Susceptibility and Pathology in Juvenile Atlantic Cod Gadus morhua to a Marine Viral Haemorrhagic Septicaemia Virus Isolated from Diseased Rainbow Trout Oncorhynchus mykiss
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Challenge Material
2.2. Experiment 1: Intra-Peritoneal Injection
2.2.1. Fish and Experimental Design
2.2.2. Tissue Sampling Experiment 1
2.3. Experiment 2: Cohabitation Infection
2.3.1. Fish and Experimental Design
2.3.2. Tissue Sampling Experiment 2
2.4. Detection of Virus Using Real Time RT-PCR (rRT-PCR)
2.5. Hematoxylin-Erythrosine-Saffron (HES) Staining and Immunohistochemistry (IHC)
2.6. Bacteriology
2.7. Statistical Analysis Mortality Data
3. Results
3.1. Experiment 1 Intra-Peritoneal Injection
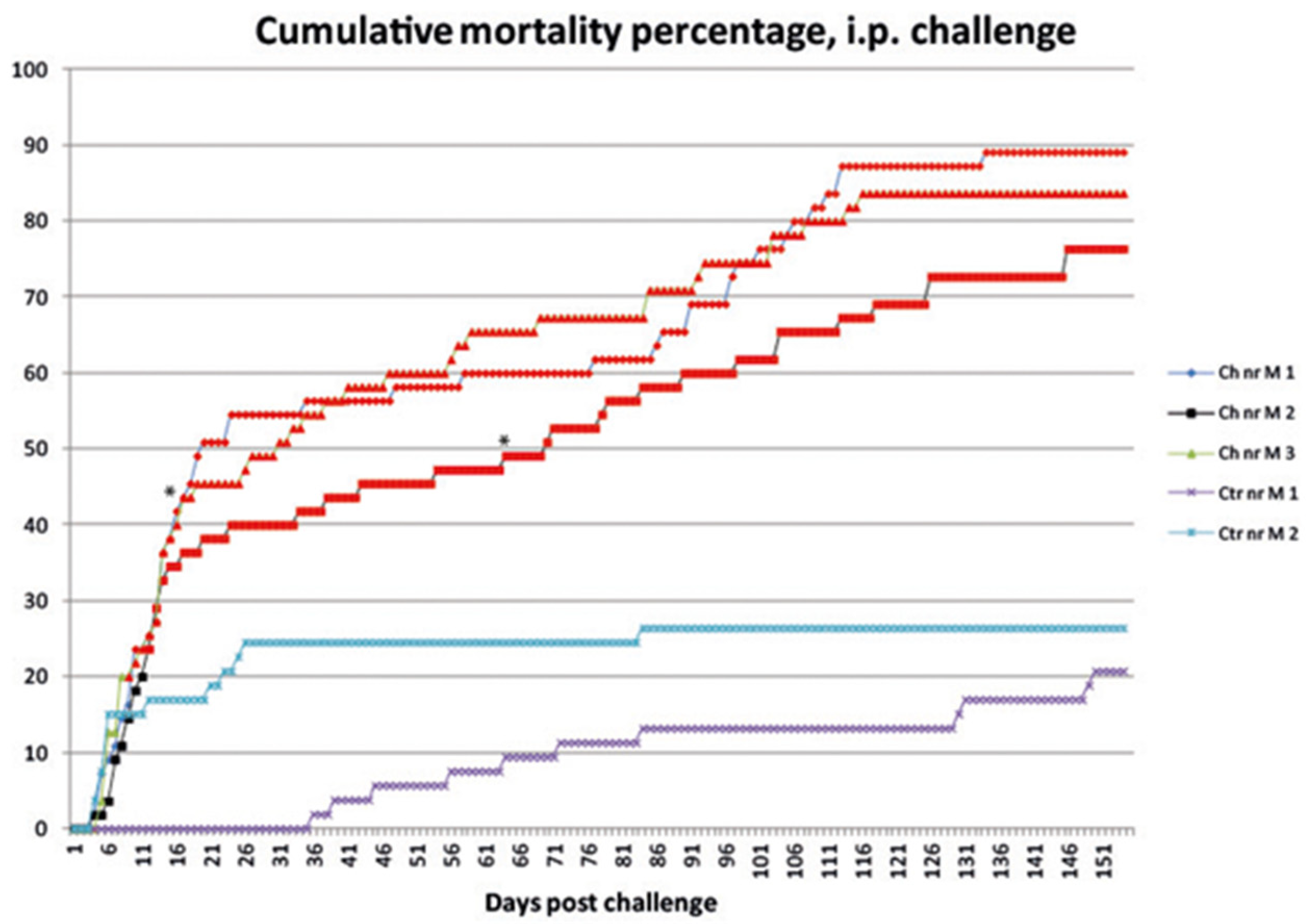
3.1.1. Mortality
3.1.2. Sampling
3.1.3. Clinical Signs and Gross Pathology
3.1.4. Virus Detection and Tissue Distribution Using rRT-PCR
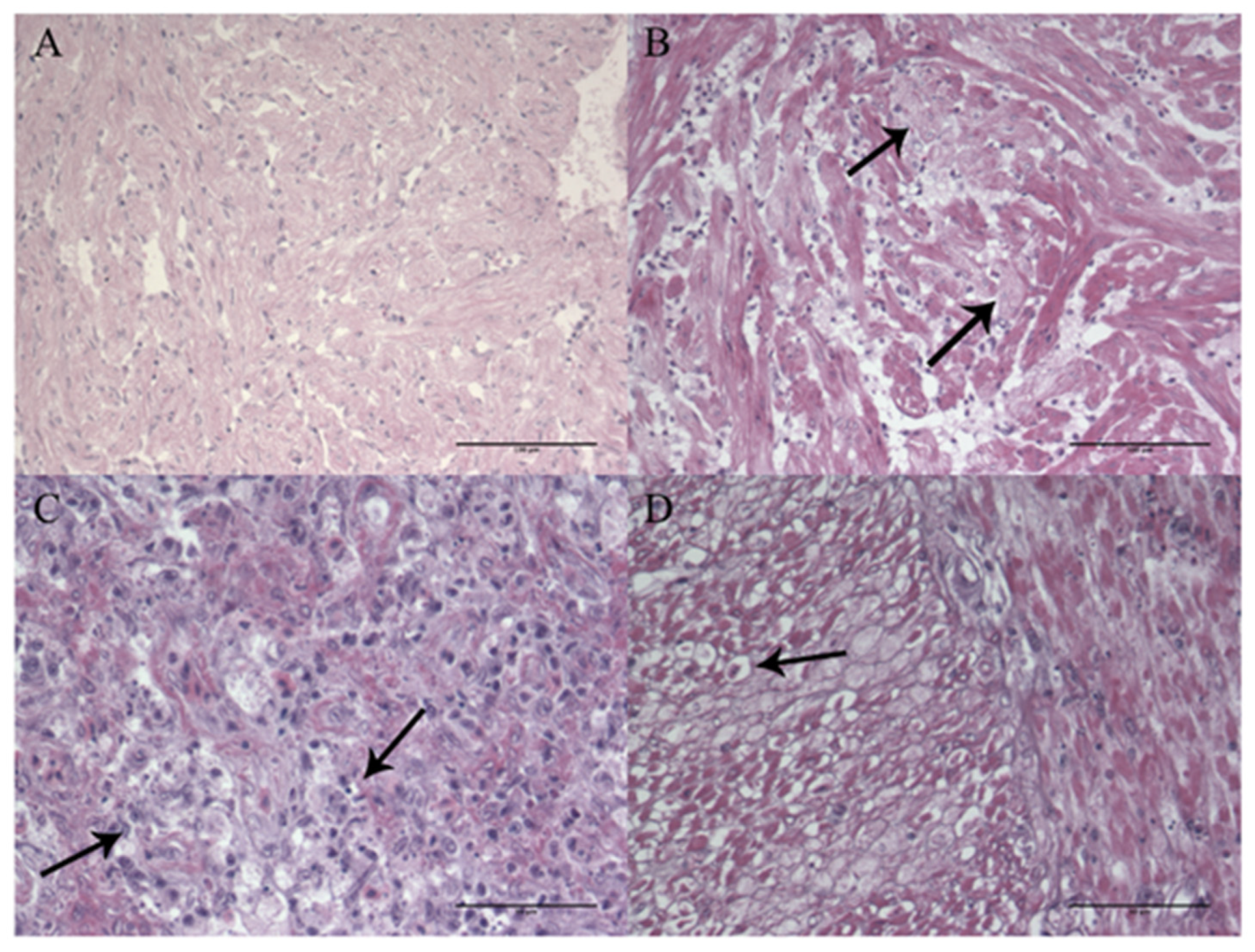
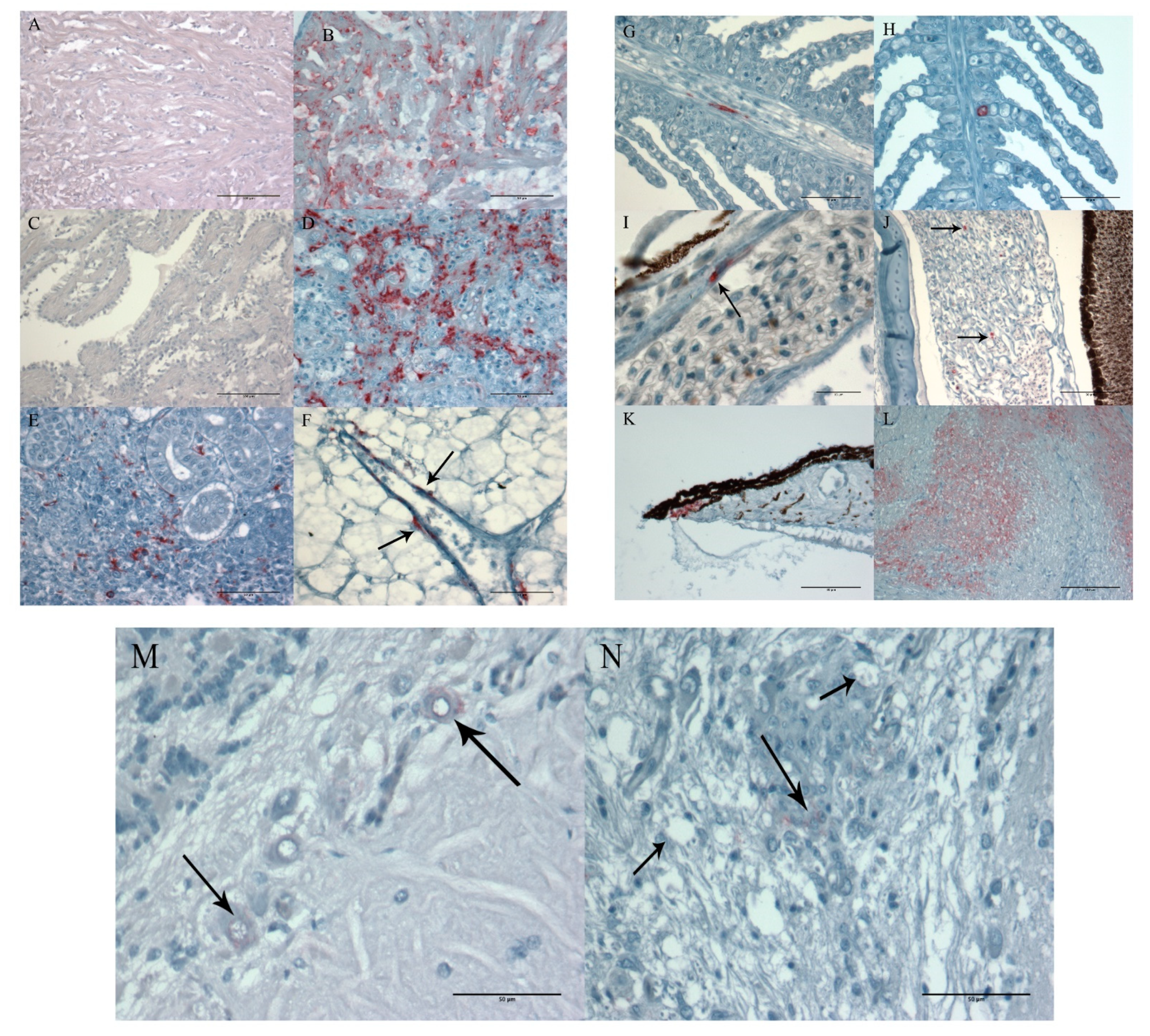
3.1.5. Histopathology and Immunohistochemistry
3.2. Experiment 2 Cohabitation Infection
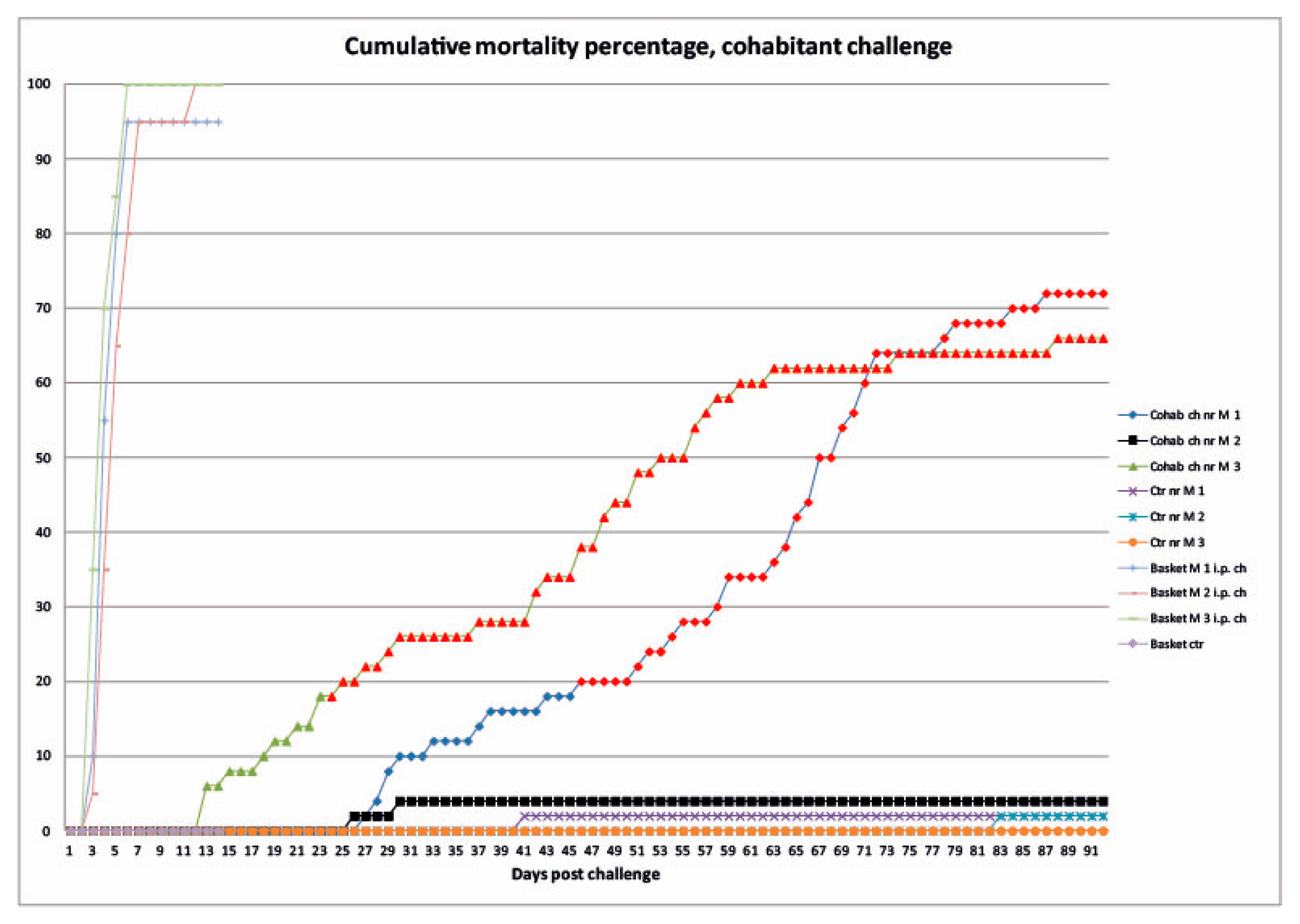
3.2.1. Mortality
3.2.2. Clinical Signs and Gross Pathology
3.2.3. Virus Detection and Tissue Distribution Using rRT-PCR
3.2.4. Histopathology and Immunohistochemistry
3.3. Bacteriology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skall, H.F.; Olesen, N.J.; Mellergaard, S. Viral haemorrhagic septicaemia virus in marine fish and its implications for fish farming—A review. J. Fish. Dis. 2005, 28, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Dale, O.B.; Ørpetveit, I.; Lyngstad, T.M.; Kahns, S.; Skall, H.F.; Olesen, N.J.; Dannevig, B.H. Outbreak of viral haemorrhagic septicaemia (VHS) in seawater-Farmed rainbow trout in Norway caused by VHS virus Genotype III. Dis. Aquat. Org. 2009, 85, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Brudeseth, B.E. Novirhabdovirus Infections of Fish—With Emphasis on VHS Pathogenesis. Ph.D. Thesis, Norwegian School of Veterinary Sciense, Oslo, Norway, 2009. [Google Scholar]
- Snow, M.; Cunningham, C.O.; Bricknell, I.R. Susceptibility of juvenile Atlantic cod Gadus morhua to viral haemorrhagic septicaemia virus isolated from wild-Caught Atlantic cod. Dis. Aquat. Org. 2000, 41, 225–229. [Google Scholar] [CrossRef]
- Skall, H.F.; Slierendrecht, W.J.; King, J.A.; Olesen, N.J. Experimental infection of rainbow trout Oncorhynchus mykiss with viral haemorrhagic septicaemia virus isolates from European marine and farmed fishes. Dis. Aquat. Org. 2004, 58, 99–110. [Google Scholar] [CrossRef]
- Snow, M.; Bain, N.; Black, J.; Taupin, V.; Cunningham, C.O.; King, J.A.; Skall, H.F.; Raynard, R.S. Genetic population structure of marine viral haemorrhagic septicaemia virus (VHSV). Dis. Aquat. Org. 2004, 61, 11–21. [Google Scholar] [CrossRef]
- Dixon, P.F. VHSV came from the marine environment: Clues from the literature, or just red herrings? Bull. Eur. Assoc. Fish. Pathol. 1999, 19, 60–65. [Google Scholar]
- Jensen, N.J.; Larsen, J.L. Ulcus-Syndrome in cod (Gadus-morhua). I. A pathological and histopathological study. Nord. Vet. Med. 1979, 31, 222–228. [Google Scholar] [PubMed]
- Smail, D.A. Isolation and identification of Viral Haemorrhagic Septicaemia (VHS) viruses from cod Gadus morhua with the ulcus syndrome and from haddock Melanogrammus aeglefinus having skin haemorrhages in the North Sea. Dis. Aquat. Org. 2000, 41, 231–235. [Google Scholar] [CrossRef]
- Smail, D.A. Isolation and Identification of Viral Haemorrhagic Septicaemia (VHS) Virus from North Sea Cod (Gadus morhua L.). 1995. Available online: http://prep.ices.dk/sites/pub/CM%20Doccuments/1995/F/1995_F15.pdf (accessed on 7 December 2021). [CrossRef]
- Mortensen, H.F.; Heuer, O.E.; Lorenzen, N.; Otte, L.; Olesen, N.J. Isolation of viral haemorrhagic septicaemia virus (VHSV) from wild marine fish species in the Baltic Sea, Kattegat, Skagerrak and the North Sea. Virus Res. 1999, 63, 95–106. [Google Scholar] [CrossRef]
- Skall, H.F.; Olesen, N.J.; Mellergaard, S. Prevalence of viral haemorrhagic septicaemia virus in Danish marine fishes and its occurrence in new host species. Dis. Aquat. Org. 2005, 66, 145–151. [Google Scholar] [CrossRef]
- Snow, M.; King, J.A.; Garden, A.; Raynard, R.S. Experimental susceptibility of Atlantic cod, Gadus morhua (L.), and Atlantic halibut, Hippoglossus hippoglossus (L.) to different genotypes of viral haemorrhagic septicaemia virus. J. Fish. Dis. 2005, 28, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Snow, M.; McKay, P.; McIntosh, R. Relative resistance of juvenile Atlantic cod to oral and immersion infection with VHSV mimicking natural routes of exposure. Bull. Eur. Assoc. Fish. Pathol. 2009, 29, 78–85. [Google Scholar]
- Bowden, T.J. A study of the susceptibility of Atlantic halibut, Hippoglossus hippoglossus (L.), to viral haemorrhagic septicaemia virus isolated from turbot, Scophthalmus maximus (L.). J. Fish. Dis. 2003, 26, 207–212. [Google Scholar] [CrossRef]
- López-Vázquez, C.; Conde, M.; Dopazo, C.P.; Barja, J.L.; Bandín, I. Susceptibility of juvenile sole Solea senegalensis to marine isolates of viral haemorrhagic septicaemia virus from wild and farmed fish. Dis. Aquat. Org. 2011, 93, 111–116. [Google Scholar] [CrossRef]
- Snow, M.; King, J.A.; Garden, A.; Shanks, A.M.; Raynard, R.S. Comparative susceptibility of turbot Scophthalmus maximus to different genotypes of viral haemorrhagic septicaemia virus. Dis. Aquat. Org. 2005, 67, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Brudeseth, B.E.; Skall, H.F.; Evensen, Ø. Differences in virulence of marine and freshwater isolates of viral hemorrhagic septicemia virus in vivo correlate with in vitro ability to infect gill epithelial cells and macrophages of rainbow trout (Oncorhynchus mykiss). J. Virol. 2008, 82, 10359–10365. [Google Scholar] [CrossRef]
- Brudeseth, B.E.; Raynard, R.S.; King, J.A.; Evensen, Ø. Sequential pathology after experimental infection with marine viral hemorrhagic septicemia virus isolates of low and high virulence in turbot (Scophthalmus maximus L.). Vet. Pathol. 2005, 42, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Puvanendran, V.; Mortensen, A.; Johansen, L.-H.; Kettunen, A.; Hansen, Ø.J.; Henriksen, E.; Heide, M. Development of cod farming in Norway: Past and current biological and market status and future prospects and directions. Rev. Aquac. 2021, 14, 308–342. [Google Scholar] [CrossRef]
- OIE. Viral haemorrhagic septicaemia. In Manual of Diagnostic Tests for Aquatic Animals; The World Organisation for Animal Health: Paris, France, 2009; Available online: www.oie.int/fileadmin/Home/eng/Internationa_Standard_Setting/docs/pdf/2.3.09.VHS.pdf (accessed on 7 December 2021).
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Für Exp. Pathol. Und Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Johansen, R.; Bergh, Ø.; Modahl, I.; Dahle, G.; Gjerset, B.; Holst, J.C.; Sandlund, N. High prevalence of viral haemorrhagic septicaemia virus (VHSV) in Norwegian spring-Spawning herring. Mar. Ecol. Prog. Ser. 2013, 478, 223–230. [Google Scholar] [CrossRef]
- Matejusova, I.; McKay, P.; McBeath, A.J.A.; Collet, B.; Snow, M. Development of a sensitive and controlled real-Time RT-PCR assay for viral haemorrhagic septicaemia virus (VHSV) in marine salmonid aquaculture. Dis. Aquat. Org. 2008, 80, 137–144. [Google Scholar] [CrossRef]
- Duesund, H.; Nylund, S.; Watanabe, K.; Ottem, K.F.; Nylund, A. Characterization of a VHS virus genotype III isolated from rainbow trout (Oncorhynchus mykiss) at a marine site on the west coast of Norway. Virol. J. 2010, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Snow, M.; Black, J.; Matejusova, I.; McIntosh, R.; Baretto, E.; Wallace, I.S.; Bruno, D.W. Detection of salmonid alphavirus RNA in wild marine fish: Implications for the origins of salmon pancreas disease in aquaculture. Dis. Aquat. Org. 2010, 91, 177–188. [Google Scholar] [CrossRef]
- Evensen, Ø.; Olesen, N.J. Immunohistochemical detection of VHS virus in paraffin-Embedded specimens of rainbow trout (Oncorhynchus mykiss); The influence of primary antibody, fixative, and antigen unmasking on method sensitivity. Vet. Pathol. 1997, 34, 253–261. [Google Scholar] [CrossRef]
- Wangen, I.H.; Karlsbakk, E.; Einen, A.C.B.; Ottem, K.F.; Nylund, A.; Mortensen, S. Fate of Francisella noatunensis, a pathogen of Atlantic cod Gadus morhua, in blue mussels Mytilus edulis. Dis. Aquat. Org. 2012, 98, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Grotmol, S.; Bergh, Ø.; Totland, G.K. Transmission of viral encephalopathy and retinopathy (VER) to yolk-Sac larvae of the Atlantic halibut Hippoglossus hippoglossus: Occurrence of nodavirus in various organs and a possible route of infection. Dis. Aquat. Org. 1999, 36, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Sandlund, N.; Bergh, Ø. Screening and characterisation of potentially pathogenic bacteria associated with Atlantic cod Gadus morhua larvae: Bath challenge trials using a multidish system. Dis. Aquat. Org. 2008, 81, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Suau, A.; Bonnet, R.; Sutren, M.; Godon, J.J.; Gibson, G.R.; Collins, M.D.; Dore, J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 1999, 65, 4799–4807. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussinee, L.; Huber, P.; Russell, S.; LePage, V.; Reid, A.; Young, K.M.; Nagy, E.; Stevenson, R.M.W.; Lumsden, J.S. Viral haemorrhagic septicaemia virus IVb experimental infection of rainbow trout, Oncorhynchus mykiss (Walbaum), and fathead minnow, Pimephales promelas (Rafinesque). J. Fish. Dis. 2010, 33, 347–360. [Google Scholar] [CrossRef]
- Iida, H.; Mori, K.; Nishizawa, T.; Arimoto, M.; Muroga, K. Fate of viral hemorrhagic septicemia virus in Japanese flounder Paralichthys olivaceus challenged by immersion. Fish. Pathol. 2003, 38, 87–91. [Google Scholar] [CrossRef]
- Lumsden, J.S.; Morrison, B.; Yason, C.; Russell, S.; Young, K.; Yazdanpanah, A.; Huber, P.; Al-Hussinee, L.; Stone, D.; Way, K. Mortality event in freshwater drum Aplodinotus grunniens from Lake Ontario, Canada, associated with viral haemorrhagic septicemia virus, Type IV. Dis. Aquat. Org. 2007, 76, 99–111. [Google Scholar] [CrossRef]
- Al-Hussinee, L.; Lord, S.; Stevenson, R.M.W.; Casey, R.N.; Groocock, G.H.; Britt, K.L.; Kohler, K.H.; Wooster, G.A.; Getchell, R.G.; Bowser, P.R.; et al. Immunohistochemistry and pathology of multiple Great Lakes fish from mortality events associated with viral hemorrhagic septicemia virus type IVb. Dis. Aquat. Org. 2011, 93, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, P.K.; Gregg, J.L.; Grady, C.A.; Taylor, L.; Winton, J.R. Chronic and persistent viral hemorrhagic septicemia virus infections in Pacific herring. Dis. Aquat. Org. 2010, 93, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Brudeseth, B.E.; Castric, J.; Evensen, Ø. Studies on pathogenesis following single and double infection with viral hemorrhagic septicemia virus and infectious hematopoietic necrosis virus in rainbow trout (Oncorhynchus mykiss). Vet. Pathol. 2002, 39, 180–189. [Google Scholar] [CrossRef]
- Oidtmann, B.; Joiner, C.; Stone, D.; Dodge, M.; Reese, R.A.; Dixon, P. Viral load of various tissues of rainbow trout challenged with viral haemorrhagic septicaemia virus at various stages of disease. Dis. Aquat. Org. 2011, 93, 93–104. [Google Scholar] [CrossRef]
- Sandlund, N.; Gjerset, B.; Bergh, Ø.; Modahl, I.; Olesen, N.J.; Johansen, R. Screening for viral hemorrhagic septicemia virus in marine fish along the Norwegian coastal line. PLoS ONE 2014, 9, e108529. [Google Scholar] [CrossRef][Green Version]
- Hershberger, P.K.; MacKenzie, A.H.; Gregg, J.L.; Wilmot, M.D.; Powers, R.L.; Purcell, M.K. Long-Term shedding from fully convalesced individuals indicates that Pacific herring are a reservoir for viral hemorrhagic septicemia virus. Dis. Aquat. Org. 2021, 144, 245–252. [Google Scholar] [CrossRef]
- Dalmo, R.A.; Ingebrigtsen, K.; Bogwald, J. Non-Specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J. Fish. Dis. 1997, 20, 241–273. [Google Scholar] [CrossRef]
- Lovy, J.; Lewis, N.L.; Hershberger, P.K.; Bennett, W.; Meyers, T.R.; Garver, K.A. Viral tropism and pathology associated with viral hemorrhagic septicemia in larval and juvenile Pacific herring. Vet. Microbiol. 2012, 161, 66–76. [Google Scholar] [CrossRef]
- Lovy, J.; Piesik, P.; Hershberger, P.K.; Garver, K.A. Experimental infection studies demonstrating Atlantic salmon as a host and reservoir of viral hemorrhagic septicemia virus type IVa with insights into pathology and host immunity. Vet. Microbiol. 2013, 166, 91–101. [Google Scholar] [CrossRef]
- Snow, M.; Smail, D.A. Experimental susceptibility of turbot Scophthalmus maximus to viral haemorrhagic septicaemia virus isolated from cultivated turbot. Dis. Aquat. Org. 1999, 38, 163–168. [Google Scholar] [CrossRef] [PubMed]
- López-Vázquez, C.; Dopazo, C.P.; Barja, J.L.; Bandín, I. Experimental infection of turbot, Psetta maxima (L.), with strains of viral haemorrhagic septicaemia virus isolated from wild and farmed marine fish. J. Fish. Dis. 2007, 30, 303–312. [Google Scholar] [CrossRef]
- Muroga, K.; Iida, H.; Mori, K.; Nishizawa, T.; Arimoto, M. Experimental horizontal transmission of viral hemorrhagic septicemia virus (VHSV) in Japanese flounder Paralichthys olivaceus. Dis. Aquat. Org. 2004, 58, 111–115. [Google Scholar] [CrossRef]
- Goodwin, A.E.; Merry, G.E. Mortality and carrier status of bluegills exposed to viral hemorrhagic septicemia virus genotype IVb at different temperatures. J. Aquat. Anim. Health 2011, 23, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Elston, R.A.; Meyers, T.R. Effect of viral hemorrhagic septicemia virus on Pacific herring in Prince William Sound, Alaska, from 1989 to 2005. Dis. Aquat. Org. 2009, 83, 223–246. [Google Scholar] [CrossRef]
- Thompson, T.M.; Batts, W.N.; Faisal, M.; Bowser, P.; Casey, J.W.; Phillips, K.; Garver, K.A.; Winton, J.; Kurath, G. Emergence of Viral hemorrhagic septicemia virus in the North American Great Lakes region is associated with low viral genetic diversity. Dis. Aquat. Org. 2011, 96, 29–43. [Google Scholar] [CrossRef]
- Bain, M.B.; Cornwell, E.R.; Hope, K.M.; Eckerlin, G.E.; Casey, R.N.; Groocock, G.H.; Getchell, R.G.; Bowser, P.R.; Winton, J.R.; Batts, W.N.; et al. Distribution of an invasive aquatic pathogen (Viral Hemorrhagic Septicemia Virus) in the Great Lakes and its relationship to shipping. PLoS ONE 2010, 5, e10156. [Google Scholar] [CrossRef] [PubMed]
- Garver, K.A.; Traxler, G.S.; Hawley, L.M.; Richard, J.; Ross, J.P.; Lovy, J. Molecular epidemiology of viral haemorrhagic septicaemia virus (VHSV) in British Columbia, Canada, reveals transmission from wild to farmed fish. Dis. Aquat. Org. 2013, 104, 93–104. [Google Scholar] [CrossRef]
- Dixon, P.F.; Feist, S.; Kehoe, E.; Parry, L.; Stone, D.M.; Way, K. Isolation of viral haemorrhagic septicaemia virus from Atlantic herring Clupea harengus from the English Channel. Dis. Aquat. Org. 1997, 30, 81–89. [Google Scholar] [CrossRef]
- Ahne, W. Experimental infection of pike (Esox lucius) with Egtvedvirus. Tierarztl. Umsch. 1980, 35, 225–229. [Google Scholar]
- Schönherz, A.A.; Hansen, M.H.H.; Jorgensen, H.B.H.; Berg, P.; Lorenzen, N.; Einer-Jensen, K. Oral transmission as a route of infection for viral haemorrhagic septicaemia virus in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish. Dis. 2012, 35, 395–406. [Google Scholar] [CrossRef] [PubMed]
| Experiment 1; i.p. Challenge | Experiment 2; Cohabitation Challenge | ||||||
|---|---|---|---|---|---|---|---|
| Fish group and tanks | Nr of fish | Sampling dpc | Sampled orgns | Fish group and tanks | Nr of cohab + i.p. injected fish | Sampling, dpc | Sampled organs |
| Ctr nr M1-2 | 53 | Mortality registration only | Ctr nr M1-3 | 50 + 20 | Mortality registration only | ||
| Ch nr M1-3 | 55 | Ch nr M1-3 | 50 + 20 | ||||
| Ctr nr S1-3 | 44 | 7, 14, 28, 84, 155 | Eyes, Gills, Heart, Liver, Spleen, Head kidney, Brain | Ctr nr S1-2 | 50 + 20 | 10, 28, 62, 92 | Eyes, Gills, Liver, Spleen, Head Kidney, Brain, Pylorus caeca, Intestine |
| Ch nr S1-3 | 55 | Ch nr S1-3 | 50 + 20 | ||||
| No. of Positive Organs | Comments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dpc | No. of Sampled Individuals | Gills | Heart | Spleen | Kidney | Eye | Brain | Liver | |
| 6 * | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | All organs sampled from individual between 6–55 dpc were tested |
| 7 | 15 | 14 | 15 | 15 | 14 | 9 | 11 | 13 | |
| 12 * | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 14 | 12 | 6 | 9 | 4 | 4 | 3 | 7 | 4 | |
| 14 * | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| 20 * | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 28 | 9 | 1 | 5 | 1 | 1 | 2 | 2 | 0 | |
| 44 * | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 55 * | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | |
| 84 | 28 | 0 of 5 | 4 | 0 of 5 | 1 of 5 | 2 of 5 | 1 | 0 of 5 | Five individuals tested positive in either brain or heart. These 5 were tested in all organs |
| 155 | 28 | 0 of 3 | 2 | 0 of 3 | 0 of 3 | 1 of 3 | 1 | 0 of 3 | Three individuals tested positive in either brain or heart. These 3 were tested in all organs |
| Days Post Hatching | Assay 1 | Assay 2 | ||
|---|---|---|---|---|
| dpc | Brain | Heart | Brain | Heart |
| 7 | 3/15 | 10/15 | 11/15 | 15/15 |
| 14 | 1/9 | 3/9 | 4/9 | 7/9 |
| 28 | 1/9 | 1/9 | 2/9 | 5/9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandlund, N.; Johansen, R.; Fiksdal, I.U.; Einen, A.C.B.; Modahl, I.; Gjerset, B.; Bergh, Ø. Susceptibility and Pathology in Juvenile Atlantic Cod Gadus morhua to a Marine Viral Haemorrhagic Septicaemia Virus Isolated from Diseased Rainbow Trout Oncorhynchus mykiss. Animals 2021, 11, 3523. https://doi.org/10.3390/ani11123523
Sandlund N, Johansen R, Fiksdal IU, Einen ACB, Modahl I, Gjerset B, Bergh Ø. Susceptibility and Pathology in Juvenile Atlantic Cod Gadus morhua to a Marine Viral Haemorrhagic Septicaemia Virus Isolated from Diseased Rainbow Trout Oncorhynchus mykiss. Animals. 2021; 11(12):3523. https://doi.org/10.3390/ani11123523
Chicago/Turabian StyleSandlund, Nina, Renate Johansen, Ingrid U. Fiksdal, Ann Cathrine B. Einen, Ingebjørg Modahl, Britt Gjerset, and Øivind Bergh. 2021. "Susceptibility and Pathology in Juvenile Atlantic Cod Gadus morhua to a Marine Viral Haemorrhagic Septicaemia Virus Isolated from Diseased Rainbow Trout Oncorhynchus mykiss" Animals 11, no. 12: 3523. https://doi.org/10.3390/ani11123523
APA StyleSandlund, N., Johansen, R., Fiksdal, I. U., Einen, A. C. B., Modahl, I., Gjerset, B., & Bergh, Ø. (2021). Susceptibility and Pathology in Juvenile Atlantic Cod Gadus morhua to a Marine Viral Haemorrhagic Septicaemia Virus Isolated from Diseased Rainbow Trout Oncorhynchus mykiss. Animals, 11(12), 3523. https://doi.org/10.3390/ani11123523







