The Potential of Metalloproteinase-9 Administration to Accelerate Mammary Involution and Boost the Immune System at Dry-Off
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, Recombinant Proteins and Growth Conditions
2.2. Purification and Quantification of rtMMP-9
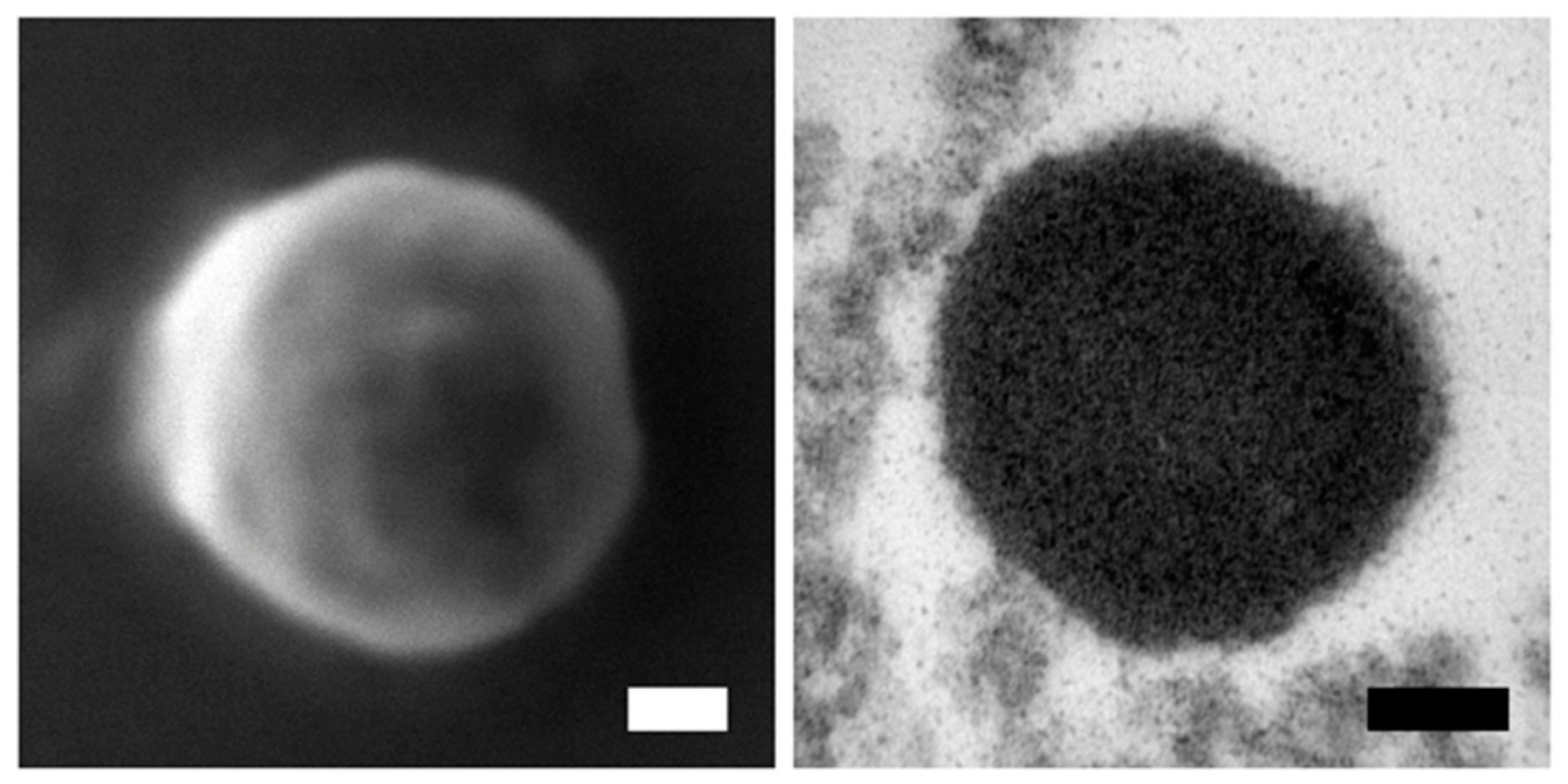
2.3. Electron Microscopy (EM)
2.4. Analysis of Metalloproteinase Activity
2.5. Intra-Mammary Infusions
2.6. Mammary Secretion Analyses
2.7. Statistical Analysis
3. Results
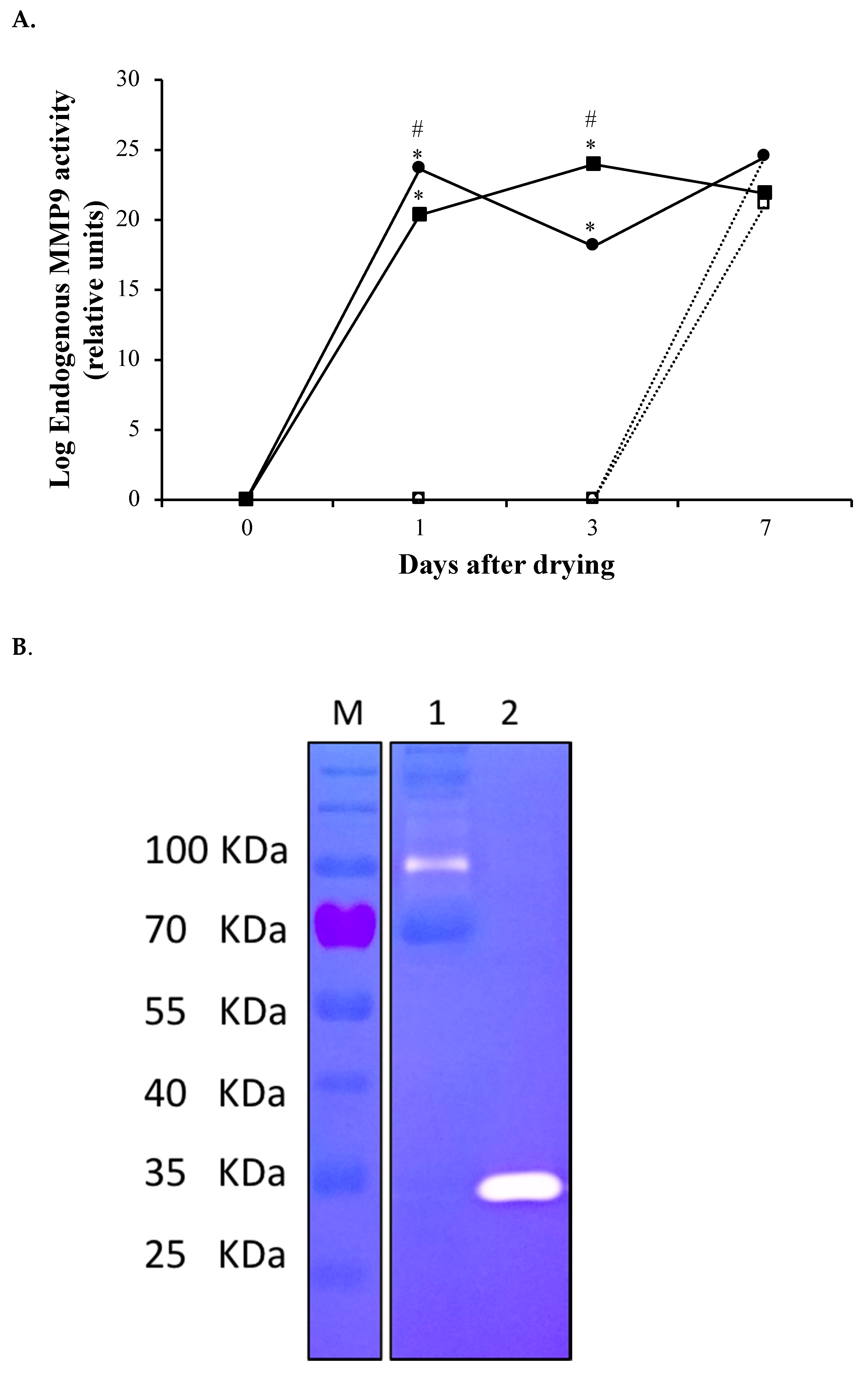
3.1. rMMP-9 Production, Activity Determination and Mammary Gland Infusion
3.2. Induced Metalloproteinase Activity in the Mammary Gland
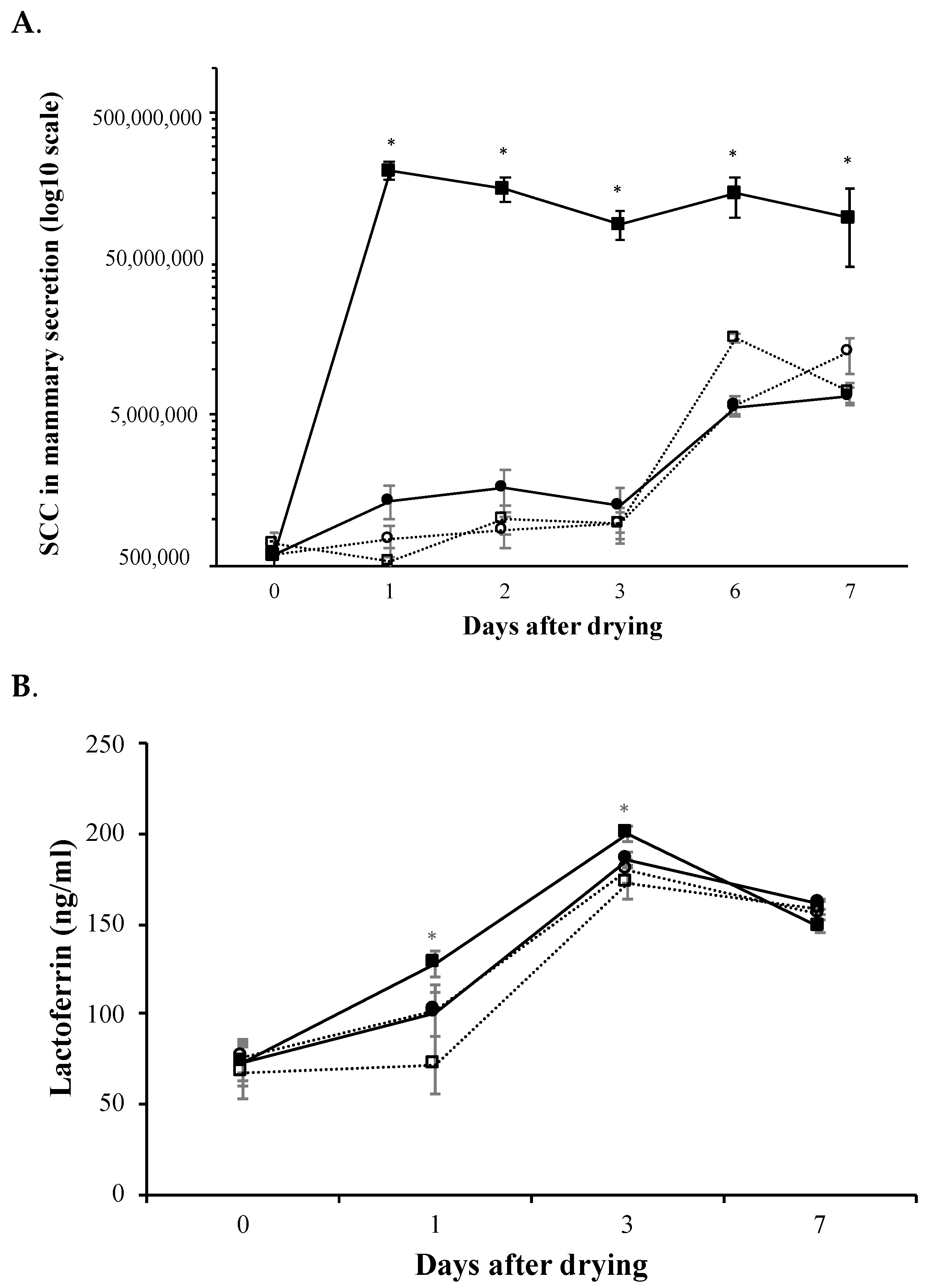
3.3. Mammary Immune Response and Involution Markers Monitorization after Intramammary Administration of rtMMP-9
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachman, K.C.; Schairer, M.I. Invited Review: Bovine studies on optimal lengths of dry periods. J. Dairy Sci. 2003, 10, 3027–3037. [Google Scholar] [CrossRef]
- Pezeshki, A.; Capuco, A.V.; De Spiegeleer, B.; Peelman, L.; Stevens, M.; Collier, R.J.; Burvenich, C. An integrated view on how the management of the dry period length of lactating cows could affect mammary biology and defense. J. Anim. Physiol. Anim. Nutr. 2010, 94, e7–e30. [Google Scholar] [CrossRef]
- Bach, A.; De-Prado, A.; Aris, A. Short communication: The effects of cabergoline administration at dry-off of lactating cows on udder engorgement, milk leakages, and lying behavior. J. Dairy Sci. 2015, 98, 7097–7101. [Google Scholar] [CrossRef] [Green Version]
- O’Driscoll, K.; Gleeson, D.; O’Brien, B.; Boyle, L. Does omission of a regular milking event affect cow comfort? Livest. Sci. 2011, 138, 132–143. [Google Scholar] [CrossRef]
- Gott, P.N.; Rajala-Schultz, P.J.; Schuenemann, G.M.; Proudfoot, K.L.; Hoga, J.S. Intramammary infections and milk leakage following gradual or abrupt cessation of milking. J. Dairy Sci. 2016, 99, 4005–4017. [Google Scholar] [CrossRef]
- Rajala-Schultz, P.J.; Hogan, J.S.; Smith, K.L. Short communication: Association between milk yield at dry-off and probability of intramammary infections at calving. J. Dairy Sci. 2005, 88, 577–579. [Google Scholar] [CrossRef]
- Burvenich, C.; Bannerman, D.D.; Lippolis, J.D.; Peelman, L.; Nonnecke, B.J.; Kehrli, M.E., Jr.; Paape, M.J. Cumulative physiological events influence the inflammatory response of the bovine udder to Escherichia coli infections during the transition period. J. Dairy Sci. 2007, 90, E39–E54. [Google Scholar] [CrossRef] [Green Version]
- Oliver, S.P.; Sordillo, L.M. Approaches to the manipulation of mammary involution. J. Dairy Sci. 1989, 72, 1647–1664. [Google Scholar] [CrossRef]
- Yu, T.C.; Chen, S.E.; Ho, T.H.; Peh, H.C.; Liu, W.B.; Tiantong, A.; Nagahata, H.; Chang, C.J. Involvement of TNF-α and MAPK pathway in the intramammary MMP-9 release via degranulation of cow neutrophils during acute mammary gland involution. Vet. Immunol. Immunopathol. 2012, 147, 161–169. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.; Wang, X.; Li, X.; Qi, S.; Zhang, Y.; Gao, M. TGF-β1 Induces EMT in Bovine Mammary Epithelial Cells Through the TGFβ1/Smad Signaling Pathway. Cell Physiol. Biochem. 2017, 43, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, G.; Bernier, P.; Dodier, L.; Delbecchi, G.; Wagner, F.; Talbot, B.G.; Lacasse, P. Local control of mammary involution: Is stanniocalcin-1 involved? J. Dairy Sci. 2009, 92, 1998–2006. [Google Scholar] [CrossRef] [Green Version]
- Capuco, A.V.; Akers, R.M. Mammary involution in dairy animals. J. Mammary Gland Biol. Neoplasia. 1999, 4, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [Green Version]
- Jena, M.K.; Jaswal, S.; Kumar, S.; Mohanty, A.K. Molecular mechanism of mammary gland involution: An update. Dev. Biol. 2019, 15, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, N.; Sympson, C.J.; Werb, Z.; Bissell, M.J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science 1995, 267, 891–893. [Google Scholar] [CrossRef] [Green Version]
- Hui, L.; Huiling, Z.; Lihui, L.; Xingai, S.; Wenjuan, Z.; Yongsen, S. The Effects of Matrix Metalloproteinase-9 on Dairy Goat Mastitis and Cell Survival of Goat Mammary Epithelial Cells. PLoS ONE 2016, 11, e0160989. [Google Scholar]
- Gifre-Renom, L.; Carratalá, J.V.; Parés, S.; Sánchez-García, L.; Ferrer-Miralles, N.; Villaverde, A.; Bach, A.; Garcia-Fruitós, E.; Arís, A. Potential of MMP-9 based nanoparticles at optimizing the cow dry period: Pulling apart the effects of MMP-9 and nanoparticles. Sci. Rep. 2020, 10, 11299. [Google Scholar] [CrossRef]
- Cano-Garrido, O.; Sánchez-Chardi, A.; Parés, S.; Giró, I.; Tatkiewicz, W.I.; Ferrer-Miralles, N.; Ratera, I.; Natalello, A.; Cubarsi, R.; Veciana, J.; et al. Functional protein-based nanomaterial produced in microorganisms recognized as safe: A new platform for biotechnology. Acta Biomater. 2016, 43, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Gifre, L.; Arís, A.; Bach, A.; Garcia--Fruitós, E. Trends in recombinant protein use in animal production. Microb. Cell Fact. 2017, 16, 40. [Google Scholar] [CrossRef] [Green Version]
- Poquet, I.; Saint, V.; Seznec, E.; Simoes, N.; Bolotin, A.; Gruss, A. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 2000, 35, 1042–1051. [Google Scholar] [CrossRef]
- Cortes-Perez, N.G.; Poquet, I.; Oliveira, M.; Gratadoux, J.J.; Madsen, S.M.; Miyoshi, A.; Corthier, G.; Azevedo, V.; Langella, P.; Bermúdez-Humarán, L.G. Construction and characterization of a Lactococcus lactis strain deficient in intracellular ClpP and extracellular HtrA proteases. Microbiology 2006, 152, 2611–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mierau, I.; Kleerebezem, M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 2005, 68, 705–717. [Google Scholar] [CrossRef]
- Peternel, S.; Grdadolnik, J.; Gaberc-Porekar, V.; Komel, R. Engineering inclusion bodies for non-denaturing extraction of functional proteins. Microb. Cell Fact. 2008, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Domènech, A.; Parés, S.; Bach, A.; Arís, A. Mammary serum amyloid A3 activates involution of the mammary gland in dairy cows. J. Dairy Sci. 2014, 97, 7595–7605. [Google Scholar] [CrossRef]
- Murcia, M.A.; Vera, A.; Martinez-Tome, M.; Munoz, A.; Hernandez-Cordoba, M.; Ortiz-Gonzalez, R. Fast determination of the Ca, Mg, K, Na and Zn contents in milk and nondairy imitation milk using ICP-AES without mineralization stage. LWT Food Sci. Technol. 1999, 323, 175–179. [Google Scholar] [CrossRef]
- Garcia-Fruitós, E. Lactic Acid Bacteria: A promising alternative for recombinant protein production. Microb. Cell. Fact. 2012, 11, 157. [Google Scholar] [CrossRef] [Green Version]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.I.; Quigley, J.P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 20262–20267. [Google Scholar] [CrossRef] [Green Version]
- Gifre-Renom, L.; Seras-Franzoso, J.; Rafael, D.; Andrade, F.; Cano-Garrido, O.; Martinez-Trucharte, F.; Ugarte-Berzal, E.; Martens, E.; Boon, L.; Villaverde, A.; et al. The Biological Potential Hidden in Inclusion Bodies. Pharmaceutics 2020, 15, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-DeSimone, N.; Hahn-Dantona, E.; Sipley, J.; Nagase, H.; French, D.L.; Quigley, J.P. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J. Biol. Chem. 1999, 274, 13066–13076. [Google Scholar] [CrossRef] [Green Version]
- Céspedes, M.V.; Fernández, Y.; Unzueta, U.; Mendoza, R.; Seras-Franzoso, J.; Sánchez-Chardi, A.; Álamo, A.; Toledo-Rubio, V.; Ferrer-Miralles, N.; Vazquez, E.; et al. Bacterial mimetics of endocrine secretory granules as immobilized in vivo depots for functional protein drugs. Sci. Rep. 2016, 6, 35765. [Google Scholar] [CrossRef]
- Seras-Franzoso, J.; Sánchez-Chardi, A.; Garcia-Fruitós, E.; Vázquez, E.; Villaverde, A. Cellular uptake and intracellular fate of protein releasing bacterial amyloids in mammalian cells. Soft. Matter. 2016, 12, 3451–3460. [Google Scholar] [CrossRef] [PubMed]
- Ponchon, B.; Lacasse, P.; Silanikove, N.; Ollier, S.; Zhao, X. Effects of intramammary infusions of casein hydrolysate, ethylene glycol-bis (beta-aminoethyl ether)-N,N,N’,N’-tetraacetic acid, and lactose at drying-off on mammary gland involution. J. Dairy Sci. 2014, 97, 779–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parés, S. Strategies for the Optimization of Cow Dry Period. Ph.D. Thesis, Autonomous University of Barcelona, Barcelona, Spain, 27 October 2017. Available online: https://www.tesisenred.net/handle/10803/458660?locale-attribute=en#page=1 (accessed on 5 November 2021).
- Dallard, B.E.; Baravalle, C.; Andreotti, C.; Ortega, H.H.; Neder, V.; Calvinho, L.F. Intramammary inoculation of Panax ginseng extract in cows at drying off enhances early mammary involution. J. Dairy Res. 2011, 78, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lanctôt, S.; Fustier, P.; Taherian, A.R.; Bisakowski, B.; Zhao, X.; Lacasse, P. Effect of intramammary infusion of chitosan hydrogels at drying-off on bovine mammary gland involution. J. Dairy Sci. 2017, 100, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Dallard, B.E.; Baravalle, C.; Ortega, H.; Ruffino, V.; Heffel, S.; Calvinho, L.F. Effect of a biological response modifier on cellular death mechanisms at drying off. J. Dairy Res. 2008, 75, 167–175. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parés, S.; Cano-Garrido, O.; Bach, A.; Ferrer-Miralles, N.; Villaverde, A.; Garcia-Fruitós, E.; Arís, A. The Potential of Metalloproteinase-9 Administration to Accelerate Mammary Involution and Boost the Immune System at Dry-Off. Animals 2021, 11, 3415. https://doi.org/10.3390/ani11123415
Parés S, Cano-Garrido O, Bach A, Ferrer-Miralles N, Villaverde A, Garcia-Fruitós E, Arís A. The Potential of Metalloproteinase-9 Administration to Accelerate Mammary Involution and Boost the Immune System at Dry-Off. Animals. 2021; 11(12):3415. https://doi.org/10.3390/ani11123415
Chicago/Turabian StyleParés, Sílvia, Olivia Cano-Garrido, Alex Bach, Neus Ferrer-Miralles, Antonio Villaverde, Elena Garcia-Fruitós, and Anna Arís. 2021. "The Potential of Metalloproteinase-9 Administration to Accelerate Mammary Involution and Boost the Immune System at Dry-Off" Animals 11, no. 12: 3415. https://doi.org/10.3390/ani11123415
APA StyleParés, S., Cano-Garrido, O., Bach, A., Ferrer-Miralles, N., Villaverde, A., Garcia-Fruitós, E., & Arís, A. (2021). The Potential of Metalloproteinase-9 Administration to Accelerate Mammary Involution and Boost the Immune System at Dry-Off. Animals, 11(12), 3415. https://doi.org/10.3390/ani11123415





