Use of Contrast-Enhanced Ultrasonography for the Characterization of Tumor Thrombi in Seven Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dunn, C.W.; Snyder, W.H.; Ring, W.S.; Latson, T.W. Pheochromocytoma with extension into the inferior vena cava: A case report. Surgery 1992, 111, 472–474. [Google Scholar]
- Anderson, C.R.; Birchard, S.J.; Powers, B.E.; Belandria, G.A.; Kuntz, C.A.; Withrow, S.J. Surgical treatment of adrenocortical tumors: 21 cases (1990–1996). J. Am. Anim. Hosp. Assoc. 2001, 37, 93–97. [Google Scholar] [CrossRef]
- Kyles, A.E.; Feldman, E.C.; De Cock, H.E.; Kass, P.H.; Mathews, K.G.; Hardie, E.M.; Nelson, R.W.; Ilkiw, J.E.; Gregory, C.R. Surgical management of adrenal gland tumors with and without associated tumor thrombi in dogs: 40 cases (1994–2001). J. Am. Vet. Med. Assoc. 2003, 223, 654–662. [Google Scholar] [CrossRef]
- Barber, L.G. Thyroid tumors in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2007, 37, 756–773. [Google Scholar] [CrossRef]
- Barrera, J.S.; Bernard, F.; Ehrhart, E.J.; Monnet, E. Evaluation of risk factors for outcome associated with adrenal gland tumors with or without invasion of the caudal vena cava and treated via adrenalectomy in dogs: 86 cases (1993–2009). J. Am. Vet. Med. Assoc. 2013, 242, 1715–1721. [Google Scholar] [CrossRef]
- Campos, M.; Ducatelle, R.; Rutteman, G.; Kooistra, H.S.; Duchateau, L.; de Rooster, H.; Peremans, K.; Daminet, S. Clinical, pathologic, and immunohistochemical prognostic factors in dogs with thyroid carcinoma. J. Vet. Intern. Med. 2014, 28, 1805–1813. [Google Scholar] [CrossRef]
- Massari, F.; Nicoli, S.; Romanelli, G.; Buracco, P.; Zini, E. Adrenalectomy in dogs with adrenal gland tumors: 52 cases (2002–2008). J. Am. Vet. Med. Assoc. 2011, 239, 216–221. [Google Scholar] [CrossRef]
- Mayhew, P.D.; Culp, W.T.N.; Balsa, I.M.; Zwingenberger, A.L. Phrenicoabdominal venotomy for tumor thrombectomy in dogs with adrenal neoplasia and suspected vena caval invasion. Vet. Surg. 2018, 47, 227–235. [Google Scholar] [CrossRef]
- Knight, R.C.; Lamb, C.R.; Brockman, D.J.; Lipscomb, V.J. Variations in surgical technique for adrenalectomy with caudal vena cava venotomy in 19 dogs. Vet. Surg. 2019, 48, 751–759. [Google Scholar] [CrossRef]
- Mayhew, P.D.; Boston, S.E.; Zwingenberger, A.L.; Giuffrida, M.A.; Runge, J.J.; Holt, D.E.; Raleigh, J.S.; Singh, A.; Culp, W.T.N.; Case, J.B.; et al. Perioperative morbidity and mortality in dogs with invasive adrenal neoplasms treated by adrenalectomy and cavotomy. Vet. Surg. 2019, 48, 742–750. [Google Scholar] [CrossRef]
- Davis, M.K.; Schochet, R.A.; Wrigley, R. Ultrasonograpic identification of vascular invasion by adrenal tumors in dogs. Vet. Radiol. Ultrasound 2012, 53, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.M.; Wisner, E.R.; Johnson, E.G.; MacLeod, J.S. Contrast-enhanced computed tomography as a preoperative indicator of vascular invasion from adrenal masses in dogs. Vet. Radiol. Ultrasound 2009, 50, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, L.; Francica, G.; Sordelli, A.; Esposito, F.; Giorgio, A.; Sorrentino, P.; de Stefano, G.; Di Sarno, A.; Ferraioli, G.; Sperlongano, P. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: Color Doppler ultrasound, contrast-enhanced ultrasound, and fine-needle biopsy. Abdom. Imaging 2006, 31, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.A.; Jang, H.; Kim, T.K. Differentiating malignant from benign thrombosis in hepatocellular carcinoma: Contrast-enhanced ultrasound. Abdom. Imaging 2014, 39, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Ghittoni, G.; Ravetta, V.; Viera, F.T.; Rosa, L.; Serassi, M.; Scabini, M.; Vercelli, A.; Tinelli, C.; Dal Bello, B.; et al. Contrast-enhanced ultrasonography and spiral computed tomography in the detection and characterization of portal vein thrombosis complicating hepato-cellular carcinoma. Eur. Radiol. 2008, 18, 1749–1756. [Google Scholar] [CrossRef]
- Sorrentino, P.; D’Angelo, S.; Tarantino, L.; Torello Viera, F.; Rosa, L.; Serassi, M.; Scabini, M.; Vercelli, A.; Tinelli, C.; Dal Bello, B.; et al. Contrast-enhanced sonography versus biopsy for the differential diagnosis of thrombosis in hepatocellular carcinoma patients. World J. Gastroenterol. 2009, 15, 2245–2251. [Google Scholar] [CrossRef]
- Danila, M.; Sporea, I.; Popescu, A.; Sirli, R.; Sendroiu, M. The value of Contrast Enhanced Ultrasound in the evaluation of the nature of portal vein thrombosis. Med. Ultrason. 2011, 13, 102–107. [Google Scholar]
- Van Gansbeke, D.; Avni, E.F.; Delcour, C.; Engelholm, L.; Struyven, J. Sonographic features of portal vein thrombosis. AJR Am. J. Roentgenol. 1985, 144, 749–752. [Google Scholar] [CrossRef]
- Besso, J.G.; Penninck, D.G.; Gliatto, J.M. Retrospective ultrasonographic evaluation of adrenal lesions in 26 dogs. Vet. Radiol. Ultrasound 1997, 38, 448–455. [Google Scholar] [CrossRef]
- Spârchez, Z.; Radu, P.; Zaharia, T.; Kacso, G.; Diaconu, B.; Grigorescu, I.; Badea, R. B-mode and contrast enhanced ultrasound guided biopsy of portal vein thrombosis. Value in the diagnosis of occult hepatocellular carcinoma in liver cirrhosis. Med. Ultrason. 2010, 12, 286–294. [Google Scholar]
- D’Anjou, M.A.; Penninck, D. Adrenal Glands. In Atlas of Small Animal Ultrasonography, 2nd ed.; Penninck, D., D’Anjou, M.A., Eds.; Wiley Blackwell Publishing: Hoboken, NJ, USA, 2015; pp. 387–401. [Google Scholar]
- Haers, H.; Saunders, J.H. Review of clinical characteristics and application of contrast-enhanced ultrasonography in dogs. J. Am. Vet. Med. Assoc. 2009, 234, 460–470. [Google Scholar] [CrossRef] [PubMed]
- D’Anjou, M.A.; Schwarz, T. Heart and Vessels. In Veterinary Computed Tomography; Schwarz, T., Saunders, J., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 229–242. [Google Scholar]
- Gregori, T.; Mantis, P.; Benigni, L.; Priestnall, S.L.; Lamb, C.R. Comparison of computed tomographic and pathologic findings in 17 dogs with primary adrenal neoplasia. Vet. Radiol. Ultrasound 2015, 56, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Pey, P.; Rossi, F.; Vignoli, M.; Duchateau, L.; Rossi, F.; Saunders, J.H. Assessment of contrast-enhanced ultrasonography and contrast-enhanced computed tomography for the evaluation of adrenal tumors in dogs. Vet. Radiol. Ultrasound 2013, 54, 408–449. [Google Scholar]
- Pey, P.; Rossi, F.; Vignoli, M.; Duchateau, L.; Marescaux, L.; Saunders, J.H. Use of contrast-enhanced ultrasonography to characterize adrenal gland tumors in dogs. Am. J. Vet. Res. 2014, 75, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Bargellini, P.; Orlandi, R.; Dentini, A.; Paloni, C.; Rubini, G.; Fonti, P.; Diana, A.; Peterson, M.E.; Boiti, C. Use of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Adrenal Tumors in Dogs. J. Am. Anim. Hosp. Assoc. 2016, 52, 132–143. [Google Scholar] [CrossRef] [PubMed]

| Case No | Breed | Sex | Age (Years) | Weight (kg) | Clinical Symptoms | CBC and Biochemistry Abnormalities | Type of Tumor |
|---|---|---|---|---|---|---|---|
| 1 | Mixed | FS | 10 | 27 | Swelling of the neck, sudden change of voice | Elevated serum alkaline phosphatase | Thyroid carcinoma |
| 2 | Mixed | MN | 11 | 14 | Swelling of the neck, sudden change of voice | None | Thyroid carcinoma |
| 3 | Welsh Terrier | MN | 7 | 15 | PU/PD, polyphagia abdominal distension | Elevated serum alkaline phosphatase | Adrenal carcinoma |
| 4 | Mixed | F | 10 | 9 | Abdominal distension, diarrhea | None | Adrenal carcinoma |
| 5 | Boxer | M | 11 | 32 | PU/PD, diarrhea | Elevated serum alkaline phosphatase | Adrenal pheochromocytoma |
| 6 | Labrador | FS | 13 | 32 | PU/PD, polyphagia abdominal distension | Elevated serum alkaline phosphatase | Adrenal pheochromocytoma |
| 7 | Mixed | MN | 13 | 33 | PU/PD, diarrhea | Elevated serum alkaline phosphatase | Retroperitoneal liposarcoma |
| Case No | Site of the Primary Tumor | Thrombus Location | B-Mode US: Primary Tumor | B-Mode US: Thrombus | CFD: Thrombus | CEUS Findings of the Thrombus | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Contrast Uptake | Aspect of the Contrast Uptake | Margination of the Thrombus | Enhancement Compared to the Vessel | Enhancement Compared to the Primary Tumor | ||||||
| 1 | Thyroid carcinoma | Thyroid veins (right and left) | 2 Hyperechoic nodules (left: 2.8 cm; right 3 cm) | Intravascular homogeneous mass with vessel distension and contiguity with the primary tumor | Intralesional Doppler signal; residual flow within the lumen of the vessel | Present | Homogeneous | Irregular and ill-defined | Earlier | Simultaneous |
| 2 | Thyroid carcinoma | Left thyroid vein | 1 Heterogeneous partially anechoic nodule on the left thyroid lobe (3 cm) | Intravascular homogeneous mass | Residual flow within the lumen of the vessel | Present | Homogeneous | Regular and well-defined | Earlier | Simultaneous |
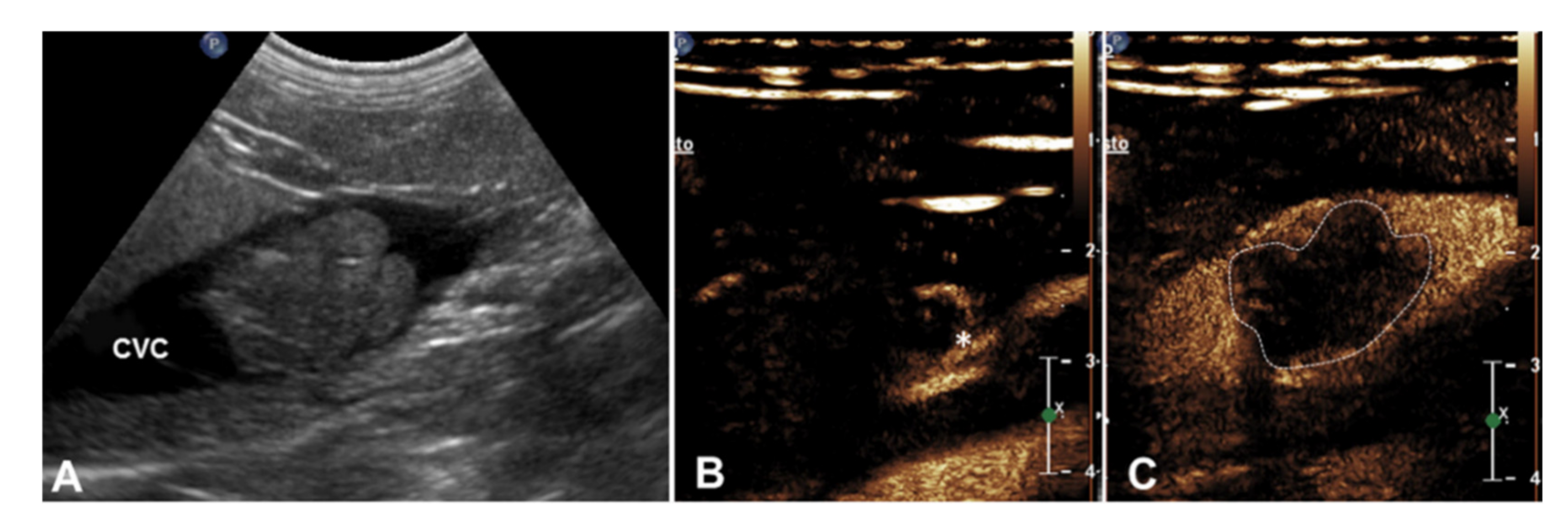
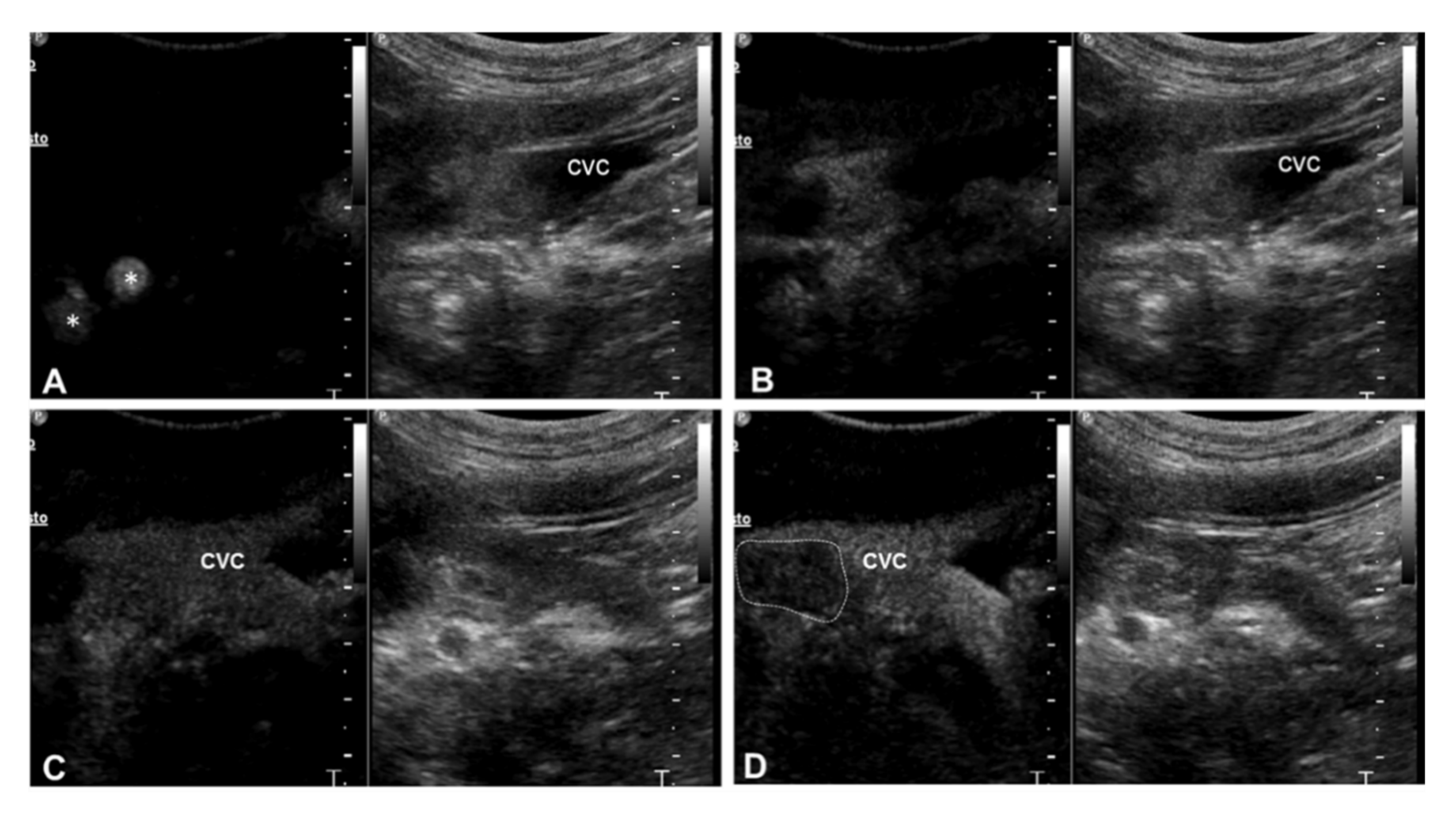
| 3 | Right adrenal carcinoma | Caudal vena cava | 1 Heterogeneous partially hyperechoic nodule with acoustic shadowing on the right adrenal gland (2.9 cm) | Intravascular homogeneous mass in contiguity with the primary tumor | Residual flow within the lumen of the vessel | Present | Heterogeneous | Irregular and ill-defined | Earlier | N/A |
| 4 | Right adrenal carcinoma | Caudal vena cava | Heterogeneous partially hyperechoic right adrenal gland with acoustic shadowing (width 1.5 cm) | Intravascular homogeneous mass with vessel distension | Residual flow within the lumen of the vessel | Present | Heterogeneous | Irregular and ill-defined | Earlier | N/A |
| 5 | Right adrenal pheochromocytoma | Caudal vena cava | 1 Heterogeneous mass on the right adrenal gland (5 cm) | Intravascular homogeneous mass with vessel distension | Residual flow within the lumen of the vessel | Present | Heterogeneous | Regular and well-defined | Earlier | Simultaneous |
| 6 | Left adrenal pheochromocytoma | Caudal vena cava | Heterogeneous left adrenal gland (width 2.3 cm) | Intravascular homogeneous mass with vessel distension | Residual flow within the lumen of the vessel | Present | Heterogeneous | Irregular and ill-defined | Earlier | N/A |
| 7 | Retroperitoneal liposarcoma | Caudal vena cava | Heterogeneous mass on the left retroperitoneal space, involving the left adrenal gland (3.5 cm) | Intravascular homogeneous mass | Intralesional Doppler signal; residual flow within the lumen of the vessel | Present | Heterogeneous | Irregular and ill-defined | Earlier | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordella, A.; Pey, P.; Linta, N.; Quinci, M.; Baron Toaldo, M.; Pisoni, L.; Bettini, G.; Diana, A. Use of Contrast-Enhanced Ultrasonography for the Characterization of Tumor Thrombi in Seven Dogs. Animals 2020, 10, 1613. https://doi.org/10.3390/ani10091613
Cordella A, Pey P, Linta N, Quinci M, Baron Toaldo M, Pisoni L, Bettini G, Diana A. Use of Contrast-Enhanced Ultrasonography for the Characterization of Tumor Thrombi in Seven Dogs. Animals. 2020; 10(9):1613. https://doi.org/10.3390/ani10091613
Chicago/Turabian StyleCordella, Alessia, Pascaline Pey, Nikolina Linta, Manuela Quinci, Marco Baron Toaldo, Luciano Pisoni, Giuliano Bettini, and Alessia Diana. 2020. "Use of Contrast-Enhanced Ultrasonography for the Characterization of Tumor Thrombi in Seven Dogs" Animals 10, no. 9: 1613. https://doi.org/10.3390/ani10091613
APA StyleCordella, A., Pey, P., Linta, N., Quinci, M., Baron Toaldo, M., Pisoni, L., Bettini, G., & Diana, A. (2020). Use of Contrast-Enhanced Ultrasonography for the Characterization of Tumor Thrombi in Seven Dogs. Animals, 10(9), 1613. https://doi.org/10.3390/ani10091613






