The Pattern of Blood–Milk Exchange for Antiparasitic Drugs in Dairy Ruminants
Abstract
Simple Summary
Abstract
1. Introduction
2. Pattern of Antiparasitic Drugs Milk Excretion in Dairy Ruminants
2.1. Benzimidazoles
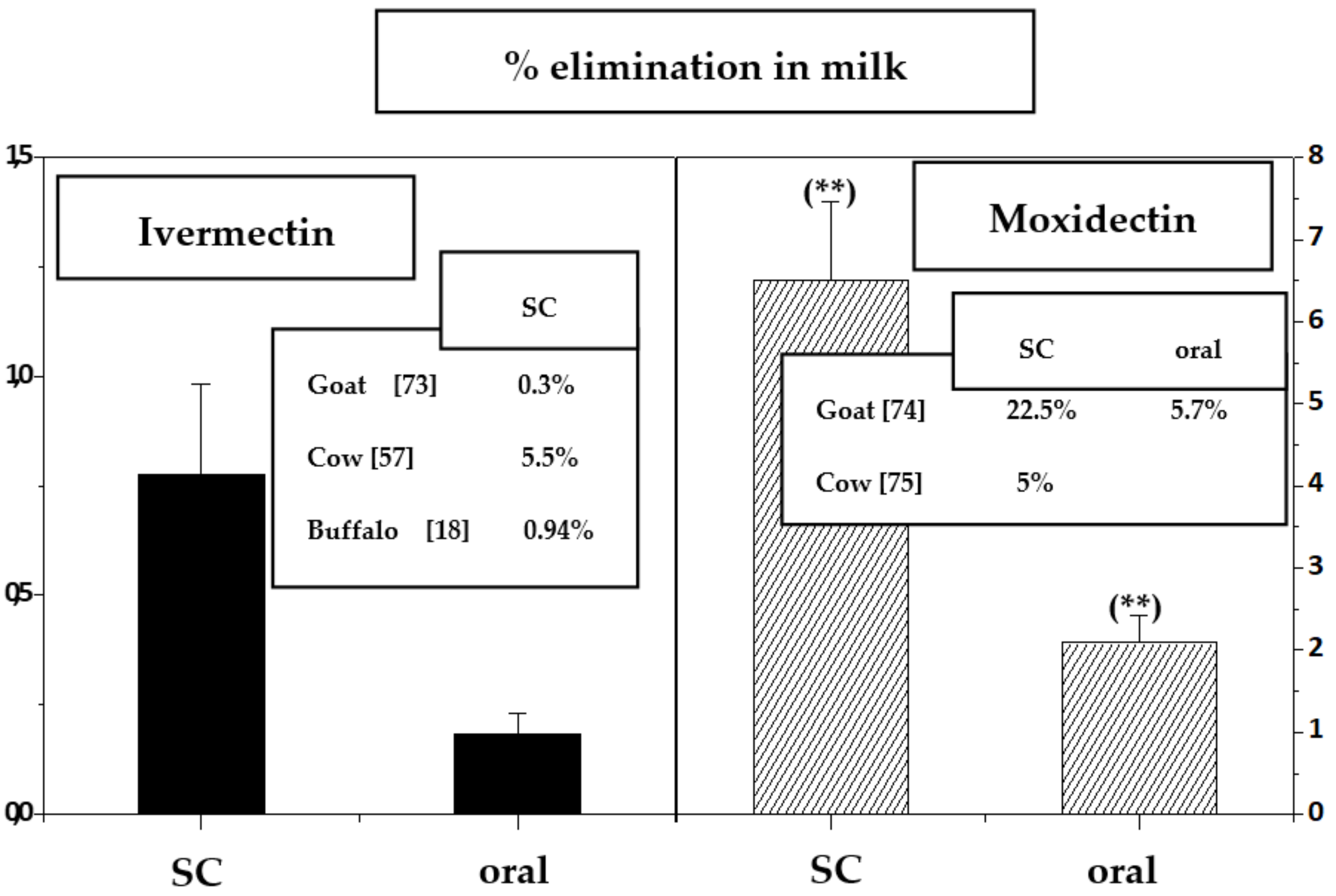
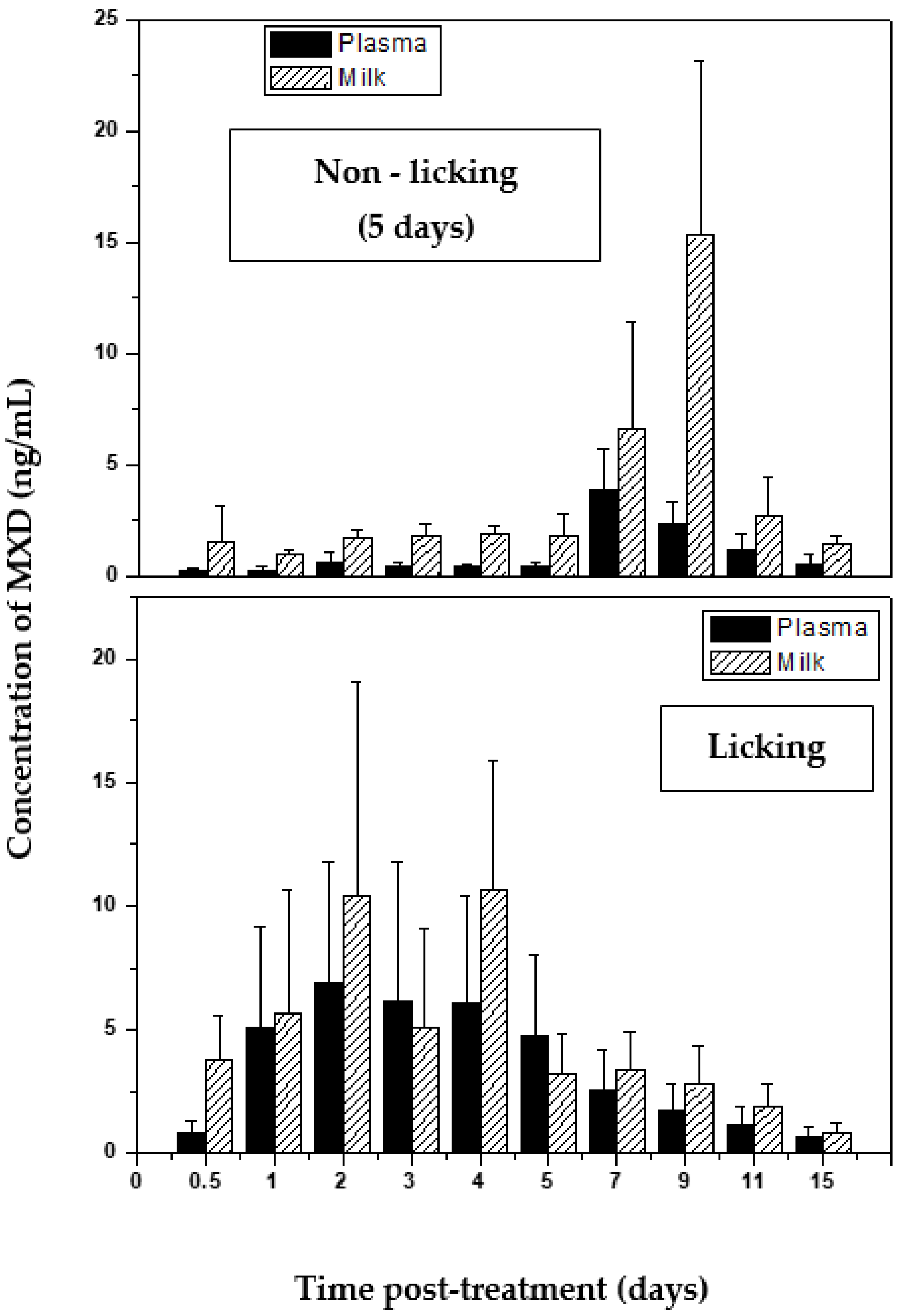
2.2. Macrocyclic Lactones
| Species | Route of Administration | Dose (mg/kg) | Cmax Plasma (ng/mL) | Cmax Milk (ng/mL) | Ratio AUC Milk/Plasma | Reference |
|---|---|---|---|---|---|---|
| cattle | pour-on | 0.5 | 43.76 ±18.23 | 5.14 ± 2.53 | 0.102 ± 0.048 | [79] |
| 0.5 | 16.16 ± 6.02 | 2.28 ± 0.85 | 0.124 ± 0.041 | [80] | ||
| oral | 0.2 | 30.02 ± 5.73 | 3.14 ± 0.88 | 0.104 ± 0.022 | [80] | |
| subcutaneous | 0.2 | 44.0 ± 24.2 | 6.4 ± 1.8 | 0.16 ± 0.01 | [94] | |
| sheep | pour-on | 0.5 | 2.28 ± 0.41 | 1.5 ± 0.32 | 0.69 ± 0.08 | [82] |
| 0.5 | 2.22 ± 0.88 | 1.37 ± 0.55 | 0.79 ± 0.12 | [83] | ||
| 1.0 | 5.25 ± 2.71 | 7.07 ± 5.16 | 1.12 ± 0.43 | [83] | ||
| goat | pour-on | 0.5 | 2.20 ± 0.52 | 0.32 ± 0.08 | 0.122 ± 0.07 | [81] |
| 1.0 | 2.98 ± 1.37 | 0.82 ± 0.08 | 0.254 ± 0.179 | [81] | ||
| oral | 0.5 | 15.48 ± 6.64 | 5.34 ± 2.23 | 0.36 ± 0.05 | [92] | |
| 1.0 | 38.10 ± 8.57 | 11.47 ± 2.23 | 0.33 ± 0.08 | [92] |
2.3. Salicylanilides
2.4. Miscellaneous: Nitroxynil and Clorsulon
3. Milk Excretion: Residues in Milk-Derived Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vercruysse, J.; Claerebout, E. Treatment vs non-treatment of helminth infections in cattle: Defining the threshold. Vet. Parasitol. 2001, 98, 195–214. [Google Scholar] [CrossRef]
- Blanco-Penedo, I.; Höglund, J.; Fall, N.; Emanuelson, U. Exposure to pasture borne nematodes affects individual milk yield in Swedish dairy herds. Vet. Parasitol. 2012, 188, 93–98. [Google Scholar] [CrossRef]
- Charlier, J.; Claerebout, E.; Duchateau, L.; Vercruysse, J. A survey to determine relationships between bulk tank milk antibodies against ostertagia ostertagi and milk production parameters. Vet. Parasitol. 2005, 129, 67–75. [Google Scholar] [CrossRef]
- Charlier, J.; Duchateau, L.; Claerebout, E.; Vercruysse, J. Predicting milk-production responses after an autumn treatment of pastured dairy herds with eprinomectin. Vet. Parasitol. 2007, 143, 322–328. [Google Scholar] [CrossRef]
- Charlier, J.; Höglund, J.; von Samson-Himmelstjerna, G.; Dorny, P.; Vercruysse, J. Gastrointestinal nematode infections in adult dairy cattle: Impact on production, diagnosis and control. Vet. Parasitol. 2009, 164, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, H.W.; Schoenmaker, G.J.W.; Kloosterman, A.; Borgsteede, F.H. Effect of anthelmintic treatment of dairy cattle on milk production related to some parameters estimating nematodes infection. Vet. Parasitol. 1989, 34, 239–253. [Google Scholar] [CrossRef]
- Nødtvedt, A.; Dohoo, I.; Sanchez, J.; Conboy, G.; DesCôteaux, L.; Keefe, G. Increase in milk yield following eprinomectin treatment at calving in pastured dairy cattle. Vet. Parasitol. 2002, 105, 191–206. [Google Scholar] [CrossRef]
- Juste Jordán, R.; García Pérez, A. Effect of treatment with netobimin on milk production of sheep. Vet. Parasitol. 1991, 38, 173–183. [Google Scholar] [CrossRef]
- Fthenakis, G.C.; Papadopulos, E.; Himonas, C.; Leontide, L.; Kritas, S.; Papatsas, J. Efficacy of moxidectin against sarcoptic mange and effects on milk yield of ewes and growth of lambs. Vet. Parasitol. 2000, 87, 207–216. [Google Scholar] [CrossRef]
- Fthenakis, G.C.; Papadopoulos, E.; Himonas, C. Effects of three anthelmintic regimes on milk yield of ewes and growth of lambs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2005, 52, 78–82. [Google Scholar] [CrossRef]
- Riviere, J.E. Absorption, Distribution, Metabolism, and Elimination. In Veterinary Pharmacology and Therapeutics, 10th ed.; Riviere, J.E., Papich, M.G., Eds.; John Willey and Sons: Hoboken, NJ, USA, 2018; pp. 8–40. [Google Scholar]
- Shoop, W.; Mrozik, H.; Fisher, M. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995, 59, 139–156. [Google Scholar] [CrossRef]
- Van Herwaarden, A.E.; Schinkel, A.H. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol. Sci. 2006, 27, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Halwachs, S.; Wassermann, L.; Honscha, W. Expresion and subcellular localization of efflux transporter ABCG2/BCRP in important tissue barriers of lactating dairy cows, sheep and goats. J. Vet. Pharmacol. Ther. 2013, 36, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, J.; Alvinerie, M.; Ménez, C.; Lespine, A. Interaction of anthelmintic drugs with P-glycoprotein in recombinant LLC-PK1-mdr1a cells. Chem. Biol. Interact. 2010, 186, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Barrera, B.; González-Lobato, L.; Otero, J.A.; Real, R.; Prieto, J.G.; Alvarez, A.I.; Merino, G. Effects or triclabendazole on secretion of danofloxacin and moxidectin into the milk of sheep: Role of triclabendazole metabolites as inhibitors of the ruminant ABCG2 transporter. Vet. J. 2013, 198, 429–436. [Google Scholar] [CrossRef]
- Mahnke, H.; Ballent, M.; Baumann, S.; Imperiale, F.; von Bergen, M.; Lanusse, C.; Lifschitz, A.L.; Honscha, W.; Halwachs, S. The ABCG2 efflux transporter in the mammary gland mediates veterinary drug secretion across the blood-milk barrier into milk of dairy cows. Drug Metab. Dispos. 2016, 44, 700–708. [Google Scholar] [CrossRef]
- Anastasio, A.; Esposito, M.; Amorena, M.; Catellani, P.; Serpe, L.; Cortesi, M. Residue study of ivermectin in plasma, milk, and mozzarella cheese following subcutaneous administration to buffalo (Bubalus bubalis). J. Agric. Food Chem. 2002, 50, 5241–5245. [Google Scholar] [CrossRef]
- Cerkvenik, V.; Bogdan Perko, B.; Rogelj, I.; Doganoc, D.; Skubic, V.; Beek, W.; Keukens, H. Fate of ivermectin residues in ewes´milk and derived products. J. Dairy Res. 2004, 71, 39–45. [Google Scholar] [CrossRef]
- Imperiale, F.A.; Busetti, M.R.; Suarez, V.H.; Lanusse, C.E. Milk excretion of ivermectin and moxidectin in dairy sheep: Assessment of drug residues during cheese elaboration and ripening period. J. Agric. Food Chem. 2004, 52, 6205–6211. [Google Scholar] [CrossRef]
- Power, C.; Danaher, M.; Sayers, R.; O´Brien, B.; Clancy, C.; Furey, A.; Jordan, K. Investigation of migration of triclabendazole residues to milk products manufactured from bovine milk, and stability therein, following lactating cow treatment. J Dairy Sci. 2013, 96, 6223–6232. [Google Scholar] [CrossRef]
- Dorny, P.; Vercruysse, J.; Jalila, A.; Sani, R.; Symoens, C. Control of haemonchosis in Malaysian goats with closantel. Vet. Parasitol. 1994, 53, 233–241. [Google Scholar] [CrossRef]
- Castro-Hermida, J.A.; González-Warleta, M.; Martínez-Sernández, V.; Ubeira, F.M.; Mezo, M. Current challenges for fasciolicide treatment in ruminant livestock. Trends Parasitol. 2021, 37, 430–444. [Google Scholar] [CrossRef]
- Regulation (EC) No 470/2009 of the European Parliament and of the Council. 6 May 2009. Available online: https://eur-lex.europa.eu (accessed on 13 September 2021).
- Commission Regulation (EU) No 37/2010 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin (Text with EEA Relevance). 22 December 2009. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- McKellar, Q.; Scott, E. The benzimidazole anthelmintic agents -A review. J. Vet. Pharmacol. Ther. 1990, 13, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Galtier, P.; Alvinerie, M.; Delatour, P. In vitro sulphoxidation of albendazole by ovine liver microsomes: Assay and frecuency of various xenobiotics. Am. J. Vet. Res. 1986, 47, 447–450, PMID: 3954232. [Google Scholar] [PubMed]
- Lanusse, C.; Prichard, R. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Met. Rev. 1993, 25, 235–279. [Google Scholar] [CrossRef]
- Hennessy, D.R.; Sangster, N.C.; Steel, J.W.; Collins, G.H. Comparative pharmacokinetic behavior of albendazole in sheep and goats. Int. J. Parasitol. 1993, 23, 321–325. [Google Scholar] [CrossRef]
- Alvarez, L.; Sánchez, S.; Lanusse, C. In vivo and ex vivo uptake of albendazole and its sulphoxide metabolite by cestode parasites: Relationship with their kinetic behavior in sheep. J. Vet. Parasitol. 1999, 22, 77–86. [Google Scholar] [CrossRef]
- Sánchez, S.; Alvarez, L.; Lanusse, C. Fasting induced changes on the pharmacokinetic behaviour of albendazole and its metabolites in cattle. J. Vet. Pharmacol. Ther. 1997, 20, 38–47. [Google Scholar] [CrossRef]
- Lanusse, C.; Virkel, G.; Sánchez, S.; Alvarez, L.; Lifschitz, A.; Imperiale, F.; Monfrinotti, A. Ricobendazole kinetics and availability following subcutaneous administration of a novel injectable formulation to calves. Res. Vet. Sci. 1998, 65, 5–10. [Google Scholar] [CrossRef]
- Barker, S.; Kappel, J. Drug residues in milk from cows administered fenbendazole as a paste, drench, or feed top-dressing. J. Vet. Pharmacol. Ther. 1997, 20, 160–162. [Google Scholar] [CrossRef]
- Brandon, D.L.; Bates, A.H.; Binder, R.G.; Montague, W.C.; Whitehand, L.C.; Barker, S.A. Analysis of fenbendazole residues in bovine milk by ELISA. J. Agric. Food Chem. 2002, 50, 5791–5796. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; Imperiale, F.; Mottier, L.; Alvarez, L.; Lanusse, C. Comparison of milk residue profiles after oral and subcutaneous administration of benzimidazole anthelmintics to dairy cows. Anal. Chim. Acta. 2005, 536, 91–99. [Google Scholar] [CrossRef]
- Romero, T.; Althaus, R.; Moya, V.J.; Beltrán, M.; Reybroeck, W.; Molina, M.P. Albendazole residues in goat´s milk: Interferences in microbial inhibitor tests used to detect antibiotics in milk. J. Food. Anal. 2017, 25, 302–305. [Google Scholar] [CrossRef] [PubMed]
- De Liguoro, M.; Longo, F.; Brambilla, G.; Cinquina, A.; Bocca, A.; Lucisano, A. Distribution of the anthelmintic drug albendazole and its major metabolites in ovine milk and milk products after a single oral dose. J. Dairy Res. 1996, 63, 533–542. [Google Scholar] [CrossRef]
- Fletouris, D.J.; Botsoglou, N.A.; Psomas, I.E.; Mantis, A. Albendazole-related drug residues in milk and their fate during cheesemaking, ripening and storage. J. Food Prot. 1998, 61, 1484–1488. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP, Albendazole. EMA: Amsterdam, The Netherlands, 2004. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- Virkel, G.; Lifschitz, A.; Pis, A.; Lanusse, C. In vitro ruminal biotransformation of benzimidazole sulphoxide anthelmintics: Enantioselective sulphoreduction in sheep and cattle. J. Vet. Pharmacol. Ther. 2002, 25, 15–23. [Google Scholar] [CrossRef]
- Lanusse, C.; Nare, B.; Gascon, L.; Prichard, R. Metabolism of albendazole and albendazole sulphoxide by ruminal and intestinal fluids of sheep and cattle. Xenobiotica 1992, 22, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Beretta, C.; Fadini, L.; MalvisiStracciari, J.; Montesissa, C. In vitro febantel transformation by sheep and cattle ruminal fluids and metabolism by hepatic subcellular fractions from different animal species. Biochem. Pharmacol. 1987, 36, 3107–3114. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP. Fenbendazole. EMA: Amsterdam, The Netherlands, 2004. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- Kappel, L.; Barker, S. Fenbendazole-related drug residues in milk from treated dairy cows. J. Vet. Pharmacol. Ther. 1996, 19, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Elitok, B.; Elitok, Ö.M.; Kabu, M. Field trial on comparative efficacy of four fasciolicides against natural liver fluke infection in cattle. Vet. Parasitol. 2006, 135, 279–285. [Google Scholar] [CrossRef]
- Loyacano, A.F.; Williams, J.C.; Gurie, J.; de Rosa, A.A. Effect of gastrointestinal nematodes and liver fluke on weight gain and reproductive performance of beef heifers. Vet. Parasitol. 2002, 107, 227–234. [Google Scholar] [CrossRef]
- Schweizer, G.; Braun, U.; Deplazes, P.; Torgerson, P.R. Estimating the financial losses due to bovine fasciolasis in Switzerland. Vet. Rec. 2005, 157, 188–193. [Google Scholar] [CrossRef]
- Imperiale, F.; Ortiz, P.; Cabrera, M.; Farias, C.; Sallovitz, J.M.; Iezzi, S.; Pérez, J.; Alvarez, L.; Lanusse, C. Residual concentrations of the flukicidal compound triclabendazole in dairy cows´milk and cheese. Food Addit.Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2011, 28, 438–445. [Google Scholar] [CrossRef]
- Hennessy, D.R.; Lacey, E.; Steel, J.W.; Prichard, K. The kinetics of triclabendazole disposition in sheep. J. Vet. Pharmacol. Ther. 1987, 10, 64–72. [Google Scholar] [CrossRef]
- Kinabo, L.D.B.; Bogan, J.A. Pharmacokinetics and efficacy of triclabendazole in goats with induced fascioliasis. J. Vet. Pharmacol. Ther. 1988, 11, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, P.K. Kinetic disposition of triclabendazole in buffalo compared to cattle. J. Vet. Pharmacol. Ther. 1995, 18, 370–374. [Google Scholar] [CrossRef]
- Virkel, G.; Lifschitz, A.; Sallovitz, J.; Pis, A.; Lanusse, C. Assessment of the main metabolism pathways for the flukicidal compound triclabendazole in sheep. J. Vet. Pharmacol. Ther. 2006, 29, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Counotte, G.H.; Reimink, A.; Redder, B.; Hasselt, H. Triclabendazole (Fasinex) residue in milk: Determination and excretion kinetics. Tijdschr. Diergeneeskd. 1990, 115, 875–881, PMID: 2219087. [Google Scholar] [PubMed]
- Power, C.; Whelan, M.; Danaher, M.; Bloemhoff, Y.; Sayers, R.; O´Brien, B.; Furey, A.; Jordan, K. Investigation of the persistence of triclabendazole residues in bovine milk following lactating-cow and dry-cow treatments. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Lanusse, C.; Gascon, L.H.; Prichard, R.K. Comparative plasma disposition kinetics of albendazole, fenbendazole, oxfendazole and their metabolites in adult sheep. J. Vet. Pharmacol. Ther. 1995, 19, 196–203. [Google Scholar] [CrossRef]
- Sánchez, S.; Alvarez, L.; Lanusse, C. Nutritional condition affects the disposition kinetics of albendazole in cattle. Xenobiotica 1996, 26, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.; Campan, M.; Galtier, P.; Alvinerie, M. Kinetic and insecticidal properties of ivermectin residues in the milk of dairy cows. J. Vet. Pharmacol. Ther. 1988, 11, 288–291. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP. Triclabendazole. EMA: Amsterdam, The Netherlands, 22 August 2014. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- Takiguchi, Y.; Mishima, H.; Okuda, M.; Terao, M.; Aoki, A.; Fukuda, R. Milbemycins, a new family of macrolide antibiotics: Fermentation, isolation and physico-chemical properties. J Antibiot. 1980, 33, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Shoop, W.J.; Egerton, C.; Eary, H.; Haines, B.; Michael, H.; Mrozik, P.; Eskola, M.; Fisher, L.; Slayton, D.; Ostlind, B.; et al. Eprinomectin: A novel avermectin for use as topical endectocide for cattle. Int. J. Parasitol. 1996, 26, 1237–1242. [Google Scholar] [CrossRef]
- Lanusse, C.; Lifschitz, A.; Virkel, G.; Alvarez, L.; Sánchez, S.; Sutra, J.F.; Galtier, P.; Alvinerie, M. Comparative plasma disposition kinetics of ivermectin, moxidectin and doramectin in cattle. J. Vet. Pharmacol. Ther. 1997, 20, 91–99. [Google Scholar] [CrossRef]
- Toutain, P.L.; Upson, D.W.; Terhune, T.N.; McKenzie, M.E. Comparative pharmacokinetics of doramectin and ivermectin in cattle. Vet. Parasitol. 1997, 72, 3–8. [Google Scholar] [CrossRef]
- Lifschitz, A.; Imperiale, F.; Virkel, G.; Muñoz Cobeñas, M.; Scherling, N.; DeLay, R.; Lanusse, C. Depletion of moxidectin tissue residues in sheep. J. Agric. Food Chem. 2000, 48, 6011–6015. [Google Scholar] [CrossRef] [PubMed]
- Sallovitz, J.; Lifschitz, A.; Imperiale, F.; Pis, A.; Virkel, G.; Lanusse, C. A detailed assessment of the pattern of moxidectin tissue distribution after pour-treatment in calves. J. Vet. Pharmacol. Ther. 2003, 26, 397–404. [Google Scholar] [CrossRef]
- Alvinerie, M.; Galtier, P. Comparative pharmacokinetic properties of moxidectin and ivermectin in different animal species. J. Vet. Pharmacol. Ther. 1997, 20, 74. [Google Scholar] [CrossRef]
- Sallovitz, J.; Lifschitz, A.; Imperiale, F.; Pis, A.; Virkel, G.; Lanusse, C. Breed differences on the plasma availability of moxidectin administered pour-on to calves. Vet. J. 2002, 164, 47–53. [Google Scholar] [CrossRef]
- Lo, P.; Fink, D.; Williams, J.; Blodinger, J. Pharmacokinetic studies of ivermectin: Effect of formulation. Vet. Res. Commun. 1985, 9, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Alvinerie, M.; Escudero, E.; Sutra, J.F.; Eeckhoutte, C.; Galtier, P. The pharmacokinetics of moxidectin after oral and subcutaneous administration to sheep. Vet. Res. 1998, 29, 113–118, PMID: 9601143. [Google Scholar] [PubMed]
- Escudero, E.; Carceles, C.; Diaz, M.; Sutra, J.F.; Galtier, P.; Alvinerie, M. Pharmacokinetics of moxidectin and doramectin in goats. Res. Vet. Sci. 1999, 67, 177–181. [Google Scholar] [CrossRef]
- Imperiale, F.; Sallovitz, J.; Farias, C.; Lifschitz, A.; Lanusse, C. Licking induced changes to the pattern of moxidectin milk elimination after topical treatment in dairy cows. J. Vet. Pharmacol. Ther. 2009, 32, 534–540. [Google Scholar] [CrossRef]
- Cerkvenik, V.; Grabnar, I.; Skubic, V.; Doganoc, D.; Beek, W.; Keukens, D.; Kosorok, M.; Pogacnik, M. 2002 Ivermectin pharmacokinetics in lactating sheep. Vet. Parasitol. 2002, 104, 175–185. [Google Scholar] [CrossRef]
- Imperiale, F.; Lifschitz, A.; Sallovitz, J.; Virkel, G.; Lanusse, C. Comparative depletion of ivermectin and moxidectin milk residues in dairy sheep after oral and subcutaneous administration. J. Dairy Res. 2004, 71, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Alvinerie, M.; Sutra, J.F.; Galtier, P. Ivermectin in goat plasma and milk after subcutaneous injection. Vet. Res. 1993, 24, 417–421, PMID: 8260963. [Google Scholar] [PubMed]
- Carceles, C.; Diaz, M.; Vicente, M.; Sutra, J.; Alvinerie, M.; Escudero, E. Milk kinetics of moxidectin and doramectin in goats. Res. Vet. Sci. 2001, 68, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Alvinerie, M.; Sutra, J.F.; Lanusse, C.; Galtier, P. Plasma profile study of moxidectin in a cow and its suckling calf. Vet. Res. 1996, 27, 545–549, PMID: 8822622. Available online: https://pubmed.ncbi.nlm.nih.gov/8822622/ (accessed on 13 September 2021). [PubMed]
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP, Moxidectin. EMA: Amsterdam, The Netherlands, 2004. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- JECFA. Proceedings of the Joint FAO/Expert Committee on Food Additives 44th Meeting, 15–24 February 2000; FAO: Rome, Italy, 2000.
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP. Eprinomectin. EMA: Amsterdam, The Netherlands, 1 July 2016. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- Alvinerie, M.; Sutra, J.F.; Galtier, P.; Mage, C. Pharmacokinetics of eprinomectin in plasma and milk following topical administration to lactating dairy cattle. Res. Vet. Sci. 1999, 67, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Pan, B.; Wang, Y.; Wang, F.; Yang, Z.; Wang, M. Plasma and milk kinetics of eprinomectin following topical or oral administration to lactating Chinese Holstein cows. Vet. Parasitol. 2010, 174, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, J.; Chartier, C.; Sutra, J.F.; Alvinerie, M. Eprinomectin in dairy goats: Dose influence on plasma levels and excretion in milk. Parasitol Res. 2001, 87, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, F.; Pis, A.; Sallovitz, A.; Busetti, M.; Suarez, V.; Lanusse, C. Pattern of eprinomectin milk excretion in dairy sheep unaffected by lactation stage: Comparative residual profiles in dairy products. J. Food Prot. 2006, 69, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Hodoscek, L.; Grabnar, I.; Milcinski, L.; Sussinger, A.; KozuhErzen, N.; Zadnik, T.; Pogacnik, M.; Cerkvenik-Flajs, V. Linearity of eprinomectin pharmacokinetics in lactating dairy sheep following pour-on administration: Excretion in milk and exposure of suckling lambs. Vet. Parasitol. 2008, 154, 129–136. [Google Scholar] [CrossRef]
- Gokbulut, C.; Bilgili, A.; Hanedan, B.; Aksit, D.; Aksoy, A.M.; Turgut, C. Breed related plasma disposition of ivermectin following subcutaneous administration in Kilis and Damascus goats. Res. Vet. Sci. 2009, 87, 445–448. [Google Scholar] [CrossRef]
- Steel, J.W. Pharmacokinetics and metabolism of avermectins in livestock. Vet. Parasitol. 1993, 48, 45–57. [Google Scholar] [CrossRef]
- Herd, R.P.; Sams, R.A.; Ashcraft, S.M. Persistence of ivermectin in plasma and faeces following treatment of cows with ivermectin sustained-release, pour-on or injectable formulations. Int. J. Parasitol. 1996, 26, 1087–1093, PMID: 8982789. Available online: https://pubmed.ncbi.nlm.nih.gov/8982789 (accessed on 13 September 2021). [CrossRef] [PubMed]
- Gayrard, V.; Alvinerie, M.; Toutain, P.L. Comparison of pharmacokinetic profiles of doramectin and ivermectin pour-on formulations in cattle. Vet.Parasitol. 1999, 81, 47–55. [Google Scholar] [CrossRef]
- Chartier, C.; Etter, E.; Pors, I.; Alvinerie, M. Activity of eprinomectin in goats against experimental infections with Haemonchus contortus, Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet. Rec. 1999, 144, 99–100. [Google Scholar] [CrossRef]
- Chartier, C.; Pors, I. Duration of activity of topical eprinomectin against experimental infections with Teladorsagia circumcincta and Trichostrongylus colubriformis in goats. Vet. Parasitol. 2004, 125, 415–419. [Google Scholar] [CrossRef]
- Cringoli, G.; Rinaldi, L.; Veneziano, V.; Capelli, G.; Rubino, R. Effectiveness of eprinomectin pour-on against gastrointestinal nematodes of naturally infected goats. Small Rumin. Res. 2004, 55, 209–213. [Google Scholar] [CrossRef]
- Lespine, A.; Sutra, J.F.; Dupuy, J.; Alvinerie, M. Eprinomectin in goat: Assessment of subcutaneous administration. Parasitol. Res. 2003, 89, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Badie, C.; Lespine, A.; Devos, J.; Sutra, J.F.; Chartier, C. Kinetics and anthelmintic efficacy of topical eprinomectin when given orally to goats. Vet. Parasitol. 2015, 209, 56–61. [Google Scholar] [CrossRef]
- Shoop, W.; Michael, B.; Egerton, J.; Mrozik, H.; Fisher, M. Titration of subcutaneously administered eprinomectin against mature and immature nematodes in cattle. J. Parasitol. 2001, 87, 1466–1469. [Google Scholar] [CrossRef]
- Baoliang, P.; Yuwan, W.; Zhende, P.; Lifschitz, A.L.; Ming, W. Pharmacokinetics of eprinomectin in plasma and milk following subcutaneous administration to lactating dairy cattle. Vet. Res. Commun. 2006, 30, 263–270. [Google Scholar] [CrossRef]
- Rehbein, S.; Kellermann, M.; Wehner, T.A. Pharmacokinetics and anthelmintic efficacy of topical eprinomectin in goats prevented from grooming. Parasitol. Res. 2014, 113, 4039–4044. [Google Scholar] [CrossRef]
- Alvinerie, M.; Lacoste, E.; Sutra, J.F.; Chartier, C. Some pharmacokinetic parameters of eprinomectin in goats following pour-on administration. Vet. Res. Commun. 1999, 23, 449–455. [Google Scholar] [CrossRef]
- Lespine, A.; Chartier, C.; Hoste, H.; Alvinerie, M. Endectocides in goats: Pharmacology, efficacy and use conditions in the context of anthelmintics resistance. Small Rumin. Res. 2012, 103, 10–17. [Google Scholar] [CrossRef]
- Martin, R. Modes of action of anthelmintic drugs. Vet. J. 1997, 154, 11–34. [Google Scholar] [CrossRef]
- Fairweather, I.; Boray, J.C. Fasciolicides: Efficacy, actions, resistance and its management. Vet. J. 1999, 158, 81–112. [Google Scholar] [CrossRef] [PubMed]
- Mohammed-Ali, N.A.; Bogan, J.A. The pharmacodynamics of the flukicidal salicylanilides, rafoxanide, closantel and oxyclosanide. J. Vet. Pharmacol. Ther. 1987, 10, 127–133. [Google Scholar] [CrossRef]
- Boray, J. Trematode infections of domestic animals. In Chemotherapy of Parasitic Diseases; Campbell, W., Rew, R., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 401–426. [Google Scholar]
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP, Closantel. EMA: Amsterdam, The Netherlands, 2014. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP, Oxyclozanide. EMA: Amsterdam, The Netherlands, 2004. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP, Rafoxanide. EMA: Amsterdam, The Netherlands, 2016. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- Hennessy, D.R.; Sangster, N.C.; Steel, J.W.; Collins, G.H. Comparative pharmacokinetic disposition of closantel in sheep and goats. J. Vet. Pharmacol. Ther. 1993, 16, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, S.; Lifschitz, A.L.; Sallovitz, J.; Nejamkin, P.; Lloberas, M.; Manazza, J.; Lanusse, C.; Imperiale, F. Closantel plasma and milk disposition in dairy goats: Assessment of drug residues in cheese and ricotta. J. Vet. Pharmacol. Ther. 2014, 37, 589–594. [Google Scholar] [CrossRef]
- Michiels, M.; Meuldermans, W.; Heykants, J. The metabolism and fate of closantel (Flukiver) in sheep and cattle. Drug Met. Rev. 1987, 18, 235–251. [Google Scholar] [CrossRef] [PubMed]
- McKellar, Q.A.; Kinabo, L.D.B. The pharmacology of flukicidal drugs. Br. Vet. J. 1991, 147, 306–321. [Google Scholar] [CrossRef]
- Sangster, N.C.; Rickard, J.M.; Hennessy, D.R.; Steel, J.W.; Collins, G.H. Disposition of oxfendazole in goats and efficacy compared with sheep. Res. Vet. Sci. 1991, 51, 258–263. [Google Scholar] [CrossRef]
- Hennessy, D.R.; Sangster, N.C.; Steel, J.W.; Collins, G.H. Comparative kinetic disposition of oxfendazole in sheep and goats before and during infection with Haemonchus contortus and Trichostrongylus colubriformis. J. Vet. Pharmacol. Ther. 1993, 16, 245–253. [Google Scholar] [CrossRef]
- Power, C.; Sayers, R.; O´Brien, B.; Clancy, C.; Furey, A.; Jordan, K.; Danaher, M. Investigation of persistence of closantel residues in bovine milk following lactating-cow and dry-cow treatment and its migration into dairy products. J. Agric. Food Chem. 2013, 61, 8703–8710. [Google Scholar] [CrossRef]
- Gokbulut, C.; Yalinkilinc, H.S.; Aksit, D.; Veneziano, V. Comparative pharmacokinetics of levamisole-oxyclozanide combination in sheep and goats following per os administration. Can. Vet. J. 2014, 78, 316–320, PMID: 25356001. [Google Scholar] [PubMed]
- Whelan, M.; Chirollo, C.; Furey, A.; Cortesi, M.L.; Anastasio, A.; Danaher, M. Investigation of the persistence of levamisole and oxyclozanide in milk and fate in cheese. J. Agric. Food Chem. 2010, 58, 12204–12209. [Google Scholar] [CrossRef]
- Fujinuma, K.; Takeba, K.; Kamata, K. Concentration in plasma and excretion in milk of lactating cows after oral administration of tribromsalan, oxyclozanide and bromofenofos. J. Food Hyg. Soc. Jpn. 2006, 47, 249–253. [Google Scholar] [CrossRef][Green Version]
- Benchaoui, H.A.; McKellar, Q.A. Determination of rafoxanide and closantel in ovine plasma by high performance liquid chromatography. Biomed.Chrom. 1993, 7, 181–183. [Google Scholar] [CrossRef]
- El-Banna, H.A.; Goudah, A.; El-Zorba, H.; Abd-El-Rahman, S. Comparative pharmacokinetics of ivermectin alone and a novel formulation of ivermectin and rafoxanide in calves and sheep. Parasitol. Res. 2008, 102, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Alvinerie, M.; Floc´h, R.; Galtier, P. Plasma protein binding of nitroxynil in several species. J. Vet. Pharmacol. Ther. 1991, 14, 170–173. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP, Nitroxinil. EMA: Amsterdam, The Netherlands, 2012. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- Whelan, M.; Bloemhoff, Y.; Furey, A.; Sayers, R.; Danaher, M. Investigation of the persistence of nitroxynil residues in milk from lactating dairy cows by ultra performance liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 7793–7797. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, G.W.; Dawson, K.; Fitzgibbon, C.C.; Martin, P.J. Efficacy of an injectable combination anthelmintic (nitroxynil + clorsulon + ivermectin) against early immature Fasciola hepatica compared to triclabendazole combination flukicides given orally or topically to cattle. Vet. Parasitol. 2009, 162, 278–284. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Committee for Medicinal Products for Veterinary Use, CVMP, Clorsulon. EMA: Amsterdam, The Netherlands, 2014. Available online: https://ec.europa.eu (accessed on 13 September 2021).
- Food and Drug Administration, U.S. Department of Health and Human Services. Multicriteria-Based Ranking Model for Risk Management of Animal Drug Residues in Milk and Milk Products. 2015. Available online: https://www.fda.gov/food/cfsan-risk-safety-assessments/multicriteria-based-ranking-model-risk-management-animal-drug-residues-milk-and-milk-products (accessed on 13 September 2021).
- Cerkvenik, V.; Doganoc, D.Z.; Skubic, V.; Beek, W.M.J.; Keukens, H.J. Thermal and long-term freezing stability of ivermectin residues in sheep milk. Eur. Food Res. Technol. 2001, 213, 72–76. [Google Scholar] [CrossRef]
- Imperiale, F.A.; Farias, C.; Pis, A.; Sallovitz, J.M.; Lifschitz, A.; Lanusse, C. Thermal stability of antiparasitic macrocyclic lactones milk residues during industrial processing. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 57–62. [Google Scholar] [CrossRef]
- Iezzi, S.; Nejamkin, P.; Sallovitz, J.; Farias, C.; Lifschitz, A.; Imperiale, F.; Lanusse, C. Evaluation of thermal stability of closantel during the cheese elaboration with goat milk. In Proceedings of the XLIII Reunión Anual de la Asociación Argentina de Farmacología Experimental (SAFE), Buenos Aires, Argentina, 4 November 2011. ISSN 2250-4079. [Google Scholar]
- Anastasio, A.; Veneziano, V.; Capurro, E.; Rinaldi, L.; Cortesi, M.L.; Rubino, R.; Danaher, M.; Cringoli, G. Fate of eprinomectin in goat milk and cheeses with different ripening times following pour-on administration. J. Food Prot. 2005, 68, 1097–1101. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imperiale, F.; Lanusse, C. The Pattern of Blood–Milk Exchange for Antiparasitic Drugs in Dairy Ruminants. Animals 2021, 11, 2758. https://doi.org/10.3390/ani11102758
Imperiale F, Lanusse C. The Pattern of Blood–Milk Exchange for Antiparasitic Drugs in Dairy Ruminants. Animals. 2021; 11(10):2758. https://doi.org/10.3390/ani11102758
Chicago/Turabian StyleImperiale, Fernanda, and Carlos Lanusse. 2021. "The Pattern of Blood–Milk Exchange for Antiparasitic Drugs in Dairy Ruminants" Animals 11, no. 10: 2758. https://doi.org/10.3390/ani11102758
APA StyleImperiale, F., & Lanusse, C. (2021). The Pattern of Blood–Milk Exchange for Antiparasitic Drugs in Dairy Ruminants. Animals, 11(10), 2758. https://doi.org/10.3390/ani11102758





