A Systematic Review of Musculoskeletal Mobilization and Manipulation Techniques Used in Veterinary Medicine
Abstract
Simple Summary
Abstract
1. Introduction
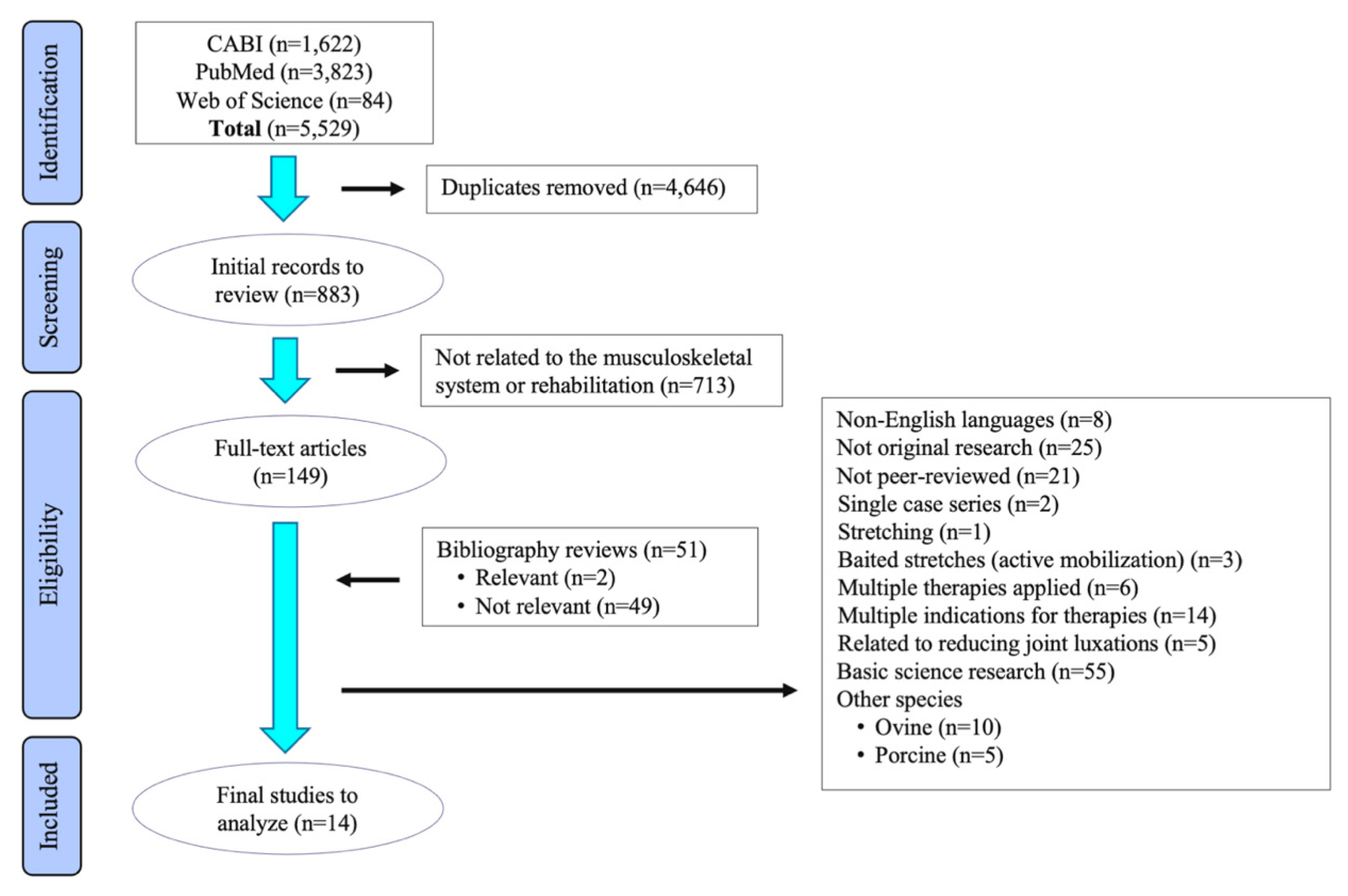
2. Materials and Methods
3. Results
3.1. Clinical Indications
3.1.1. Mobilization
3.1.2. Manipulation
3.2. Dosages
3.2.1. Mobilization
3.2.2. Manipulation
3.3. Outcome Parameters
3.3.1. Observation
3.3.2. Physical Examination
3.3.3. Muscle Tone and Activity
3.3.4. Spinal Reflexes
3.3.5. Mechanical Nociceptive Thresholds
3.3.6. Passive Joint Range of Motion
3.3.7. Thoracolumbar Flexibility
3.3.8. Motion Analysis
3.3.9. Visual Analog Scales
3.3.10. Owner Surveys of Performance
3.3.11. Thermography
3.3.12. Lameness Evaluation
3.4. Clinical Efficacy
3.4.1. Physical Examination
3.4.2. Muscle Tone and Activity
3.4.3. Spinal Reflexes
3.4.4. Mechanical Nociceptive Thresholds
3.4.5. Passive Joint Range of Motion
3.4.6. Thoracolumbar Flexibility
3.4.7. Motion Analysis
3.4.8. Visual Analog Scales
3.4.9. Owner Surveys of Performance
3.4.10. Thermography
3.4.11. Lameness Evaluation
4. Discussion
4.1. Quality
4.2. Treatment Methods
4.3. Clinical Indications
4.4. Dosages
4.5. Outcome Parameters
4.6. Perceived Efficacy
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haussler, K.K. Equine manual therapies in sport horse practice. Vet. Clin. N. Am. Equine Pract. 2018, 34, 375–389. [Google Scholar] [CrossRef]
- Bergenstrahle, A.; Nielsen, B.D. Attitude and behavior of veterinarians surrounding the use of complementary and alternative veterinary medicine in the treatment of equine musculoskeletal pain. J. Equine Vet. Sci. 2016, 45, 87–97. [Google Scholar] [CrossRef]
- Koh, R.; Montalbano, C.; Gamble, L.J.; Walden, K.; Rouse, J.; Liu, C.C.; Wakshlag, L.G.; Wakshlag, J.J. Internet survey of feeding, dietary supplement, and rehabilitative medical management use in flyball dogs. Can. Vet. J. 2020, 61, 375–381. [Google Scholar] [PubMed] [PubMed Central]
- Lange, C.D.; Axiak Flammer, S.; Gerber, V.; Kindt, D.; Koch, C. Complementary and alternative medicine for the management of orthopaedic problems in Swiss Warmblood horses. Vet. Med. Sci. 2017, 3, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Thirkell, J.; Hyland, R. A survey examining attitudes towards equine complementary therapies for the treatment of musculoskeletal injuries. J. Equine Vet. Sci. 2017, 59, 82–87. [Google Scholar] [CrossRef]
- Wilson, J.M.; McKenzie, E.; Duesterdieck-Zellmer, K. International survey regarding the use of rehabilitation modalities in horses. Front. Vet. Sci. 2018, 5, 120. [Google Scholar] [CrossRef]
- Hesbach, A.L. Manual therapy in veterinary rehabilitation. Top. Companion Anim. Med. 2014, 29, 20–23. [Google Scholar] [CrossRef]
- Haussler, K.K. Review of manual therapy techniques in equine practice. J. Equine Vet. Sci. 2009, 29, 849–869. [Google Scholar] [CrossRef]
- Gruenenfelder, F.I.; Boos, A.; Mouwen, M.; Steffen, F. Evaluation of the anatomic effect of physical therapy exercises for mobilization of lumbar spinal nerves and the dura mater in dogs. Am. J. Vet. Res. 2006, 67, 1773–1779. [Google Scholar] [CrossRef]
- Acutt, E.V.; le Jeune, S.S.; Pypendop, B.H. Evaluation of the effects of chiropractic on static and dynamic muscle variables in sport horses. J. Equine Vet. Sci. 2019, 73, 84–90. [Google Scholar] [CrossRef]
- Coates, J.C. Manual therapy. In Canine Sports Medicine and Rehabilitation, 2nd ed.; Zink, M.C., Van Dyke, J.B., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 120–135. [Google Scholar] [CrossRef]
- Edge-Hughes, L. Canine thoracic costovertebral and costotransverse joints: Three case reports of dysfunction and manual therapy guidelines for assessment and treatment of these structures. Top. Companion Anim. Med. 2014, 29, 1–5. [Google Scholar] [CrossRef][Green Version]
- Haussler, K.K. Joint mobilization and manipulation for the equine athlete. Vet. Clin. N. Am. Equine Pract. 2016, 32, 87–101. [Google Scholar] [CrossRef]
- Haussler, K.K. The role of manual therapies in equine pain management. Vet. Clin. N. Am. Equine Pract. 2010, 26, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.G.; Walker, J.R.; Levine, D. Joint mobilization. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 1287–1316. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Reed, W.R.; Long, C.R.; Kawchuk, G.N.; Sozio, R.S.; Pickar, J.G. Neural responses to physical characteristics of a high-velocity, low-amplitude spinal manipulation: Effect of thrust direction. Spine 2018, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.Y.; Reed, W.R.; Long, C.R.; Kawchuk, G.N.; Pickar, J.G. Effects of thrust amplitude and duration of high-velocity, low-amplitude spinal manipulation on lumbar muscle spindle responses to vertebral position and movement. J. Manip. Physiol. Ther. 2013, 36, 68–77. [Google Scholar] [CrossRef][Green Version]
- Olson, V.L. Evaluation of joint mobilization treatment. A method. Phys. Ther. 1987, 67, 351–356. [Google Scholar] [CrossRef]
- Ahern, T.J. Cervical vertebral mobilization under anesthetic (CVMUA): A physical therapy for the treatment of cervico-spinal pain and stiffness. J. Equine Vet. Sci. 1994, 14, 540–545. [Google Scholar] [CrossRef]
- Pusey, A.; Colles, C.; Brooks, J. Osteopathic treatment of horses—a retrospective study. Br. Osteopath. J. 1995, 16, 30–32. [Google Scholar]
- Colles, C.M.; Nevin, A.; Brooks, J. The osteopathic treatment of somatic dysfunction causing gait abnormality in 51 horses. Equine Vet. Educ. 2014, 26, 148–155. [Google Scholar] [CrossRef]
- Haussler, K.K.; Manchon, P.T.; Donnell, J.R.; Frisbie, D.D. Effects of low-level laser therapy and chiropractic care on back pain in Quarter Horses. J. Equine Vet. Sci. 2020, 86, 102891. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; McGowan, C.M.; Hyytiäinen, H.K. Effect of caudal traction on mechanical nociceptive thresholds of epaxial and pelvic musculature on a group of horses with signs of back pain. J. Equine Vet. Sci. 2020, 93, 103197. [Google Scholar] [CrossRef]
- Sullivan, K.A.; Hill, A.E.; Haussler, K.K. The effects of chiropractic, massage and phenylbutazone on spinal mechanical nociceptive thresholds in horses without clinical signs. Equine Vet. J. 2008, 40, 14–20. [Google Scholar] [CrossRef]
- Haussler, K.K.; Erb, H.N. Pressure algometry: Objective assessment of back pain and effects of chiropractic treatment. Proc. Am. Ass. Equine Pract. 2003, 49, 66–70. [Google Scholar]
- Wakeling, J.M.; Barnett, K.; Price, S.; Nankervis, K. Effects of manipulative therapy on the longissimus dorsi in the equine back. Equine Compar. Exerc. Physiol. 2006, 3, 153–160. [Google Scholar] [CrossRef]
- Taylor, F.; Tabor, G.; Williams, J.M. Altered thoracolumbar position during application of craniocaudal spinal mobilisation in clinically sound leisure horses. Compar. Exerc. Physiol. 2019, 15, 49–53. [Google Scholar] [CrossRef]
- Haussler, K.K.; Martin, C.E.; Hill, A.E. Efficacy of spinal manipulation and mobilisation on trunk flexibility and stiffness in horses: A randomised clinical trial. Equine Vet. J. Suppl. 2010, 38, 695–702. [Google Scholar] [CrossRef]
- Haussler, K.K.; Hill, A.E.; Puttlitz, C.M.; McIlwraith, C.W. Effects of vertebral mobilization and manipulation on kinematics of the thoracolumbar region. Am. J. Vet. Res. 2007, 68, 508–516. [Google Scholar] [CrossRef]
- Gomez Alvarez, C.B.; L’Ami J, J.; Moffat, D.; Back, W.; van Weeren, P.R. Effect of chiropractic manipulations on the kinematics of back and limbs in horses with clinically diagnosed back problems. Equine Vet. J. 2008, 40, 153–159. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Beneciuk, J.M.; Bishop, M.D.; Coronado, R.A.; Penza, C.W.; Simon, C.B.; George, S.Z. Unraveling the mechanisms of manual therapy: Modeling an approach. J. Orthop. Sports Phys. Ther. 2018, 48, 8–18. [Google Scholar] [CrossRef]
- Herzog, W. The biomechanics of spinal manipulation. J. Bodyw. Mov. Ther. 2010, 14, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.R.; Long, C.R.; Kawchuk, G.N.; Pickar, J.G. Neural responses to the mechanical parameters of a high-velocity, low-amplitude spinal manipulation: Effect of preload parameters. J. Manip. Physiol. Ther. 2014, 37, 68–78. [Google Scholar] [CrossRef]
- Edgecombe, T.L.; Kawchuk, G.N.; Long, C.R.; Pickar, J.G. The effect of application site of spinal manipulative therapy (SMT) on spinal stiffness. Spine J. 2015, 15, 1332–1338. [Google Scholar] [CrossRef][Green Version]
- Reed, W.R.; Long, C.R.; Kawchuk, G.N.; Pickar, J.G. Neural responses to the mechanical characteristics of high velocity, low amplitude spinal manipulation: Effect of specific contact site. Man. Ther. 2015, 20, 797–804. [Google Scholar] [CrossRef][Green Version]
- Colloca, C.J.; Gunzburg, R.; Freeman, B.J.; Szpalski, M.; Afifi, M.; Moore, R.J. Biomechancial quantification of pathologic manipulable spinal lesions: An in vivo ovine model of spondylolysis and intervertebral disc degeneration. J. Manip. Physiol. Ther. 2012, 35, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.S.; Colloca, C.J.; Moore, R.J.; Gunzburg, R.; Harrison, D.E.; Harrison, D.D. Three-dimensional vertebral motions produced by mechanical force spinal manipulation. J. Manip. Physiol. Ther. 2006, 29, 425–436. [Google Scholar] [CrossRef]
- Funabashi, M.; Nougarou, F.; Descarreaux, M.; Prasad, N.; Kawchuk, G.N. Spinal tissue loading created by different methods of spinal manipulative therapy application. Spine 2017, 42, 635–643. [Google Scholar] [CrossRef]
- Kawchuk, G.N.; Perle, S.M. The relation between the application angle of spinal manipulative therapy (SMT) and resultant vertebral accelerations in an in situ porcine model. Man. Ther. 2009, 14, 480–483. [Google Scholar] [CrossRef]
- Lane, D.M.; Hill, S.A. Effectiveness of combined acupuncture and manual therapy relative to no treatment for canine musculoskeletal pain. Can. Vet. J. 2016, 57, 407–414. [Google Scholar]
- Thoresen, A.N. Case Reports: Effect of osteopathic manipulations on performance in 374 horses with suspected sacroiliac and/or hip joint dysfunction and back pain: 2006–2007. Z. Für Ganzheitl. Tiermed. 2009, 4, 128–135. [Google Scholar] [CrossRef]
- Heo, S.; Park, Y.; Lee, H. Concurrent validity of a universal goniometer and a double meter inclinometer for passive range of motion in Beagle dogs. J. Vet. Clin. 2017, 34, 241–244. [Google Scholar] [CrossRef]
- Liljebrink, Y.; Bergh, A. Goniometry: Is it a reliable tool to monitor passive joint range of motion in horses? Equine Vet. J. 2010, 42, 676–682. [Google Scholar] [CrossRef]
- Jaeger, G.H.; Marcellin-Little, D.J.; Depuy, V.; Lascelles, B.D. Validity of goniometric joint measurements in cats. Am. J. Vet. Res. 2007, 68, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Riedler, D.C.; Zsoldos, R.R.; Robel, M.; Jobst, I.D.; Licka, T.F. Movement caused by electrical stimulation of the lumbosacral region in standing horses. J. Equine Vet. Sci. 2020, 91, 103116. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C. The Canine Orthopedic Index. Step 3: Responsiveness testing. Vet. Surg. 2014, 43, 247–254. [Google Scholar] [CrossRef]
- Reid, J.; Nolan, A.M.; Scott, E.M. Measuring pain in dogs and cats using structured behavioural observation. Vet. J. 2018, 236, 72–79. [Google Scholar] [CrossRef]
- Lascelles, B.D.X.; Brown, D.C.; Conzemius, M.G.; Gill, M.; Oshinsky, M.L.; Sharkey, M. Measurement of chronic pain in companion animals: Discussions from the Pain in Animals Workshop (PAW) 2017. Vet. J. 2019, 250, 71–78. [Google Scholar] [CrossRef]
- van Loon, J.P.A.M.; Macri, L. Objective assessment of chronic pain in horses using the Horse Chronic Pain Scale (HCPS): A scale-construction study. Animals 2021, 11, 1826. [Google Scholar] [CrossRef]
- Lawson, A.L.; Opie, R.R.; Stevens, K.B.; Knowles, E.J.; Mair, T.S. Application of an equine composite pain scale and its association with plasma adrenocorticotropic hormone concentrations and serum cortisol concentrations in horses with colic. Equine Vet. Educ. 2020, 32, 20–27. [Google Scholar] [CrossRef]
- Ask, K.; Rhodin, M.; Tamminen, L.-M.; Hernlund, E.; Haubro Andersen, P. Identification of body behaviors and facial expressions associated with induced orthopedic pain in four equine pain scales. Animals 2020, 10, 2155. [Google Scholar] [CrossRef]
- Lesimple, C.; Fureix, C.; De Margerie, E.; Seneque, E.; Menguy, H.; Hausberger, M. Towards a Postural Indicator of Back Pain in Horses (Equus caballus). PLoS ONE 2012, 7, e44604. [Google Scholar] [CrossRef]
- Page, I.; Biner, E.; Descarreaux, M. Vertebral displacements and muscle activity during manual therapy: Distinct behaviors between spinal manipulation and mobilization. J. Manip. Physiol. Ther. 2018, 41, 753–761. [Google Scholar] [CrossRef]
- Colloca, C.J.; Keller, T.S.; Black, P.; Normand, M.C.; Harrison, D.E.; Harrison, D.D. Comparison of mechanical force of manually assisted chiropractic adjusting instruments. J. Manip. Physiol. Ther. 2005, 28, 414–422. [Google Scholar] [CrossRef]
- Gemmell, H.; Miller, P. Comparative effectiveness of manipulation, mobilisation and the activator instrument in treatment of non-specific neck pain: A systematic review. ChiroPract. Osteopath. 2006, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Shearar, K.A.; Colloca, C.J.; White, H.L. A randomized clinical trial of manual versus mechanical force manipulation in the treatment of sacroiliac joint syndrome. J. Manip. Physiol. Ther. 2005, 28, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Digiorgi, D. Spinal manipulation under anesthesia: A narrative review of the literature and commentary. ChiroPract. Man. Ther. 2013, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Hawk, C.; Whalen, W.; Farabaugh, R.J.; Daniels, C.J.; Minkalis, A.L.; Taylor, D.N.; Anderson, D.; Anderson, K.; Crivelli, L.S.; Cark, M.; et al. Best practices for chiropractic management of patients with chronic musculoskeletal pain: A clinical practice guideline. J. Altern. Complement. Med. 2020, 26, 884–901. [Google Scholar] [CrossRef] [PubMed]
- Triano, J.J.; Budgell, B.; Bagnulo, A.; Roffey, B.; Bergmann, T.; Cooperstein, R.; Gleberzon, B.; Good, C.; Perron, J.; Tepe, R. Review of methods used by chiropractors to determine the site for applying manipulation. ChiroPract. Man. Ther. 2013, 21, 36. [Google Scholar] [CrossRef]
- Reix, C.E.; Burn, C.C.; Pritchard, J.C.; Barr, A.R.; Whay, H.R. The range and prevalence of clinical signs and conformation associated with lameness in working draught donkeys in Pakistan. Equine Vet. J. 2014, 46, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Gulda, D.; Lik, M. The use of manual therapy in canine discipline—Agility. Pol. J. Nat. Sci. 2018, 33, 487–501. [Google Scholar]
- Marcellin-Little, D.J.; Levine, D. Principles and application of range of motion and stretching in companion animals. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 57–72. [Google Scholar] [CrossRef]
- Haussler, K.K. Chiropractic evaluation and management. Vet. Clin. N. Am. Equine Pract. 1999, 15, 195–209. [Google Scholar] [CrossRef]
- Reusing, M.; Brocardo, M.; Weber, S.; Villanova, J. Goniometric evaluation and passive range of joint motion in chondrodystrophic and non-chondrodystrophic dogs of different sizes. VCOT Open 2020, 3, e66–e71. [Google Scholar] [CrossRef]
- Hofstetter, L.; Hausler, M.; Wirth, B.; Swanenburg, J. Instrumented measurement of spinal stiffness: A systematic literature review of reliability. J. Manip. Physiol. Ther. 2018, 41, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Haussler, K.K. Pressure algometry for the detection of mechanical nociceptive thresholds in horses. Animals 2020, 10, 2195. [Google Scholar] [CrossRef] [PubMed]
- Niederer, D.; Engel, T.; Pfeifer, A.C.; Arampatzis, A.; Beck, H.; Wippert, P.M.; Schiltenwolf, M.; Mayer, F. Which functional outcomes can be measured in low back pain trials and therapies? A prospective 2-year factor-, cluster-, and reliability-multicenter analysis on 42 variables in 1049 individuals. Spine 2021. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.M.; Scott, R.M.; Thomson, C.B.; Mesa, E.; Evans, R.; Conzemius, M.G. Evaluation of the environmental bias on accelerometer-measured total daily activity counts and owner survey responses in dogs with osteoarthritis. Vet. Comp. Orthop. Traumatol. 2017, 30, 385–390. [Google Scholar] [CrossRef]
- Muller-Quirin, J.; Dittmann, M.T.; Roepstorff, C.; Arpagaus, S.; Latif, S.N.; Weishaupt, M.A. Riding soundness-comparison of subjective with objective lameness assessments of owner-sound horses at trot on a treadmill. J. Equine Vet. Sci. 2020, 95, 103314. [Google Scholar] [CrossRef]
- van Loon, J.; van Dierendonck, M.C. Pain assessment in horses after orthopaedic surgery and with orthopaedic trauma. Vet. J. 2019, 246, 85–91. [Google Scholar] [CrossRef]
- Gross, A.; Miller, J.; D’Sylva, J.; Burnie, S.J.; Goldsmith, C.H.; Graham, N.; Haines, T.; Bronfort, G.; Hoving, J.L.; COG. Manipulation or mobilisation for neck pain: A Cochrane Review. Man. Ther. 2010, 15, 315–333. [Google Scholar] [CrossRef]
- Coulter, I.D.; Crawford, C.; Hurwitz, E.L.; Vernon, H.; Khorsan, R.; Suttorp Booth, M.; Herman, P.M. Manipulation and mobilization for treating chronic low back pain: A systematic review and meta-analysis. Spine J. 2018, 15, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Licciardone, J.C. Osteopathic research: Elephants, enigmas, and evidence. Osteopath. Med. Prim. Care 2007, 1, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crook, T.; McGowan, C.; Pead, M. Effect of passive stretching on the range of motion of osteoarthritic joints in 10 Labrador retrievers. Vet. Rec. 2007, 160, 545–547. [Google Scholar] [CrossRef] [PubMed]
| Indication | Treatment Methods | Species [Citation] |
|---|---|---|
| Carpal Stiffness | Joint immobilization-remobilization | Canine [19] |
| Cervical Pain or stiffness | Osteopathy (with sedation, general anesthesia) | Equine [20,21,22] |
| Thoracolumbar | ||
| Acute pain | Manipulation | Equine [23] |
| Chronic pain | Manipulation, Tail traction | Equine [10,24,25,26] |
| Muscle hypertonicity | Manipulation | Equine [27] |
| Stiffness | Caudal trunk displacement, Osteopathy (with sedation, general anesthesia), Manipulation | Equine [20,21,22,28,29,30,31] |
| Study [Citation] | Study Design | Study Sample | Intervention | Outcome Parameters | Main Results | Study Quality |
|---|---|---|---|---|---|---|
| Canine Mobilization | ||||||
| Olson, 1987 [19] | RCT | Subjects: 10 dogs Inclusion: Unilateral carpal immobilization with splint × 6 weeks Exclusion: NA | Treatment: Carpal flexion, traction and craniocaudal translation (n = 6): 3 sets of 20 oscillations, once daily × 4 weeks Control: No carpal remobilization (n = 4) | Outcomes: Passive carpal joint ROM, motion analysis of carpal joint angle at walk Follow up: 4 weeks Drop out: 2 in control group | Increased carpal passive ROM and flexion-extension joint angles at walk over time (p < 0.05), but no group differences Mobilization: 140° Control: 138° | Moderate |
| Equine Mobilization | ||||||
| Ahern, 1994 [20] | Cohort, Retrospective | Subjects: 86 horses Inclusion: Axial skeleton pain Exclusion: NA | Treatment: Cervical vertebral mobilization under anesthesia—single treatment session Control: NA | Outcomes: Owner survey Follow up: 6–18 months Drop out: 17 of 103 surveys (17%) | Clinical improvement: 95% within 2 weeks; 5% within 6 weeks Maintained improvement: 88% pain free Unsuccessful: 12% return of clinical signs | Low |
| Pusey, 1995 [22] | Cohort, Retrospective | Subjects: 127 horses Inclusion: Axial skeleton stiffness Exclusion: NA | Treatment: Osteopathic treatment under sedation (82%) Mobilization under anesthesia (17%) Control: NA Note: Treatment not described | Outcomes: Owner survey Follow up: >12 months Lost to follow up (2%) | Long-term responses: Improved: 75% No change: 18% Worse: 5% | Low |
| Colles, 2014 [21] | Cohort | Subjects: 51 horses Inclusion: Unresponsive chronic lameness or gait abnormality, neck or back stiffness, muscle tone altered, tenderness, thermographic asymmetries (>1.5 °C) Exclusion: NA | Treatment: Osteopathic treatment under sedation every 2–6 weeks (average 6, range 1–14) Mobilization under anesthesia: Single treatment (67%), 2 treatments (22%), 3 treatments (2%) Control: NA | Outcomes: Owner survey Follow up: 6–12 weeks (short term); 6 months to 7 years (long term) Lost to follow up (37%) | Short-term responses (6–12 weeks): Return to work: 90% Improved performance: 20% Reduced performance: 18% Failed to respond: 10% Long-term responses (>6 months): Return to prior level of work: 53% Reduced level of work: 31% Poor response: 16% | Low |
| Taylor, 2019 [27,28] | Cohort | Subjects: 13 horses Inclusion: Normal horses Exclusion: Back pain, lameness, analgesics, reduced performance × 6 months | Treatment: Caudal truncal displacement—single treatment session Control: NA | Outcomes: Spinal angles and displacement at 5 thoracolumbar sites Time points: Pre- and post-Tx | Increased thoracolumbar flexion (3.4°) Reduced thoracolumbar lordosis (11 mm) | Moderate |
| Long, 2020 [26] | Cohort | Subjects: 11 horses Inclusion: Back pain, lameness score 0–2 (out of 5) Exclusion: NA | Treatment: Caudal tail traction—single treatment session Control: NA | Outcomes: MNTs at 5 bilateral trunk sites (T18-L3 and pelvis) Time points: Pre- and post-Tx | Percent increase in MNT values: Thoracic (83%) Lumbar (50%) Pelvic (52%) | Moderate |
| Equine Manipulation | ||||||
| Haussler, 2003 [23] | RCT | Subjects: 26 actively ridden English collegiate horses Inclusion: Back stiffness Exclusion: Acute back pain, lameness | Treatment: HVLA (n = 12)—once weekly × 3 weeks Control: No Tx (n = 12) | Outcomes: MNT at 52 axial skeleton sites Time points: Baseline, 7 and 14 days Dropout (N = 2, 8%) | Differences in pooled MNT values between treatment and control groups Inside treatment area (T13-L6) = Increased 11 ± 4% (5 of 7 sites) Outside treatment area = Increased 3 ± 8% (2 of 7 sites) | High |
| Wakeling, 2006 [24,27] | RCT | Subjects: 26 collegiate horses Inclusion: Epaxial muscle fasciculations, hypertonicity, pain, informed consent Exclusion: Overt lameness, concurrent therapies, chronic back problems, history of spinal pathology or foot problems | Treatment: HVLA (n = 9), Reflex inhibition (n = 8)—single treatment sessions Control: No Tx (n = 9) | Outcomes: Epaxial muscle tonometry and EMG: T16 bilaterally Time points: Pre- and post-Tx | HVLA: Reduced muscle tone (13%), decreased EMG intensity (21%) Reflex inhibition: Reduced muscle tone (12%), decreased EMG intensity (18%) Control: No change muscle tone (0.3%) or EMG intensity (6%) | High |
| Haussler, 2007 [28,30] | RCT: Cross-over design | Subjects: 10 horses Inclusion: Acute back pain model (Steinman pin implantation in spinous processes) Exclusion: NA | Treatment: HVLA + mobilization (n = 10)—single treatment session Control: Mobilization only (n = 10) Note: Insufficient washout period, concurrent use of NSAIDs | Outcomes: Vertical trunk displacements, applied force, stiffness Time points: Baseline, post pin implantation, post-Tx, 7-day washout period | HVLA: Increased vertical displacement (15%), increased applied force (18%) Control: Increased vertical displacement (0%), decreased applied force (2%) | Moderate |
| Gomez-Alvarez, 2008 [29] | Cohort | Subjects: 10 Warmblood horses Inclusion: Back pain, asymmetric motion, atrophy Exclusion: Lameness, poor prognosis to applied therapy | Treatment: HVLA—single treatment session Control: NA | Outcomes: Vertebral ROM from neck to pelvis; Limb joint angles; Stride length and duration during walk and trot Time points: Pre-Tx, 1-h post Tx, 3 weeks post Tx | Stride length: No change Neck angle: No change Limb kinematics: Walk—No change Trot—Increased hip flexion (3°), Increased forelimb flexion (3 cm) FE: Walk—No change; Trot—Increased ROM at T13 and T17 1 -hour post Tx but decreased 3 weeks post Tx LB: Walk—Decreased ROM at T13 and T17 3-weeks post-Tx; Trot—Increased ROM at L3 1-h post-Tx AR: Increased pelvic symmetry | Moderate |
| Sullivan, 2008 [25,30] | RCT | Subjects: 38 horses Inclusion: No overt back pain Exclusion: Lameness | Treatment: Instrumented HVLA (n = 8)—single treatment session Massage therapy (n = 8)—single treatment session Phenylbutazone (n = 7): 2g PO BID × 7 days Control: Inactive control (n = 7) Active control (n = 8) | Outcomes: MNTs at 7 thoracolumbar and sacral sites (T3-S2) Time points: Baseline, 1, 3 and 7 days post-Tx Note: Owners (21%) refused to allocate horses to HVLA or NSAID groups | Percent increase in MNT values at Day 7: Instrumented HVLA: 27% Massage therapy: 12% Phenylbutazone: 8% Inactive control: 1% Active control: 0% | Moderate |
| Haussler, 2010 [25,29] | RCT | Subjects: 24 actively ridden English collegiate horses Inclusion: Normal horses Exclusion: Acute back pain, lameness | Treatment: HVLA + mobilization (n = 12)—once weekly × 3 weeks Control: Mobilization only (n = 12) | Outcomes: Vertical trunk displacements, applied force, stiffness Time points: Pre- and post-Tx | Percent change at 3 weeks: HVLA: Increased vertical displacement (40%), increased applied force (20%), increased stiffness (7%) Control: Increased vertical displacement (19%), decreased applied force (4%), decreased stiffness (15%) | High |
| Acutt, 2019 [10] | Cohort | Subjects: 6 show jumping horses Inclusion: Painful response to local palpation Exclusion: Lameness, neck or back pain, pathology | Treatment: HVLA—single treatment session Control: NA | Outcomes: Inertial sensor system, static bioimpedance, dynamic acoustic myography, diagnostic acupuncture examination Time points: Baseline, 24, 48, and 72 h post-Tx | Percent change over time: Local pain response: Absent immediately post-Tx and at 72 h Lameness: No change Static bioimpedance: Altered at 24 and 72 h post-Tx Dynamic acoustic myography: Altered at walk and trot | Moderate |
| Haussler, 2020 [31] | RCT | Subjects: 61 Western pleasure horses Inclusion: Back pain, stiffness, muscle hypertonicity, poor performance Exclusion: Lameness >3 (out of 5) | Treatment: 3 treatment sessions over 3–5 days HVLA (n = 12) HVLA + Low level laser therapy (n = 11) Low level laser therapy alone (n = 11) Note: Incomplete randomization, concurrent medications or treatments, lacked a negative control | Outcomes: Visual analog scale, back pain, epaxial muscle tone, trunk stiffness, MNTs Time points: Baseline, 3 sessions over 3–5 days Dropout (44%) | Percent change over time: HVLA: Improved thoracic (28%) and pelvic (28%) reflexes, No significant change pain (13%), hypertonicity (17%), stiffness (18%) HVLA + Laser: Decreased pain (14%), hypertonicity (55%), stiffness (54%) Laser alone: Decreased pain (41%), hypertonicity (20%), stiffness (25%) | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haussler, K.K.; Hesbach, A.L.; Romano, L.; Goff, L.; Bergh, A. A Systematic Review of Musculoskeletal Mobilization and Manipulation Techniques Used in Veterinary Medicine. Animals 2021, 11, 2787. https://doi.org/10.3390/ani11102787
Haussler KK, Hesbach AL, Romano L, Goff L, Bergh A. A Systematic Review of Musculoskeletal Mobilization and Manipulation Techniques Used in Veterinary Medicine. Animals. 2021; 11(10):2787. https://doi.org/10.3390/ani11102787
Chicago/Turabian StyleHaussler, Kevin K., Amie L. Hesbach, Laura Romano, Lesley Goff, and Anna Bergh. 2021. "A Systematic Review of Musculoskeletal Mobilization and Manipulation Techniques Used in Veterinary Medicine" Animals 11, no. 10: 2787. https://doi.org/10.3390/ani11102787
APA StyleHaussler, K. K., Hesbach, A. L., Romano, L., Goff, L., & Bergh, A. (2021). A Systematic Review of Musculoskeletal Mobilization and Manipulation Techniques Used in Veterinary Medicine. Animals, 11(10), 2787. https://doi.org/10.3390/ani11102787







