Overview of Research Development on the Role of NF-κB Signaling in Mastitis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
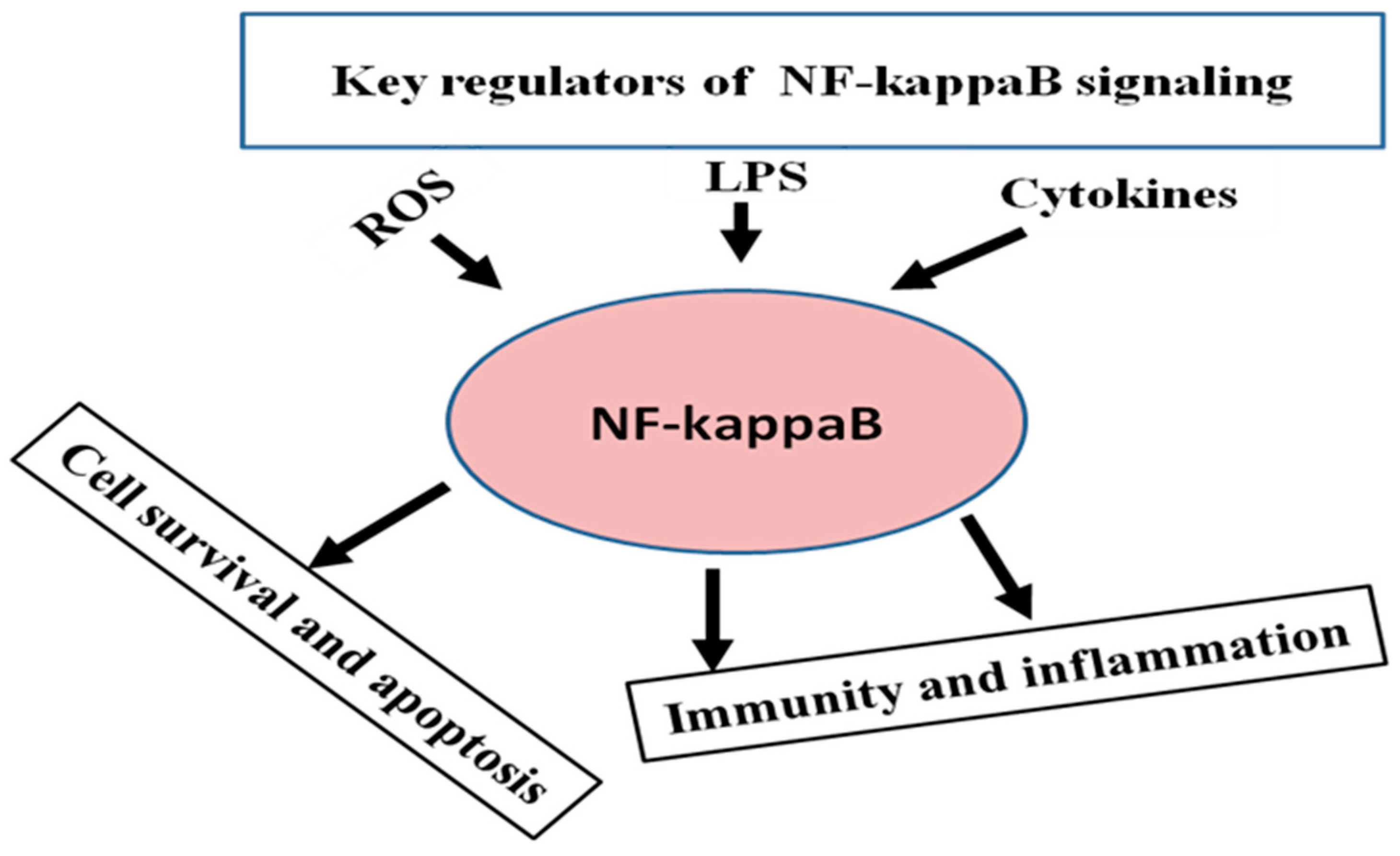
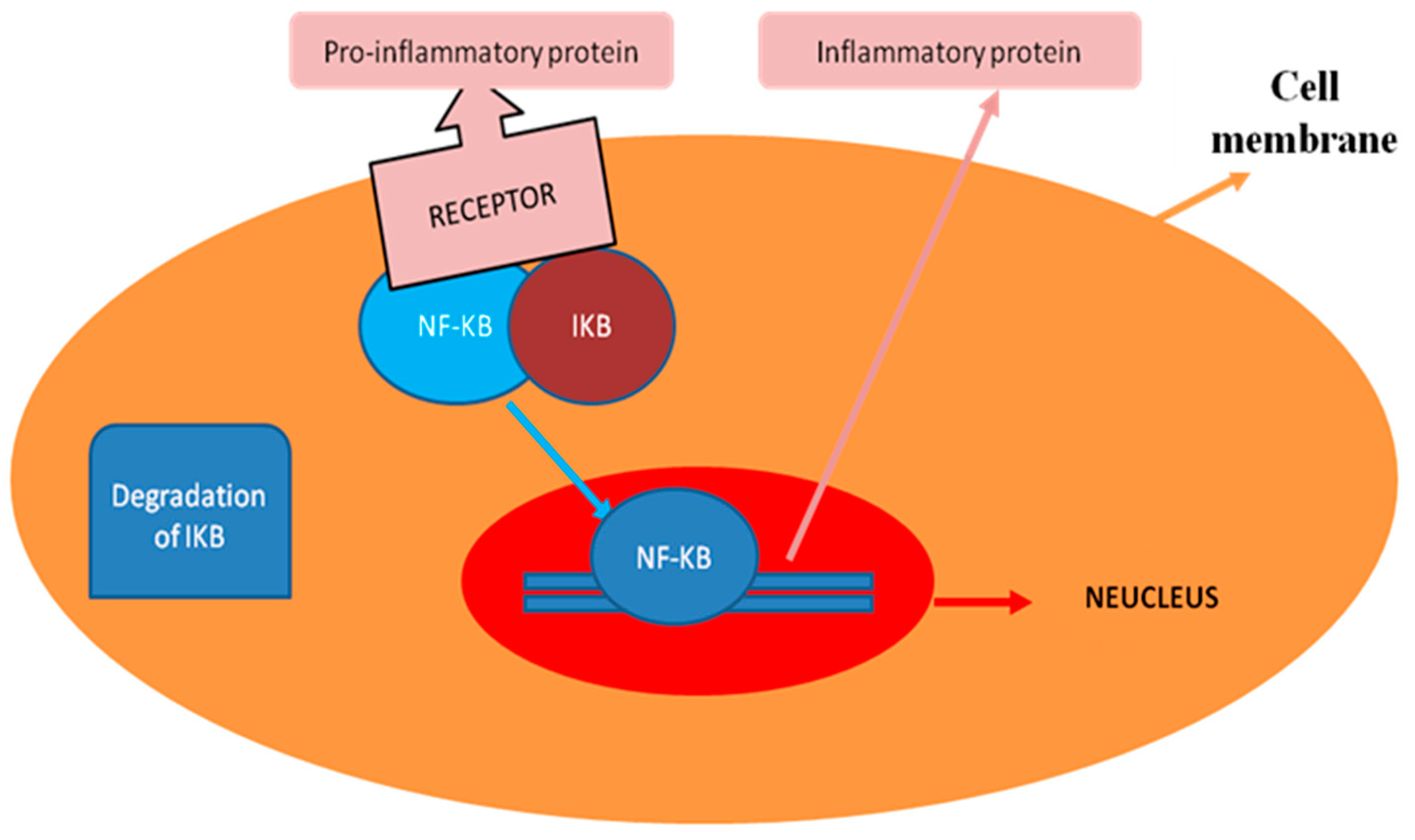
3. General Regulatory Pattern of NF-κB Signaling
4. Role of NF-κB Signaling in Normal Physiology of Mammary Gland Development
5. Role of NF-κB Signaling in Mastitis
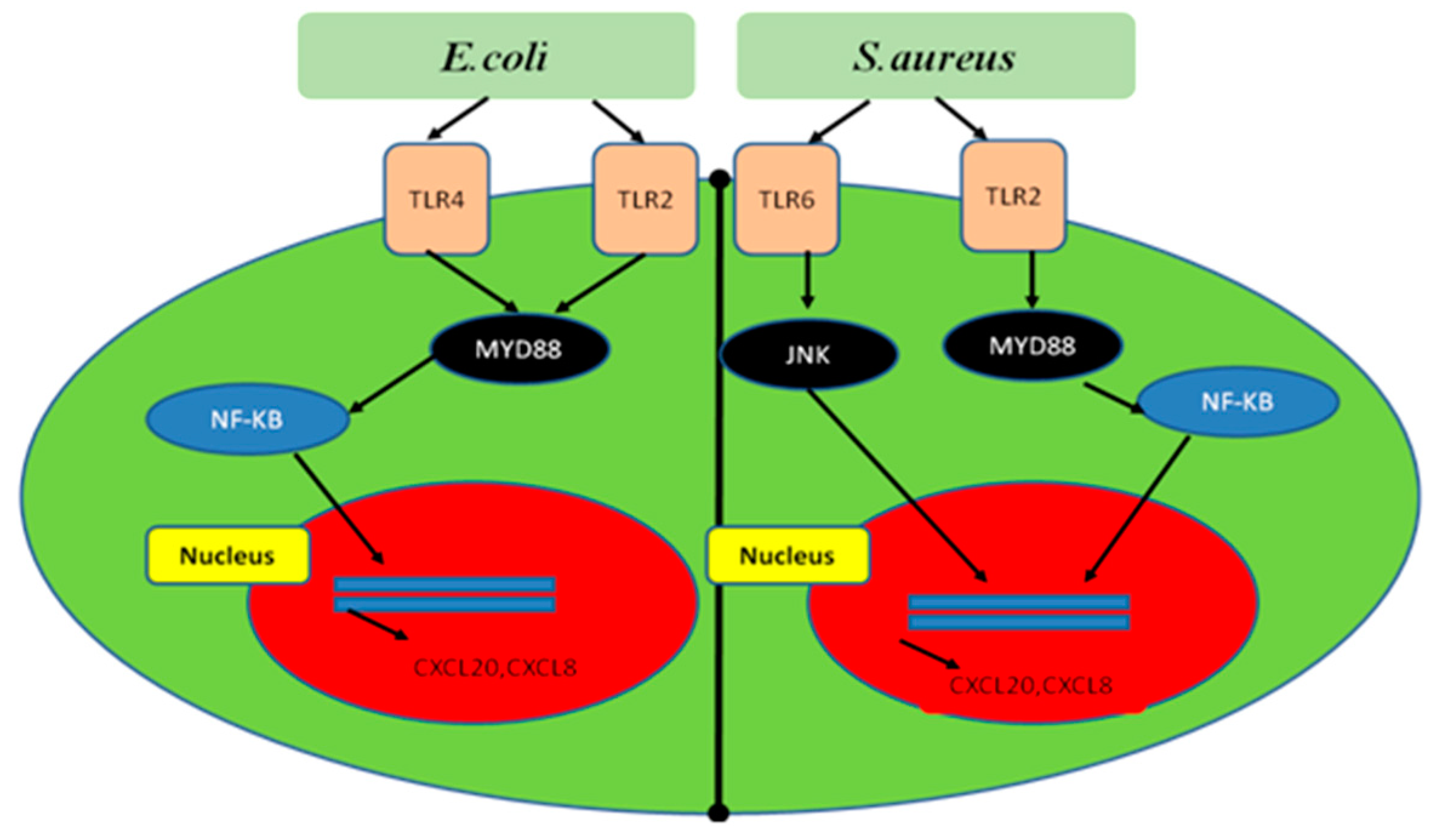
5.1. Mechanism of NF-κB Signaling Activation by Bacteria during Mastitis
5.2. Mechanism of NF-κB Signaling Activation by Inflammatory Cytokines
5.3. Bovine Myeloid Differentiation Primary Response 88 (MYD88), NFKBIA and TRAPPC9 Role as a Regulator of Lipopolysaccharide (LPS)-Induced NF-κB Signaling Pathways
5.4. NF-κB Regulates the Immunity and Inflammatory Linked Genes during Mastitis
5.5. Research Progress on Target of NF-κB Signaling as a Therapeutic in Mastitis Control
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lacasse, P. Mammary tissue damage during bovine mastitis: Causes and control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine mastitis: Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, A.M.; Liski, E.; Pyörälä, S.; Taponen, S. Pathogen-specific production losses in bovine mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef] [PubMed]
- Skarbye, A.P.; Krogh, M.A.; Sørensen, J.T. The effect of individual quarter dry-off in management of subclinical mastitis on udder condition and milk production in organic dairy herds: A randomized field trial. J. Dairy Sci. 2018, 101, 11186–11198. [Google Scholar] [CrossRef] [PubMed]
- Havixbeck, J.J.; Rieger, A.M.; Wong, M.E.; Hodgkinson, J.W.; Barreda, D.R. Neutrophil contributions to the induction and regulation of the acute inflammatory response in teleost fish. J. Leukoc. Biol. 2016, 99, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, S.; Cubeddu, T.; Pagnozzi, D.; Rocca, S.; Cacciotto, C.; Alberti, A.; Marogna, G.; Uzzau, S.; Addis, M.F. Neutrophil extracellular traps in sheep mastitis. Vet. Res. 2015, 46, 59. [Google Scholar] [CrossRef]
- Lavon, Y.; Leitner, G.; Kressel, Y.; Ezra, E.; Wolfenson, D. Comparing effects of bovine Streptococcus and Escherichia coli mastitis on impaired reproductive performance. J. Dairy Sci. 2019, 102, 10587–10598. [Google Scholar] [CrossRef]
- Poutrel, B.; Bareille, S.; Lequeux, G.; Leboeuf, F. Prevalence of Mastitis Pathogens in France: Antimicrobial Susceptibility of Staphylococcus aureus, Streptococcus uberis and Escherichia coli. J. Vet. Sci. Technol. 2018, 9, 2. [Google Scholar] [CrossRef]
- Biswas, S.; Chakravarti, S.; Barui, A. Emergence of coagulase positive methicillin resistant Staphylococcus Aureus isolated from buffalo mastitis milk samples. Explor. Anim. Med. Res. 2018, 8, 190–196. [Google Scholar]
- Li, T.; Lu, H.; Wang, X.; Gao, Q.; Dai, Y.; Shang, J.; Li, M. Molecular characteristics of Staphylococcus aureus causing bovine mastitis between 2014 and 2015. Front. Cell. Infect. Microbiol. 2017, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Günther, J.; Talbot, R.; Petzl, W.; Zerbe, H.; Schuberth, H.J.; Seyfert, H.M.; Glass, E.J. Escherichia coli- and Staphylococcus aureus-induced mastitis differentially modulate transcriptional responses in neighbouring uninfected bovine mammary gland quarters. BMC Genom. 2013, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.Q.; Zhao, Y.Q.; Shang, C.C.; Yao, Y.L.; Tian, T.T.; Li, J.; Chen, D.K. Dynamics of cytokines associated with IL-17 producing cells in serum and milk in mastitis of experimental challenging with Staphylococcus aureus and Escherichia coli in dairy goats. J. Anim. Vet. Adv. 2012, 11, 475–479. [Google Scholar]
- Pumipuntu, N.; Kulpeanprasit, S.; Santajit, S.; Tunyong, W.; Kong-ngoen, T.; Hinthong, W.; Indrawattana, N. Screening method for Staphylococcus aureus identification in subclinical bovine mastitis from dairy farms. Vet. World 2017, 10, 721–726. [Google Scholar] [CrossRef]
- Wellnitz, O.; Bruckmaier, R.M. The innate immune response of the bovine mammary gland to bacterial infection. Vet. J. 2012, 192, 148–152. [Google Scholar] [CrossRef]
- Brenaut, P.; Lefèvre, L.; Rau, A.; Laloë, D.; Pisoni, G.; Moroni, P.; Bevilacqua, C.; Martin, P. Contribution of mammary epithelial cells to the immune response during early stages of a bacterial infection to Staphylococcus aureus. Vet. Res. 2014, 45, 16. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Albiger, B.; Dahlberg, S.; Henriques-Normark, B.; Normark, S. Role of the innate immune system in host defence against bacterial infections: Focus on the Toll-like receptors. J. Intern. Med. 2007, 261, 511–528. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef]
- Bhattarai, D.; Worku, T.; Dad, R.; Rehman, Z.U.; Gong, X.; Zhang, S. Mechanism of pattern recognition receptors (PRRs) and host pathogen interplay in bovine mastitis. Microb. Pathog. 2018, 120, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Q.; Haan, B.J.; De Zhang, H.; Faas, M.M. Identification of TLR2 / TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, B.; Hu, G.; Kan, X.; Li, Y.; Gong, Q.; Xu, D.; Ma, H.; Cao, Y.; Huang, B.; et al. Vanillin protects the blood–milk barrier and inhibits the inflammatory response in LPS-induced mastitis in mice. Toxicol. Appl. Pharmacol. 2019, 365, 9–18. [Google Scholar] [CrossRef]
- Doyle, S.L.; O’Neill, L.A.J. Toll-like receptors: From the discovery of NF-κB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2006, 72, 1102–1113. [Google Scholar] [CrossRef]
- Liu, C.Y.; Gao, X.X.; Chen, L.; You, Q.X. Rapamycin suppresses Abeta25–35- or LPSinduced neuronal inflammation via modulation of NF-kappaB signaling. Neuroscience 2017, 355, 188–199. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kB and Rel proteins: Evolutionary conserved mediators of the immune response. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Tripathi, P.; Aggarwal, A. NF-kB transcription factor: A key player in the generation of immune response. Curr. Sci. 2006, 90, 25. [Google Scholar]
- Fitzgerald, D.C.; Meade, K.G.; McEvoy, A.N.; Lillis, L.; Murphy, E.P.; MacHugh, D.E.; Baird, A.W. Tumour necrosis factor-α (TNF-α) increases nuclear factor κB (NFκB) activity in and interleukin-8 (IL-8) release from bovine mammary epithelial cells. Vet. Immunol. Immunopathol. 2007, 116, 59–68. [Google Scholar] [CrossRef]
- Renard, P.; Zachary, M.D.; Bougelet, C.; Mirault, M.E.; Haegeman, G.; Remacle, J.; Raes, M. Effects of antioxidant enzyme modulations on interleukin-1-induced nuclear factor kappa B activation. Biochem. Pharmacol. 1997, 53, 149–160. [Google Scholar] [CrossRef]
- Chandel, N.S.; Trzyna, W.C.; McClintock, D.S.; Schumacker, P.T. Role of Oxidants in NF-κB Activation and TNF-α Gene Transcription Induced by Hypoxia and Endotoxin. J. Immunol. 2000, 165, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 18, 3–79. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Fu, Z.H.; Liu, S.Q.; Qin, M.B.; Huang, J.A.; Xu, C.Y.; Wu, W.H.; Zhu, L.Y.; Qin, N.; Lai, M.Y. NIK- and IKKβ-binding protein contributes to gastric cancer chemoresistance by promoting epithelial-mesenchymal transition through the NF-κB signaling pathway. Oncol. Rep. 2018, 39, 2721–2730. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, X.; Zhang, M.; Yin, H.; Jiang, K.; Wu, H.; Dai, A.; Yang, S. Nuciferine alleviates LPS-induced mastitis in mice via suppressing the TLR4-NF-κB signaling pathway. Inflamm. Res. 2018, 67, 903–911. [Google Scholar] [CrossRef]
- Stelwagen, K.; Singh, K. The role of tight junctions in mammary gland function. J. Mammary Gland Biol. Neoplasia 2014, 19, 131–138. [Google Scholar] [CrossRef]
- Quesnell, R.R.; Erickson, J.; Schultz, D.B. Apical electrolyte concentration modulates barrier function and tight junction protein localization in bovine mammary epithelium. Am. J. Physiol. Cell. Physiol. 2007, 292, C305–C318. [Google Scholar] [CrossRef]
- Song, X.; Zhang, W.; Wang, T.; Jiang, H.; Zhang, Z.; Fu, Y.; Yang, Z.Y.; Cao, Y.; Zhang, N. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation 2014, 37, 1588–1598. [Google Scholar] [CrossRef]
- Guo, W.; Liu, B.; Yin, Y.; Kan, X.; Gong, Q.; Li, Y.; Cao, Y.; Wang, J.; Xu, D.; Ma, H.; et al. Licochalcone A protects the blood-milk barrier integrity and relieves the inflammatory response in LPS-induced mastitis. Front. Immunol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Glynn, D.J.; Hutchinson, M.R.; Ingman, W.V. Toll-Like Receptor 4 Regulates Lipopolysaccharide-Induced Inflammation and Lactation Insufficiency in a Mouse Model of Mastitis1. Biol. Reprod. 2014, 90, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Hong, D.; Zhang, T.; Duan, H.; Wei, P.; Guo, X.; Mu, X. Cynatratoside-C from Cynanchum atratum displays anti-inflammatory effect via suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Chem. Biol. Interact. 2018, 279, 187–195. [Google Scholar] [CrossRef]
- Notebaert, S.; Carlsen, H.; Janssen, D.; Vandenabeele, P.; Blomhoff, R.; Meyer, E. In vivo imaging of NF-κB activity during Escherichia coli-induced mammary gland infection. Cell. Microbiol. 2008, 10, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, L.; Sun, Y.; Huang, S.; Tang, J.; Yu, P.; Wang, G. Altered Molecular Expression of the TLR4 / NF- κ B Signaling Pathway in Mammary Tissue of Chinese Holstein Cattle with Mastitis. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.B.; Wang, C.R.; Liu, Z.K.; Wang, J.Y. LPS induces pro-inflammatory response in mastitis mice and mammary epithelial cells: Possible involvement of NF-κB signaling and OPN. Pathol. Biol. 2015, 63, 11–16. [Google Scholar] [CrossRef]
- Connelly, L.; Barham, W.; Pigg, R.; Saint-Jean, L.; Sherrill, T.; Cheng, D.S.; Chodosh, L.A.; Blackwell, T.S.; Yull, F.E. Activation of nuclear factor kappa B in mammary epithelium promotes milk loss during mammary development and infection. J. Cell. Physiol. 2010, 222, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Brantley, D.M.; Chen, C.L.; Muraoka, R.S.; Bushdid, P.B.; Bradberry, J.L.; Kittrell, F.; Medina, D.; Matrisian, L.M.; Kerr, L.D.; Yull, F.E. Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Mol. Biol. Cell. 2001, 12, 1445–1455. [Google Scholar] [CrossRef]
- Brantley, D.M.; Yull, F.E.; Muraoka, R.S.; Hicks, D.J.; Cook, C.M.; Kerr, L.D. Dynamic expression and activity of NF-kappaB during post-natal mammary gland morphogenesis. Mech. Dev. 2000, 97, 149–155. [Google Scholar] [CrossRef]
- Baxter, F.O.; Neoh, K.; Tevendale, M.C. The beginning of the end: Death signaling in early involution. J. Mammary. Gland Biol. Neoplasia. 2007, 12, 3–13. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Clarkson, R.W.; Heeley, J.L.; Chapman, R.; Aillet, F.; Hay, R.T.; Wyllie, A.; Watson, C.J. NF-kappaB inhibits apoptosis in murine mammary epithelia. J. Biol. Chem. 2000, 275, 12737–12742. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, S.; Harper, C.V.; Semprini, S.; Wilding, M.; Adamson, A.; Spiller, D.G.; Nelson, G.; Mullins, J.J.; White, M.R.H.; Davis, J.R.E. Tumor Necrosis Factor-α Activates the Human Prolactin Gene Promoter via Nuclear Factor-κB Signaling. Endocrinology 2006, 147, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Terzidou, V.; Lee, Y.; Lindstrom, T.; Johnson, M.; Thornton, S.; Phillip, R.B. Regulation of the human oxytocin receptor by nuclear factor-kB and CCAAT/enhancer-binding protein-b. J. Clin. Endocrinol. Metab. 2006, 91, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Boutet, P.; Sulon, J.; Closset, R.; Detilleux, J.; Beckers, J.F.; Bureau, F.; Lekeux, P. Prolactin-induced activation of nuclear factor kappaB in bovine mammary epithelial cells: Role in chronic mastitis. J. Dairy Sci. 2007, 90, 155–164. [Google Scholar] [CrossRef]
- Notebaert, S.; Demon, D.; Vanden Berghe, T.; Vandenabeele, P.; Meyer, E. Inflammatory mediators in Escherichia coli-induced mastitis in mice. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 551–565. [Google Scholar] [CrossRef]
- Atabai, K.; Matthay, M.A. The pulmonary physician in critical care 5: Acute lung injury and the acute respiratory distress syndrome: Definitions and epidemiology. Thorax 2002, 57, 452–458. [Google Scholar] [CrossRef]
- Barbalat, R.; Lau, L.; Locksley, R.M.; Barton, G.M. Toll-like receptor 2 on inflammatory monocytes induces type i interferon in response to viral but not bacterial ligands. Nat. Immunol. 2009, 10, 1200–1209. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, R.; Cao, Y.; Guo, M.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Yang, Z.; Zhang, N. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2014, 20, 54–58. [Google Scholar] [CrossRef]
- Guo, Y.F.; Xu, N.N.; Sun, W.; Zhao, Y.; Li, C.Y.; Guo, M.Y. Luteolin reduces inflammation in Staphylococcus aureus-induced mastitis by inhibiting NF-kB activation and MMPs expression. Oncotarget 2017, 8, 28481–28493. [Google Scholar] [CrossRef]
- Jiang, K.F.; Zhao, G.; Deng, G.Z.; Wu, H.C.; Yin, N.N.; Chen, X.Y.; Qiu, C.W.; Peng, X.L. Polydatin ameliorates Staphylococcus aureus-induced mastitis in mice via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB pathway. Acta Pharmacol. Sin. 2017, 38, 211–222. [Google Scholar] [CrossRef]
- Li, D.; Fu, Y.; Zhang, W.; Su, G.; Liu, B.; Guo, M.; Li, F.; Liang, D.; Liu, Z.; Zhang, X.; et al. Salidroside attenuates inflammatory responses by suppressing nuclear factor-kappaB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflamm. Res. 2013, 62, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Watters, T.M.; Kenny, E.F.; O’Neill, L.A. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol. Cell Biol. 2007, 85, 411–41910. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Zhang, Y.; Zhang, X.; Wu, D.; Liu, Z.; Li, S.; Yang, Z. Morin alleviates LPS-induced mastitis by inhibiting the PI3K/AKT, MAPK, NF-κB and NLRP3 signaling pathway and protecting the integrity of blood-milk barrier. Int. Immunopharmacol. 2020, 78, 105972. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Baichwal, V.R. NF-κB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 1997, 65, 111–137. [Google Scholar]
- Scheidereit, C. IkappaB kinase complexes: Gateways to NF-kappaB activation and transcription. Oncogene 2006, 25, 6685–6705. [Google Scholar] [CrossRef]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar]
- Boulanger, D.; Bureau, F.; Mélotte, D.; Mainil, J.; Lekeux, P. Increased nuclear factor κB activity in milk cells of mastitis-affected cows. J. Dairy Sci. 2003, 86, 1259–1267. [Google Scholar] [CrossRef]
- Wellnitz, O.; Kerr, D.E. Cryopreserved bovine mammary cells to model epithelial response to infection. Vet. Immunol. Immunopathol. 2004, 101, 191–202. [Google Scholar] [CrossRef]
- Wang, X.G.; Ju, Z.H.; Hou, M.H.; Jiang, Q.; Yang, C.H.; Zhang, Y.; Sun, Y.; Li, R.L.; Wang, C.F.; Zhong, J.F.; et al. Correction: Deciphering Transcriptome and Complex Alternative Splicing Transcripts in Mammary Gland Tissues from Cows Naturally Infected with Staphylococcus aureus Mastitis. PLoS ONE 2016, 11, e0167666. [Google Scholar] [CrossRef]
- Blum, S.E.; Heller, E.D.; Jacoby, S.; Krifucks, O.; Leitner, G. Comparison of the immune responses associated with experimental bovine mastitis caused by different strains of Escherichia coli. J. Dairy Res. 2017, 84, 190–197. [Google Scholar] [CrossRef]
- Porcherie, A.; Cunha, P.; Trotereau, A.; Roussel, P.; Gilbert, F.B.; Rainard, P.; Germon, P. Repertoire of Escherichia coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells. Vet. Res. 2012, 43, 14. [Google Scholar] [CrossRef] [PubMed]
- Riollet, C.; Rainard, P.; Poutrel, B. Differential induction of complement fragment C5a and inflammatory cytokines during intramammary infections with Escherichia coli and Staphylococcus aureus. Clin. Diagn. Lab. Immunol. 2000, 7, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zerbe, H.; Petzl, W.; Brunner, R.M.; Günther, J.; Draing, C.; von Aulock, S.; Schuberth, H.J.; Seyfert, H.M. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E. coli, but S. aureus fails to both activate NF-κB in mammary epithelial cells and to quickly induce TNFα and interleukin-8 (CXCL8) expression in the udder. Mol. Immunol. 2008, 45, 1385–1397. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 2001, 1, 625–635. [Google Scholar] [CrossRef]
- Cates, E.A.; Connor, E.E.; Mosser, D.M.; Bannerman, D.D. Functional characterization of bovine TIRAP and MyD88 in mediating bacterial lipopolysaccharide-induced endothelial NF-κB activation and apoptosis. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 477–490. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C.; Wei, Z.; He, X.; Kou, J.; Zhou, E.; Yang, Z.; Fu, Y. Morin suppresses inflammatory cytokine expression by downregulation of nuclear factorkappaB and mitogen-activated protein kinase (MAPK) signaling pathways in lipopolysaccharide-stimulated primary bovine mammary epithelial cells. J. Dairy. Sci. 2016, 99, 3016–3022. [Google Scholar] [CrossRef]
- Vallabhapurapu, S.; Karin, M. Regulation and Function of NF-κB Transcription Factors in the Immune System. Annu. Rev. Immunol. 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Fang, L.; Hou, Y.; An, J.; Li, B.; Song, M.; Wang, X.; Sørensen, P.; Dong, Y.; Liu, C.; Wang, Y.; et al. Genome-wide transcriptional and post-transcriptional regulation of innate immune and defense responses of bovine mammary gland to Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2016, 6, 193. [Google Scholar] [CrossRef]
- Bodnar, B.; DeGruttola, A.; Zhu, Y.; Lin, Y.; Zhang, Y.; Mo, X.; Hu, W. Emerging role of NIK/IKK2-binding protein (NIBP)/trafficking protein particle complex 9 (TRAPPC9) in nervous system diseases. Transl. Res. 2020, 224, 55–70. [Google Scholar] [CrossRef]
- Mir, A.; Kaufman, L.; Noor, A.; Motazacker, M.M.; Jamil, T.; Azam, M.; Kahrizi, K.; Rafiq, M.A.; Weksberg, R.; Nasr, T.; et al. Identification of Mutations in TRAPPC9, which Encodes the NIK- and IKK-β-Binding Protein, in Nonsyndromic Autosomal-Recessive Mental Retardation. Am. J. Hum. Genet. 2009, 85, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zhang, J.; Xu, C.; Peng, P.; Tan, L.; Liu, S.; Huang, J. Knockdown of NIK and IKKβ-binding protein (NIBP) reduces colorectal cancer metastasis through down-regulation of the canonical NF-κB signaling pathway and suppression of MAPK signaling mediated through ERK and JNK. PLoS ONE 2017, 12, e0170595. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, P.; Liu, J.; Zhang, Q.; Zhang, Y.; Ding, X.; Jiang, L.; Wang, Y.; Zhang, Y.; Sun, D. Genome-wide association study in Chinese Holstein cows reveal two candidate genes for somatic cell score as an indicator for mastitis susceptibility. BMC Genetics 2015, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wei, Y.; Khan, Z.M.; Wang, X.; Yu, Y. Molecular marker study of inflammatory reaction in Bovine mammary epithelium cell line induced by methicillin-resistant staphylococcus areus (MRSA). Acta Veterin Zootecn Sina 2016, 47, 1995–2010. [Google Scholar]
- Wu, D.; Zhang, X.; Liu, L.; Guo, Y. Key CMM Combinations in Prescriptions for Treating Mastitis and Working Mechanism Analysis Based on Network Pharmacology. Evid. Based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Paape, M.J.; Lee, J.W.; Zhao, X.; Hope, J.C.; Rainard, P. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin. Diagn. Lab. Immunol. 2004, 11, 463–472. [Google Scholar] [CrossRef]
- Bruckmaier, R.M. Gene expression of factors related to the immune reaction in response to intramammary Escherichia coli lipopolysaccharide challenge. J. Dairy Res. 2005, 72, 120–124. [Google Scholar] [CrossRef]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granié, C.; Rupp, R.; Rainard, P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet. Res. 2013, 44, 40. [Google Scholar] [CrossRef]
- Jia, T.; Leiner, I.; Dorothee, G.; Brandl, K.; Pamer, E.G. MyD88 and Type I Interferon Receptor-Mediated Chemokine Induction and Monocyte Recruitment during Listeria monocytogenes Infection. J. Immunol. 2009, 183, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Scumpia, K.M.; Scumpia, P.O.; Delano, M.J.; Weinstein, J.S.; Cuenca, A.G.; Wynn, J.L.; Moldawer, L.L. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J. Exp. Med. 2010, 207, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Moore, T.A.; Newstead, M.W.; Deng, J.C.; Lukacs, N.W.; Standiford, T.J. IP-10 mediates selective mononuclear cell accumulation and activation in response to intrapulmonary transgenic expression and during adenovirus-induced pulmonary inflammation. J. Interf. Cytokine Res. 2005, 25, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Watson, A.D.; Kerr, D.E. Genome-wide expression analysis of lipopolysaccharide-induced mastitis in a mouse model. Infect. Immun. 2006, 74, 1907–1915. [Google Scholar] [CrossRef]
- Kosciuczuk, E.M.; Lisowski, P.; Jarczak, J.; Majewska, A.; Rzewuska, M.; Zwierzchowski, L.; Bagnicka, E. Transcriptome profiling of Staphylococci-infected cow mammary gland parenchyma. BMC Vet. Res. 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Griesbeck-Zilch, B.; Osman, M.; Kühn, C.; Schwerin, M.; Bruckmaier, R.H.; Pfaffl, M.W.; Hammerle-Fickinger, A.; Meyer, H.H.D.; Wellnitz, O. Analysis of key molecules of the innate immune system in mammary epithelial cells isolated from marker-assisted and conventionally selected cattle. J. Dairy Sci. 2009, 92, 4621–4633. [Google Scholar] [CrossRef]
- Islam, M.A.; Takagi, M.; Fukuyama, K.; Komatsu, R.; Albarracin, L.; Nochi, T.; Suda, Y.; Ikeda-Ohtsubo, W.; Rutten, V.; van Eden, W.; et al. Transcriptome analysis of the inflammatory responses of bovine mammary epithelial cells: Exploring immunomodulatory target genes for bovine mastitis. Pathogens 2020, 9, 200. [Google Scholar] [CrossRef]
- Rainard, P.; Riollet, C. Innate immunity of the bovine mammary gland. Vet. Res. 2006, 37, 369–400. [Google Scholar] [CrossRef]
- Waller, K.P. Modulation of endotoxin-induced inflammation in the bovine teat using antagonists inhibitors to leukotrienes, platelet activating factor and interleukin 1beta. Vet. Immunol. Immunopathol. 1997, 57, 239–251. [Google Scholar] [CrossRef]
- Bulgari, O.; Dong, X.; Roca, A.L.; Caroli, A.M.; Loor, J.J. Innate immune responses induced by lipopolysaccharide and lipoteichoic acid in primary goat mammary epithelial cells. J. Anim. Sci. Biotechnol. 2017, 8, 29. [Google Scholar] [CrossRef]
- Wang, W.; Hu, X.; Shen, P.; Zhang, N.; Fu, Y. Sodium houttuyfonate inhibits LPS-induced inflammatory response via suppressing TLR4/NF-ĸB signaling pathway in bovine mammary epithelial cells. Microb. Pathog. 2017, 107, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bi, C.; Wang, Y.; Sun, J.; Meng, X.; Li, J. Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-ΚB and MAPK signaling pathways. BMC Vet. Res. 2018, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Streicher, K.L. Mammary gland immunity and mastitis susceptibility. J. Mammary Gland Biol. Neoplasia 2002, 7, 135–146. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.; Zahoor, A.; Shaukat, A.; Chen, Y.; Guo, M. Upregulated-gene expression of Pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Tropica 2020, 207, 105458. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Torres, R. Role of interleukin-1β during pain and inflammation. Brain Res. Rev. 2009, 60, 57. [Google Scholar] [CrossRef]
- Dai, H.; Coleman, D.N.; Hu, L.; Martinez-Cortés, I.; Wang, M.; Parys, C.; Shen, X.; Loor, J.J. Methionine and arginine supplementation alter inflammatory and oxidative stress responses during lipopolysaccharide challenge in bovine mammary epithelial cells in vitro. J. Dairy Sci. 2020, 103, 676–689. [Google Scholar] [CrossRef]
- Zimmermann, K.K.; Spassov, S.G.; Strosing, K.M.; Ihle, P.M.; Engelstaedter, H.; Hoetzel, A.; Faller, S. Hydrogen Sulfide Exerts Anti-oxidative and Anti-inflammatory Effects in Acute Lung Injury. Inflammation 2018, 41, 249–259. [Google Scholar] [CrossRef]
- Benedetti, F.; Curreli, S.; Krishnan, S.; Davinelli, S.; Cocchi, F.; Scapagnini, G.; Gallo, R.C.; Zella, D. Anti-inflammatory effects of H2S during acute bacterial infection: A review. J. Transl. Med. 2017, 15, 100. [Google Scholar] [CrossRef]
- Zhi-Zhong, X.; Yang, L.; Jin-Song, B. Hydrogen sulfide and cellular redox homeostasis. Oxidative Med. Cell. Longev. 2016, 1–12. [Google Scholar] [CrossRef]
- Liu, W.; Xu, C.; You, X.; Olson, D.M.; Chemtob, S.; Gao, L.; Ni, X. Hydrogen sulfide delays LPS-Induced preterm birth in mice via anti-inflammatory pathways. PLoS ONE 2016, 11, e0152838. [Google Scholar] [CrossRef]
- Ahire, J.J.; Mokashe, N.U.; Patil, H.J.; Chaudhari, B.L. Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J. Food Sci. Technol. 2013, 50, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yang, C.; Lin, S.; Zhao, G.; Zhang, T.; Guo, S.; Jiang, K.; Wu, H.; Qiu, C.; Guo, M.; et al. Sodium houttuyfonate inhibits LPS-induced mastitis in mice via the NF-κB signalling pathway. Mol. Med. Rep. 2019, 19, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, L.; Wang, F.; Zheng, X.; Yuan, C.; Niu, Q.; Li, Z.; Deng, L.; Zheng, B.; Li, C.; et al. Exogenous hydrogen sulfide prevents lipopolysaccharide-induced inflammation by blocking the TLR4/NF-κB pathway in MAC-T cells. Gene 2019, 710, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, J.; Coelho, G.; Crespo, M.E.; Cruz, T.; Rodríguez-Cabezas, M.E.; Concha, A.; Gonzalez, M.; Zarzuelo, A. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment. Pharmacol. Ther. 2001, 15, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit pro-inflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef]
- Yu, S.; Liu, X.; Yu, D.; Changyong, E.; Yang, J. Morin Protects LPS-Induced Mastitis via Inhibiting NLRP3 Inflammasome and NF-κB Signaling Pathways. Inflammation 2020, 43, 1293–1303. [Google Scholar] [CrossRef]
- Zhan, K.; Yang, T.; Feng, B.; Zhu, X.; Chen, Y.; Huo, Y.; Zhao, G. The protective roles of tea tree oil extracts in bovine mammary epithelial cells and polymorphonuclear leukocytes. J. Anim. Sci. Biotechnol. 2020, 11, 62. [Google Scholar] [CrossRef]
- Mahla, R.S.; Reddy, M.C.; Vijaya Raghava Prasad, D.; Kumar, H. Sweeten PAMPs: Role of sugar complexed PAMPs in innate immunity and vaccine biology. Front. Immunol. 2013, 4, 248. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, H.; Yang, B.; Tao, H. Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chem. 2010, 120, 1138–1142. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-κB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Ruifeng, G.; Yunhe, F.; Zhengkai, W.; Ershun, Z.; Yimeng, L.; Minjun, Y.; Xiaojing, S.; Zhengtao, Y.; Naisheng, Z. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur. J. Pharmacol. 2014, 729, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.T.H.; Elsasser, H.T.; Biswas, D.; Moyes, M.K. The effect of citrus-derived oil on bovine blood neutrophil function and gene expression in vitro. J. Dairy Sci. 2014, 98, 1–99127. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, P.; Gong, P.; Lv, A.; Zhang, Z.; Liu, F. 8-Methoxypsoralen protects bovine mammary epithelial cells against lipopolysaccharide-induced inflammatory injury via suppressing JAK/STAT and NF-κB pathway. Microbiol.Immunol. 2019, 63, 427–437. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, R.; Cong, Y.; Yang, Z.; Zhou, E.; Wei, Z.; Zhang, N. Oxymatrine Lightened the Inflammatory Response of LPS-Induced Mastitis in Mice through Affecting NF-κB and MAPKs Signaling Pathways. Inflammation 2014, 37, 2047–2055. [Google Scholar] [CrossRef]
- Ershun, Z.; Yunhe, F.; Zhengkai, W.; Yongguo, C.; Naisheng, Z.; Zhengtao, Y. Cepharanthine Attenuates Lipopolysaccharide-Induced Mice Mastitis by Suppressing the NF-κB Signaling Pathway. Inflammation 2014, 32, 331–337. [Google Scholar] [CrossRef]
- Su, S.; Xiaoyu, L.; Siting, L.; Pengfei, M.; Yingying, H.; Yanli, D.; Hongyan, D.; Shibin, F.; Jinchun, L.; Xichun, W.; et al. Rutin protects against lipopolysaccharide-induced mastitis by inhibiting the activation of the NF-κB signaling pathway and attenuating endoplasmic reticulum stress. Inflammopharmacology 2018, 27, 77–88. [Google Scholar] [CrossRef]
- Li, D.; Zhang, N.; Cao, Y.; Zhang, W.; Su, G.; Sun, Y.; Yang, Z. Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-κB and MAPKs signal pathways. Eur. J. Pharmacol. 2013, 705, 79–85. [Google Scholar] [CrossRef]
- He, X.; Liu, W.; Shi, M.; Yang, Z.; Zhang, X.; Gong, P. Docosahexaenoic acid attenuates LPS-stimulated inflammatory response by regulating the PPARγ/NF-κB pathways in primary bovine mammary epithelial cells. Res. Vet. Sci. 2017, 112, 7–12. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wei, Z.; Zhou, E.; Chen, L.; Kou, J.; Wang, J.; Yang, Z. Baicalein attenuates inflammatory responses by suppressing TLR4 mediated NF-κB and MAPK signaling pathways in LPS-induced mastitis in mice. Internat. Immunopharmacol. 2015, 28, 470–476. [Google Scholar] [CrossRef] [PubMed]
| Authors | Agent | Function | Targets |
|---|---|---|---|
| Sun et al. [113] | H2S | Block TLR4, ROS, NF-κB | |
| Garcia et al. [125] | Citrus oils | Antibacterial | Down-regulate TLR2, NFKBIA, IL8, TNF-α |
| Wang et al. [78] | Morin | Anti-inflammatory | Inhibit IL-6, TNF-α, IL-1β, suppress NF-κB phosphorylation |
| Li et al. [126] | 8-Methoxypsoralen | Anti-inflammatory | Inhibit IL-6, TNF-α, IL-8, IL-1β, suppress NF-κB phosphorylation |
| Chen et al. [36] | Nuciferine | Anti-inflammatory | Inhibit TLR4, TNF-α, IL-1β, suppress NF-κB phosphorylation |
| Yang et al. [127] | Oxymatrine | Anti-inflammatory | Suppress NF-κB phosphorylation |
| Ershun et al. [128] | Cepharanthine | Anti-inflammatory | Inhibit IL6, TNF-α, IL-1β, suppress NF-κB phosphorylation |
| Su et al. [129] | Rutin | Decrease level of IL-1β, IL-6, and TNF-α, suppress NF-κB phosphorylation | |
| Liu et al. [112] | Sodium houttuyfonate | Antinflammatory | Inhibit NF-κB phosphorylation |
| Li et al. [130] | Emodin ameliorates | Anti-inflammatory, antibacterial | Decrease level of IL-1β, IL-6, and TNF-α, suppress NF-κB phosphorylation |
| Hu et al. [42] | Cynatratoside-C from Cynanchum atratum | Anti-inflammatory | Suppress TLR4, inhibit NF-κB phosphorylation |
| He et al. [131] | Docosahexaenoic acid | Anti-inflammatory | Decrease level of IL-1β, IL-6, and TNF-α, suppress NF-κB phosphorylation |
| He et al. [132] | Baicalein | Anti-inflammatory | Suppress TLR4, inhibit NF-κB phosphorylation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.Z.; Khan, A.; Xiao, J.; Ma, J.; Ma, Y.; Chen, T.; Shao, D.; Cao, Z. Overview of Research Development on the Role of NF-κB Signaling in Mastitis. Animals 2020, 10, 1625. https://doi.org/10.3390/ani10091625
Khan MZ, Khan A, Xiao J, Ma J, Ma Y, Chen T, Shao D, Cao Z. Overview of Research Development on the Role of NF-κB Signaling in Mastitis. Animals. 2020; 10(9):1625. https://doi.org/10.3390/ani10091625
Chicago/Turabian StyleKhan, Muhammad Zahoor, Adnan Khan, Jianxin Xiao, Jiaying Ma, Yulin Ma, Tianyu Chen, Dafu Shao, and Zhijun Cao. 2020. "Overview of Research Development on the Role of NF-κB Signaling in Mastitis" Animals 10, no. 9: 1625. https://doi.org/10.3390/ani10091625
APA StyleKhan, M. Z., Khan, A., Xiao, J., Ma, J., Ma, Y., Chen, T., Shao, D., & Cao, Z. (2020). Overview of Research Development on the Role of NF-κB Signaling in Mastitis. Animals, 10(9), 1625. https://doi.org/10.3390/ani10091625







