 2011 National Toxicology Program Satellite Symposium. Toxicol. Pathol. 2011, 40, 321–344. [Google Scholar] [CrossRef]
- Sweet, M.; Kirkham, N.; Bendall, M.; Currey, L.; Bythell, J.; Heupel, M. Evidence of melanoma in wild marine fish populations. PLoS ONE 2012, 7, e41989. [Google Scholar] [CrossRef] [PubMed]
- Rovnak, J.; Quackenbush, S.L. Walleye dermal sarcoma virus: Molecular biology and oncogenesis. Viruses 2010, 2, 1984–1999. [Google Scholar] [CrossRef]
- Neiffer, D.L.; Stamper, M.A. Fish sedation, anesthesia, analgesia, and euthanasia: Considerations, methods, and types of drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef]
- Siniard, W.C.; Sheley, M.F.; Stevens, B.N.; Parker-Graham, C.A.; Roy, M.A.; Sinnott, D.M.; Watson, K.D.; Marinkovich, M.J.; Robertson, J.A.; Frei, S.; et al. Immunohistochemical analysis of pigment cell tumors in two cyprinid species. J. Vet. Diagn. Investig. 2019, 31, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Iaria, C.; Ieni, A.; Corti, I.; Puleio, R.; Brachelente, C.; Mazzullo, G.; Lanteri, G. Immunohistochemical study of four fish tumors. J. Aquat. Anim. Health 2019, 31, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, H.G. Nerve sheath tumors in an isolated goldfish population. Cancer Res. 1952, 12, 890–899. [Google Scholar] [PubMed]
- Iaria, C.; Saoca, C.; Guerrera, M.C.; Ciulli, S.; Brundo, M.V.; Piccione, G.; Lanteri, G. Occurrence of diseases in fish used for experimental research. Lab Anim. 2019, 53, 619–629. [Google Scholar] [CrossRef]
- Sirri, R.; Diana, A.; Scarpa, F.; Brachelente, C.; Vitellozzi, G.; Ceredi, L.; Mandrioli, L. Ultrasonographic and pathologic study of schwannoma in a goldfish (Carassius auratus). Vet. Clin. Pathol. 2015, 44, 586–591. [Google Scholar] [CrossRef]
- Bebak, J.A.; Evans, J.J.; Weber, E.P.S., 3rd; Wolf, J.C. Pathology in practice. Benign peripheral nerve sheath tumor. J. Am. Vet. Med. Assoc. 2012, 240, 827–829. [Google Scholar] [CrossRef]
- Marino, F.; Germanà, A.; Bambir, S.; Helgason, S.; De Vico, G.; Macrì, B. Calretinin and S-100 expression in goldfish, Carassius auratus (L.), schwannoma. J. Fish Dis. 2007, 30, 251–253. [Google Scholar] [CrossRef]
- Masahito, P.; Ishikawa, T.; Sugano, H. Pigment cells and pigment cell tumors in fish. J. Investig. Dermatol. 1989, 92, 266–270. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Egusa, S. Dermal fibrosarcoma in goldfish Carassius auratus (L.). J. Fish Dis. 1980, 3, 249–254. [Google Scholar] [CrossRef]
- Constantino, F.; De Ocampo, A.A.; García-Márquez, L.J. Dermal fibroma in goldfish, Carassius auratus (L.). J. Fish Dis. 1999, 22, 223–226. [Google Scholar] [CrossRef]
- Rezaie, A.; Dezfuly, Z.T.; Peyghan, R. Fibrosarcoma in a goldfish (Carassius auratus): A case report. Pathology 2017, 9, 45–48. [Google Scholar]
- Shokrpoor, S.; Sasani, F.; Rahmati-Holasoo, H.; Zargar, A. Concurrence of a fibroma and myxoma in an oranda goldfish (Carassius auratus). Bull. Eur. Assoc. Fish Pathol. 2016, 36, 263–268. [Google Scholar]
- Vergneau-Grosset, C.; Summa, N.; Rodriguez, C.O.; Cenani, A.; Sheley, M.F.; McCarthy, M.A.; Tanner, J.C.M.; Phillips, K.L.; Hunt, G.B.; Groff, J.M. Excision and subsequent treatment of a leiomyoma from the periventiduct of a koi (Cyprinus carpio koi). J. Exot. Pet Med. 2016, 25, 194–202. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Shayegh, H.; Geramizadeh, B. Cutaneous leiomyoma in a goldfish Carassius auratus. Fish Pathol. 2015, 50, 112–114. [Google Scholar] [CrossRef]
- Sirri, R.; Pretto, T.; Montesi, F.; Berton, V.; Mandrioli, L.; Barbé, T. Hikui disease in nine koi carp (Cyprinus carpio): First description of a cutaneous perivascular wall tumour. Vet. Dermatol. 2016, 27, 301-e74. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Schmidt, R.E. Spindle-cell tumour resembling haemangiopericytoma in a common goldfish, Carassius auratus (L.). J. Fish Dis. 1991, 14, 499–502. [Google Scholar] [CrossRef]
- Jakab, C.S.; Gálfi, P.; Jerzsele, A.; Szabó, Z.; Németh, T.; Sterczer, A.; Rusvai, M.; Ózsvári, L. Expression of claudin-1 in canine peripheral nerve sheath tumours and perivascular wall tumours. Immunohistochemical study. Histol. Histopathol. 2012, 27, 905–917. [Google Scholar] [PubMed]
- Hendrick, M.J.; Mahaffey, E.A.; Moore, F.M.; Vos, J.H.; Walder, E.J. Histological Classification of Mesenchymal Tumors of Skin and Soft Tissues of Domestic Animals, 2nd ed.; International Histological Classification of Tumors of Domestic Animals; Second Series/World Health Organization; Armed Forces Institute of Pathology: Washington, DC, USA, 1998; Available online: https://www.biblio.com/book/histological-classification-mesenchymal-tumors-skin-soft/d/614709579 (accessed on 5 September 2021).
- Roccabianca, P.; Schulman, F.Y.; Avallone, G.; Foster, R.A.; Scruggs, J.L.; Dittmer, K.; Kiupel, M. Surgical Pathology of Tumors of Domestic Animals, 1st ed.; Kiupel, M., Ed.; Davis-Thompson Foundation: Gurnee, IL, USA, 2020; ISBN 978-1-7337491-2-1. Available online: https://davisthompsonfoundation.org/bookstore/surgical-pathology-of-tumors-of-domestic-animals-vol-3-tumors-of-soft-tissue-2/ (accessed on 5 September 2021).
- Spitsbergen, J.M.; Frattini, S.A.; Bowser, P.R.; Getchell, R.G.; Coffee, L.L.; Wolfe, M.J.; Fisher, J.P.; Marinovic, S.J.; Harr, K.E. Epizootic neoplasia of the lateral line system of lake trout (Salvelinus namaycush) in New York’s Finger Lakes. Vet. Pathol. 2013, 50, 418–433. [Google Scholar] [CrossRef]
- Chijiwa, K.; Uchida, K.; Tateyama, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet. Pathol. 2004, 41, 307–318. [Google Scholar] [CrossRef]
- Gjurčević, E.; Kužir, S.; Sfacteria, A.; Drašner, K.; Marino, F. Spontaneous multicentric myxoma of the dermal nerve sheaths in farmed European eels Anguilla anguilla. Dis. Aquat. Organ. 2014, 111, 173–176. [Google Scholar] [CrossRef]
- Schöniger, S.; Summers, B.A. Localized, plexiform, diffuse, and other variants of neurofibroma in 12 dogs, 2 horses, and a chicken. Vet. Pathol. 2009, 46, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Corrêa, J.; Cardoso, R. Immunohistochemical markers for schwannomas, neurofibromas and malignant peripheral nerve sheath tumors—what can the recent literature tell us? Arq. Bras. Neurocir. Brazilian Neurosurg. 2018, 37, 105–112. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Folpe, A.L.; Giannini, C.; Perry, A. Pathology of peripheral nerve sheath tumors: Diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012, 123, 295–319. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.B.; Jensen, H.E.; Leifsson, P.S. Immunohistochemistry for 2′,3′-cyclic nucleotide-3′-phosphohydrolase in 63 bovine peripheral nerve sheath tumors. Vet. Pathol. 2011, 48, 796–802. [Google Scholar] [CrossRef]
- Sirri, R.; Bianco, C.; Beraldo, P.; Mandrioli, L.; Pulvirenti, I.; Brachelente, C.; Galeotti, M.; Sarli, G. Rhabdomyosarcoma of soft tissues in an adult brook trout (Salvelinus fontinalis). J. Comp. Pathol. 2015, 153, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Macrì, D.; Lanteri, G.; Manganaro, M.; Monaco, S.; Germanà, A. Neurofibroma in a striped mullet: Histochemical and immunohistochemical study. J. Aquat. Anim. Health 2010, 22, 92–94. [Google Scholar] [CrossRef]
- Duncan, T.E.; Harkin, J.C. Electron microscopic studies of goldfish tumors previously termed neurofibromas and schwannomas. Am. J. Pathol. 1969, 55, 191–202. [Google Scholar] [PubMed]
- Balko, J.A.; Wilson, S.K.; Lewbart, G.A.; Gaines, B.R.; Posner, L.P. Propofol as an immersion anesthetic and in a minimum anesthetic concentration (Mac) reduction model in goldfish (Carassius auratus). J. Zoo Wildl. Med. 2017, 48, 48–54. [Google Scholar] [CrossRef]
- Armando, F.; Godizzi, F.; Razzuoli, E.; Leonardi, F.; Angelone, M.; Corradi, A.; Meloni, D.; Ferrari, L.; Passeri, B. Epithelial to mesenchymal transition (EMT) in a laryngeal squamous cell carcinoma of a horse: Future perspectives. Animals 2020, 10, 2318. [Google Scholar] [CrossRef]
- Miettinen, M.M.; Antonescu, C.R.; Fletcher, C.D.M.; Kim, A.; Lazar, A.J.; Quezado, M.M.; Reilly, K.M.; Stemmer-Rachamimov, A.; Stewart, D.R.; Viskochil, D.; et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in neurofibromatosis 1 Patients—A consensus overview. Hum. Pathol 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Tecilla, M.; Gambini, M.; Forlani, A.; Caniatti, M.; Ghisleni, G.; Roccabianca, P. Evaluation of cytological diagnostic accuracy for canine splenic neoplasms: An investigation in 78 cases using STARD guidelines. PLoS ONE 2019, 14, e022494559. [Google Scholar] [CrossRef] [PubMed]
- Dennis, M.M.; McSporran, K.D.; Bacon, N.J.; Schulman, F.Y.; Foster, R.A.; Powers, B.E. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet. Pathol. 2011, 48, 73–84. [Google Scholar] [CrossRef]
- Bowser, P.R.; Casey, J.W.; Casey, R.N.; Quackenbush, S.L.; Lofton, L.; Coll, J.A.; Cipriano, R.C. Swimbladder leiomyosarcoma in Atlantic salmon (Salmo salar) in North America. J. Wildl. Dis. 2012, 48, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Morikawa, S.; Kiriyama, T.; Kitaori, H. An epizootic occurrence of rhabdomyoma and a case of ganglioneuroma in hatchery-reared ayu, Plecoglossus altivelis Temminck & Schlegel. J. Fish Dis. 1983, 6, 195–200. [Google Scholar]
- Avallone, G.; Stefanello, D.; Ferrari, R.; Roccabianca, P. The controversial histologic classification of canine subcutaneous whorling tumours: The path to perivascular wall tumours. Vet. Comp. Oncol. 2019, 18, 3–8. [Google Scholar] [CrossRef]
- Karamchandani, J.R.; Nielsen, T.O.; Van De Rijn, M.; West, R.B. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Giudice, C.; Ceciliani, F.; Rondena, M.; Stefanello, D.; Grieco, V. Immunohistochemical investigation of PNL2 reactivity of canine melanocytic neoplasms and comparison with Melan A. J. Vet. Diagn. Investig. 2010, 22, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.J.; Jankovsky, J.M.; Rohrbach, B.W.; LeBlanc, A.K. C-kit Expression in canine mucosal melanomas. Vet. Pathol. 2012, 49, 760–765. [Google Scholar] [CrossRef]
- Johnson, G.C.; Coates, J.R.; Wininger, F. Diagnostic immunohistochemistry of canine and feline intracalvarial tumors in the age of brain biopsies. Vet. Pathol. 2014, 51, 146–160. [Google Scholar] [CrossRef]
- Schöniger, S.; Valentine, B.A.; Fernandez, C.J.; Summers, B.A. Cutaneous schwannomas in 22 horses. Vet. Pathol. 2011, 48, 433–442. [Google Scholar] [CrossRef]
- Zorick, T.S.; Syroid, D.E.; Brown, A.; Gridley, T.; Lemke, G. Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development 1999, 126, 1397–1406. [Google Scholar] [CrossRef]
- Zois, C.E.; Harris, A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.; Mannoor, K.; Cao, J.; Qadri, F.; Song, X. SOX2 in cancer stemness: Tumor malignancy and therapeutic potentials. J. Mol. Cell Biol. 2018, 12, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Magro, G.; Amico, P.; Vecchio, G.M.; Caltabiano, R.; Castaing, M.; Kacerovska, D.; Kazakov, D.V.; Michal, M. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: A clinicopathologic study of 94 cases. Virchows Arch. 2010, 456, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Higham, C.S.; Dombi, E.; Rogiers, A.; Bhaumik, S.; Pans, S.; Connor, S.E.J.; Miettinen, M.; Sciot, R.; Tirabosco, R.; Brems, H. The characteristics of 76 atypical neurofibromas as precursors to neurofibromatosis 1 associated malignant peripheral nerve sheath tumors. Neuro-oncology 2018, 20, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Beert, E.; Brems, H.; Daniëls, B.; De Wever, I.; Van Calenbergh, F.; Schoenaers, J.; Debiec-Rychter, M.; Gevaert, O.; De Raedt, T.; Van Den Bruel, A.; et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer 2011, 50, 1021–1032. [Google Scholar] [CrossRef]
- Lemberg, K.M.; Wang, J.; Pratilas, C.A. From Genes to -omics: The evolving molecular landscape of malignant peripheral nerve sheath tumor. Genes 2020, 11, 691. [Google Scholar] [CrossRef]
- Rhodes, S.D.; He, Y.; Smith, A.; Jiang, L.; Lu, Q.; Mund, J.; Li, X.; Bessler, W.; Qian, S.; Dyer, W. Cdkn2a (Arf) loss drives NF1-associated atypical neurofibroma and malignant transformation. Hum. Mol. Genet. 2019, 28, 2752–2762. [Google Scholar] [CrossRef]
- Shin, J.; Padmanabhan, A.; de Groh, E.D.; Lee, J.-S.; Haidar, S.; Dahlberg, S.; Guo, F.; He, S.; Wolman, M.A.; Granato, M.; et al. Zebrafish neurofibromatosis type 1 genes have redundant functions in tumorigenesis and embryonic development. Dis. Model. Mech. 2012, 5, 881–894. [Google Scholar] [CrossRef]
- Teixeira, S.; Amorim, I.; Rema, A.; Faria, F.; Gärtner, F. Molecular heterogeneity of canine cutaneous peripheral nerve sheath tumors: A drawback in the diagnosis refinement. In Vivo 2016, 30, 819–827. [Google Scholar] [CrossRef][Green Version]
- Dawe, C.J. Oncozoons and the search for carcinogenindicator fishes. Environ. Health Perspect. 1987, 71, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Anders, K.; Yoshimizu, M. Role of viruses in the induction of skin tumours and tumour-like proliferations of fish. Dis. Aquat. Organ. 1994, 19, 215–232. [Google Scholar] [CrossRef]
- Campbell, T.W. Exotic Animal Hematology and Cytology, 4th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2015; ISBN 9781118611272. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118993705 (accessed on 5 September 2021).
- Anders, K.; Hilger, I.; Moller, H. Lentivirus-like particles in connective tissue tumours of fish from German coastal waters. Dis. Aquat. Organ. 1991, 11, 151–154. [Google Scholar] [CrossRef]
- Rahn, J.J.; Gibbs, P.D.L.; Schmale, M.C. Patterns of transcription of a virus-like agent in tumor and non-tumor tissues in bicolor damselfish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 138, 401–409. [Google Scholar] [CrossRef]


| CASE ID | Azan Trichrome Stain a | Alcian Blue Stain (pH 2.5) | PAS Stain c | PAS-Diastase Stain | Gomori’s Reticulin Stain d | Bielschowsky Stain f | Ziehl-Neelsen |
|---|---|---|---|---|---|---|---|
| #1 | Collagen: + b Muscular cells: − | ++ (locally extensive, S) | ++ (M) | − | ++ | − | − |
| #2 | Collagen: − Muscular cells: − | − | + (M) | − | + | − | − |
| #3 | Collagen: + Muscular cells: − | +/++ (multifocal, M) | ++ (S) | − | ++/+++ | + | − |
| #4 | Collagen: + Muscular cells: − | + (locally extensive, W) | ++ (S) | − | +/++ | + | − |
| #5 | Collagen: + Muscular cells: − | − | + (M) | − | ++ | − | − |
| #6 | Collagen: + b Muscular cells: − | + (focal, W) | +++ (S) | − | +++ e | − | − |
| CASE ID | S100 Protein a | CNPase b | n-NF c | p-NF d | c-Kit/CD117 | PNL-2 | GFAP | EGR2/Krox20 | SOX2 | Calretinin e |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | +++ | +++ (M–S) | ++ (M–S) | − | − | − | − | − | − | − |
| #2 | + | + (W–M) | ++ (S) | − | − | − | − | − | − | − |
| #3 | ++ | ++ (M–S) | − | − | − | − | − | − | − | − |
| #4 | +++ | ++ (W–M) | − | + (W) | − | − | − | − | − | − |
| #5 | + | ++ (W–M) | +++ (S) | − | − | − | − | − | − | − |
| #6 | ++ | ++ (W–M) | + (S) | + (M) | − | − | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armando, F.; Pigoli, C.; Gambini, M.; Ghidelli, A.; Ghisleni, G.; Corradi, A.; Passeri, B.; Caniatti, M.; Grieco, V.; Baumgärtner, W.; et al. Peripheral Nerve Sheath Tumors Resembling Human Atypical Neurofibroma in Goldfish (Carassius auratus, Linnaeus, 1758). Animals 2021, 11, 2621. https://doi.org/10.3390/ani11092621
Armando F, Pigoli C, Gambini M, Ghidelli A, Ghisleni G, Corradi A, Passeri B, Caniatti M, Grieco V, Baumgärtner W, et al. Peripheral Nerve Sheath Tumors Resembling Human Atypical Neurofibroma in Goldfish (Carassius auratus, Linnaeus, 1758). Animals. 2021; 11(9):2621. https://doi.org/10.3390/ani11092621
Chicago/Turabian StyleArmando, Federico, Claudio Pigoli, Matteo Gambini, Andrea Ghidelli, Gabriele Ghisleni, Attilio Corradi, Benedetta Passeri, Mario Caniatti, Valeria Grieco, Wolfgang Baumgärtner, and et al. 2021. "Peripheral Nerve Sheath Tumors Resembling Human Atypical Neurofibroma in Goldfish (Carassius auratus, Linnaeus, 1758)" Animals 11, no. 9: 2621. https://doi.org/10.3390/ani11092621
APA StyleArmando, F., Pigoli, C., Gambini, M., Ghidelli, A., Ghisleni, G., Corradi, A., Passeri, B., Caniatti, M., Grieco, V., Baumgärtner, W., & Puff, C. (2021). Peripheral Nerve Sheath Tumors Resembling Human Atypical Neurofibroma in Goldfish (Carassius auratus, Linnaeus, 1758). Animals, 11(9), 2621. https://doi.org/10.3390/ani11092621









