Assessment of Gastrointestinal Parasites and Productive Parameters on Sheep Fed on a Ration Supplemented with Guazuma ulmifolia Leaves in Southern Mexico
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Environment, Animal, Plant Material, and Experiment
2.2. Sample Collection and Chemical Analyses
2.3. Calculations and Statistical Analyses
3. Results
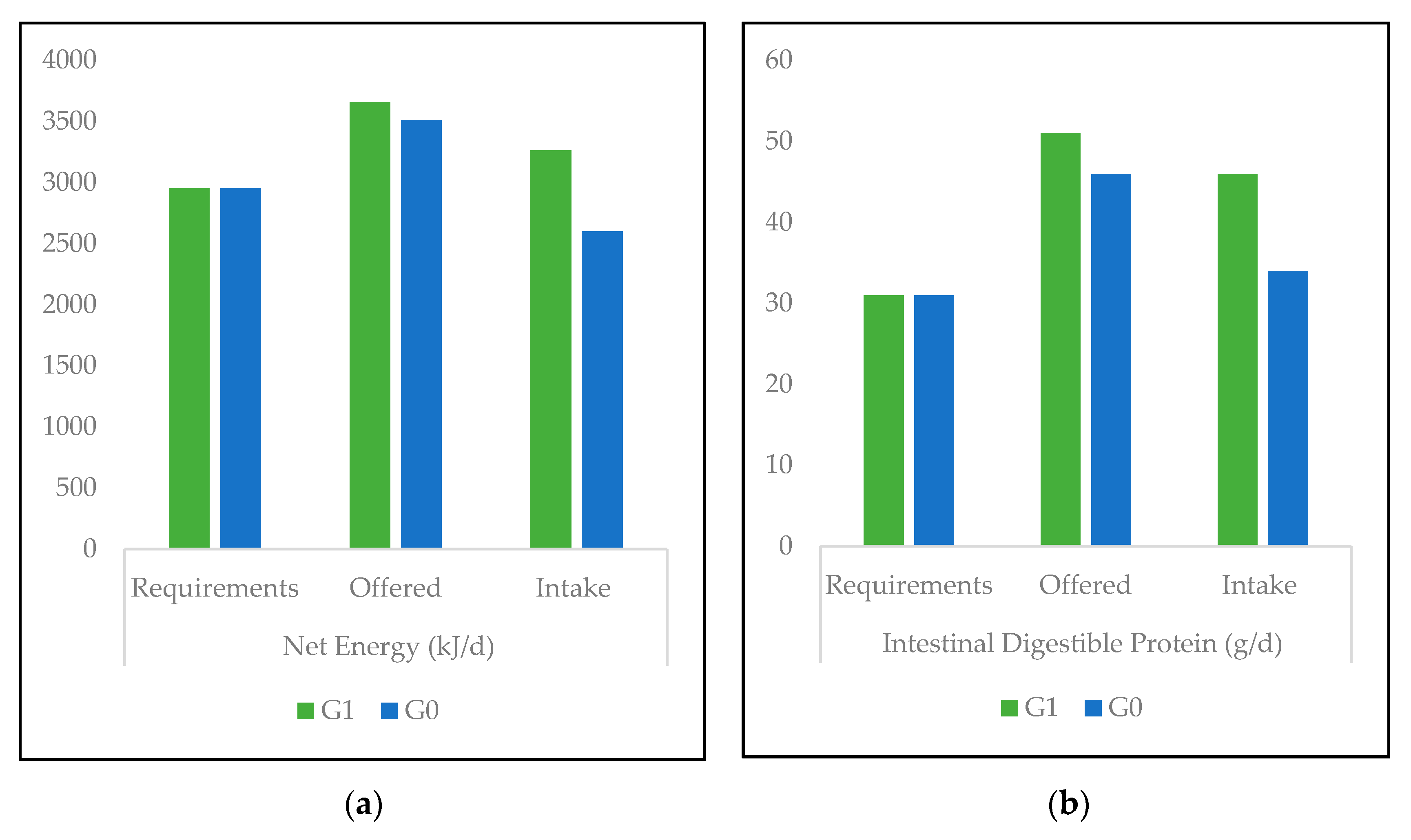
3.1. Nutrient Values and Diet-Associated Calculations
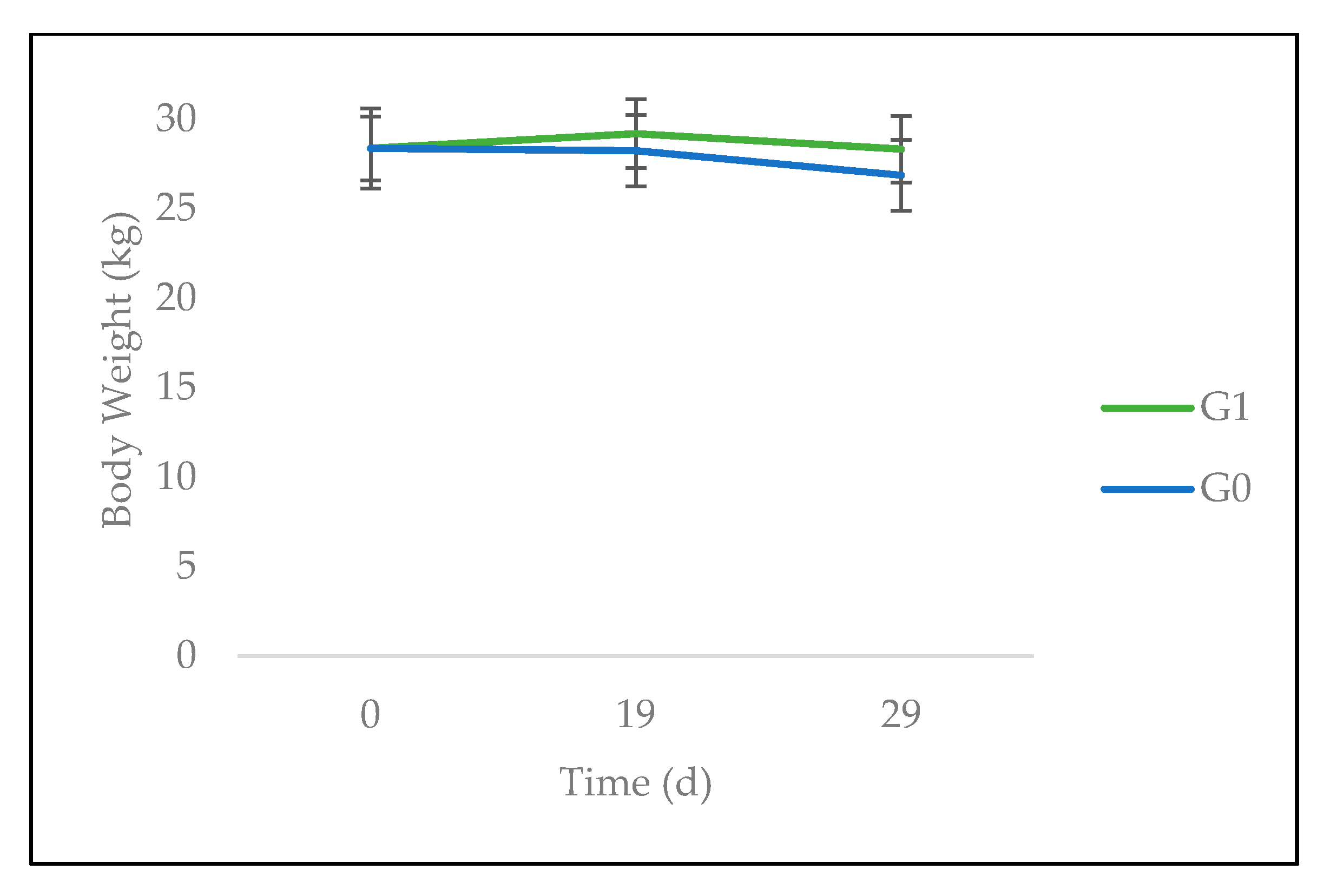
3.2. Feed Intake and Animal Performance
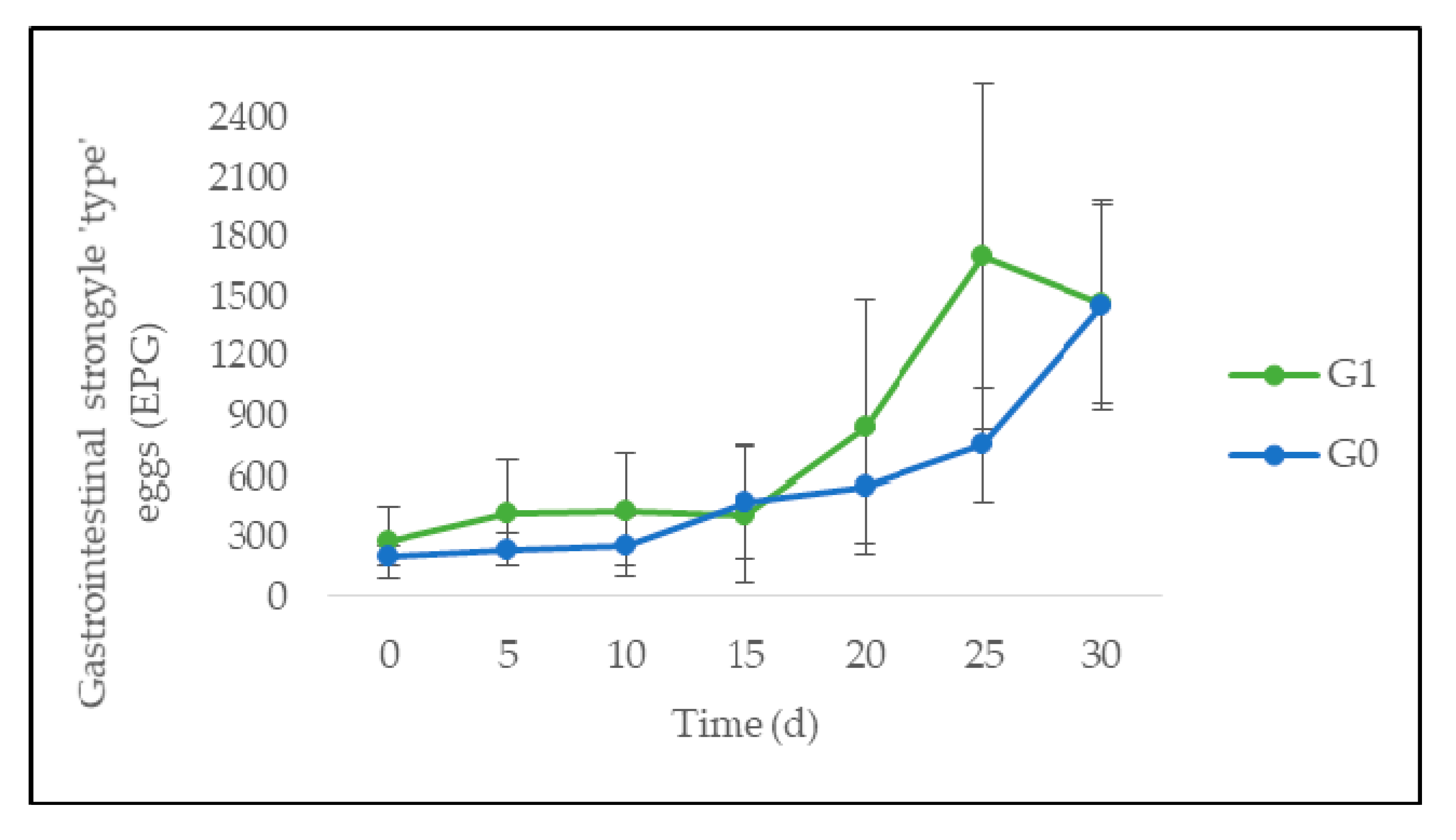
3.3. Quantitative Parasitological Analysis
- Gastrointestinal strongyle-“type” eggs: term which includes GI strongyle eggs with morphological similarities, such as, for the most common genus, Haemonchus, Ostertagia, Trichostrongylus, and Cooperia [6];
- Strongyloides papillosus eggs;
- Eimeria spp. oocysts.
4. Discussion
4.1. Nutrient Values and Diet-Associated Calculations
4.2. Feed Intake, Animal Performance, and Gastrointestinal Parasites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vázquez-García, V. Sheep Production in the Mixed-Farming Systems of Mexico: Where are the Women? Rangelands 2013, 35, 41–46. [Google Scholar] [CrossRef]
- Nahed, T.J.; Gomez, H.; Pinto, R.; Guevara, F.; Medina, F.; Ibrahim, M.; Grande, D. Research and development of silvopastoral systems in a village in the buffer zone of the Ocote Biosphere Reserve, Chiapas, Mexico. Res. J. Biol. Sci. 2010, 5, 499–507. [Google Scholar] [CrossRef]
- Rubio, E.E.S.; Pérez Rodríguez, D.; Reyes, L.O.; Buenfil, G.Z. Evaluación del potencial forrajero de árboles y arbustos tropicales para la alimentación de ovinos. Téc. Pecu. Méx. 2004, 42, 129–144. [Google Scholar]
- Pinto-Ruiz, R.; Gómez-Castro, H.; Guevara-Hernández, F.; Hernández-Sánchez, D.; Ruíz-Sesma, B. Preferencia y Conducta ingestiva de ovinos alimentados con frutos arbóreos tropicales. Rev. Cient. FCV-LUZ 2014, 24, 158–163. [Google Scholar]
- Martínez-Alfaro, J.C.; Valdés-Oyervides, F.J.; Ruíz-Zarate, F. Preferencia y conducta ingestiva de la oveja Pelibuey sobre el follaje de árboles tropicales. In Proceedings of the 1st Congreso Mesoamericano de Investigación, Tuxtla Gutiérrez, Mexico, 1–3 October 2014; Universidad Autónoma de Chiapas: Tuxtla Gutiérrez, Mexico, 2014. [Google Scholar]
- Mavrot, F.; Hertzberg, H.; Torgerson, P. Effect of gastro-intestinal nematode infection on sheep performance: A systematic review and meta-analysis. Parasit. Vectors 2015, 8, 557. [Google Scholar] [CrossRef]
- Pugh, D.G.; Baird, A.N.; Edmondson, M.A.; Passler, T. Internal parasites of sheep, goats, and cervids. In Sheep, Goat, and Cervid Medicine, 3rd ed.; Elsevier: St. Louis, MO, USA, 2021; pp. 276–326. [Google Scholar]
- Herrera-Manzanilla, F.A.; Ojeda-Robertos, N.F.; González-Garduño, R.; Cámara-Sarmiento, R. Gastrointestinal nematode populations with multiple anthelmintic resistance in sheep farms from the hot humid tropics of Mexico. Vet. Parasitol. Reg. Stud. Rep. 2017, 9, 29–33. [Google Scholar] [CrossRef]
- Mondragón-Ancelmo, J.; Olmedo-Juárez, A.; Reyes-Guerrero, D.E.; Ramírez-Vargas, G.; Ariza-Román, A.E.; López-Arellano, M.E.; Mendoza de Gives, P.; Napolitano, F. Detection of gastrointestinal nematode populations resistant to albendazole and ivermectin in sheep. Animals 2019, 9, 775. [Google Scholar] [CrossRef]
- Santiago-Figueroa, I.; Lara-Bueno, A.; González-Garduño, R.; López-Arellano, M.E.; Luis, J.; Rosa-Arana, D.; Maldonado-Simán, E.D.J. Anthelmintic resistance in hair sheep farms in a sub-humid tropical climate, in the Huasteca Potosina, Mexico. Vet. Parasitol. Reg. Stud. Rep. 2019, 17, 100–292. [Google Scholar] [CrossRef]
- De Graef, J.; Claerebout, E.; Geldhof, P. Anthelmintic resistance of gastrointestinal cattle nematodes. Vlaams Diergeneeskd. Tijdschr. 2013, 82, 113–123. [Google Scholar]
- Abbott, K.A.; Taylor, M.; Stubbings, L.A. Sustainable Worm Control Strategies for Sheep: A Technical Manual for Veterinary Surgeons and Advisers, 4th ed.; SCOPS: Worcestershire, UK, 2012; pp. 18–19, 33–41, 50–53. [Google Scholar]
- Terrill, T.H.; Miller, J.E.; Burke, J.M.; Mosjidis, J.A.; Kaplan, R.M. Experiences with integrated concepts for the control of Haemonchus contortus in sheep and goats in the United States. Vet. Parasitol. 2012, 186, 28–37. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Mueller-Harvey, I.; Sotiraki, S.; Louvandini, H.; Thamsborg, S.M.; Terrill, T.H. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Vet. Parasitol. 2015, 212, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Hoste, H.; Torres-Acosta, J.F.J.; Quijada, J.; Chan-Perez, I.; Dakheel, M.M.; Kommuru, D.S.; Terrill, T.H.; Mueller-Harvey, I. Interactions between nutrition and infections with Haemonchus contortus and related gastrointestinal nematodes in small ruminants. Adv. Parasitol. 2016, 93, 239–351. [Google Scholar] [CrossRef] [PubMed]
- Kommuru, D.S.; Barker, T.; Desai, S.; Burke, J.M.; Ramsay, A.; Mueller-Harvey, I.; Miller, J.E.; Mosjidis, J.A.; Kamisetti, N.; Terrill, T.H. Use of pelleted sericea lespedeza (Lespedeza cuneata) for natural control of coccidia and gastro-intestinal nematodes in weaned goats. Vet. Parasitol. 2014, 204, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Saratsis, A.; Voutzourakis, N.; Theodosiou, T.; Stefanakis, A.; Sotiraki, S. The effect of sainfoin (Onobrychis viciifolia) and carob pods (Ceratonia siliqua) feeding regimes on the control of lamb coccidiosis. Parasitol. Res. 2016, 115, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.P.; et al. Benefits of condensed tannins in forage legumes fed to ruminants: Importance of structure, concentration, and diet composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef]
- León-Castro, Y.; Olivares-Pérez, J.; Rojas-Hernández, S.; Villa-Mancera, A.; Valencia-Almazán, M.T.; Hernández-Castro, E.; Córdova-Izquierdo, A.; Jiménez-Guillén, R. Effect of three fodder trees on Haemonchus contortus control and weight variations in kids. Ecosist. Recur. Agropecu. 2015, 2, 193–201. [Google Scholar]
- Feedbase, Bastard Cedar (Guazuma ulmifolia), Aerial Part, Fresh: Individual Data. Available online: http://www.feedbase.com/feedsample.php?Lang=E&name=5723&databasis=2 (accessed on 1 April 2020).
- Alarcon-Aguilara, F.J.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Salazar-Olivo, L.A. The anti-diabetic properties of Guazuma ulmifolia Lam are mediated by the stimulation of glucose uptake in normal and diabetic adipocytes without inducing adipogenesis. J. Ethnopharmacol. 2008, 118, 252–256. [Google Scholar] [CrossRef]
- Caballero-George, C.; Vanderheyden, P.M.; De Bruyne, T.; Shahat, A.A.; Van den Heuvel, H.; Solis, P.N.; Gupta, M.P.; Claeys, M.; Pieters, L.; Vauquelin, G.; et al. In vitro inhibition of [3H]-angiotensin II binding on the human AT1 receptor by proanthocyanidins from Guazuma ulmifolia bark. Planta Med. 2002, 68, 1066–1071. [Google Scholar] [CrossRef]
- Magos, G.A.; Mateos, J.C.; Páez, E.; Fernández, G.; Lobato, C.; Márquez, C.; Enríquez, R.G. Hypotensive and vasorelaxant effects of the procyanidin fraction from Guazuma ulmifolia bark in normotensive and hypertensive rats. J. Ethnopharmacol. 2008, 117, 58–68. [Google Scholar] [CrossRef]
- Hör, M.; Rimpler, H.; Heinrich, M. Inhibition of intestinal chloride secretion by proanthocyanidins from Guazuma ulmifolia. Planta Med. 1995, 61, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, B.; Trabadela, C.; Sánchez-Fidalgo, S.; Quílez, A.; Miño, P.; De la Puerta, R.; Martín-Calero, M.J. The aerial parts of Guazuma ulmifolia Lam. protect against NSAID-induced gastric lesions. J. Ethnopharmacol. 2007, 114, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Camporese, A.; Balick, M.J.; Arvigo, R.; Esposito, R.G.; Morsellino, N.; De Simone, F.; Tubaro, A. Screening of anti-bacterial activity of medicinal plants from Belize (Central America). J. Ethnopharmacol. 2003, 87, 103–107. [Google Scholar] [CrossRef]
- Navarro, M.C.; Montilla, M.P.; Cabo, M.M.; Galisteo, M.; Cáceres, A.; Morales, C.; Berger, I. Antibacterial, antiprotozoal and antioxidant activity of five plants used in Izabal for infectious diseases. Phytother. Res. 2003, 17, 325–329. [Google Scholar] [CrossRef]
- Felipe, A.M.; Rincão, V.P.; Benati, F.J.; Linhares, R.E.; Galina, K.J.; De Toledo, C.E.; Lopes, G.C.; De Mello, J.C.; Nozawa, C. Antiviral effect of Guazuma ulmifolia and Stryphnodendron adstringens on poliovirus and bovine herpesvirus. Biol. Pharm. Bull. 2006, 29, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Secretaría De Agricultura, Ganadería, Desarrollo Rural, Pesca Y Alimentación. NORMA Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Técnicas Para la Producción, Cuidado y uso de los Animales de Laboratorio; Diario Oficial de la Federación, 22 de agosto 2001, Primera sección; Secretaría de Gobernación: Ciudad de México, México, 2001. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen, 5th ed.; Universidad Nacional Autónoma de México, Instituto de Geografía: Cuidad de México, México, 2004. [Google Scholar]
- Thienpont, D.; Rochette, F.; Vanparijs, O.F.J. Diagnosing Helminthiasis by Coprological Examination, 2nd ed.; Janssen Research Foundation: Beerse, Belgium, 1986. [Google Scholar]
- Nozière, L.D.P.; Sauvant, D.; Delaby, L. INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018. [Google Scholar]
- Thompson, J.; Meyer, H. Body Condition Scoring of Sheep; Oregon State University Extension Service: Corvallis, OR, USA, 1994. [Google Scholar]
- Becker, A.C.; Kraemer, A.; Epe, C.; Strube, C. Sensitivity and efficiency of selected coproscopical methods—Sedimentation, combined zinc sulfate sedimentation-flotation, and McMaster method. Parasitol. Res. 2016, 115, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; AOAC: Gaitherburg, MD, USA, 2000. [Google Scholar]
- Agence Française de Normalisation. Norm NF U44-171: Sludge, organic soil conditioners, growth media, determination of dry matter. In AFNOR Guideline; AFNOR Editions; Agence Française de Normalisation: Paris, France, 1982. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics-adaptation and validation of methyl cellulose precipitable tannin assay and modified somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef]
- SAS/STAT®, Version 9.4; Statistical Analysis System Institute Inc.: Cary, NC, USA, 2002–2012.
- National Research Council. Nutrient Requirements of Small Ruminants Sheep, Goats, Cervids, and New World Camelids; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Meschy, F. Nutrition Minérale des Ruminants; Editions Quae: Versailles, France, 2010; pp. 19–36, 56. [Google Scholar]
- Agabriel, J. Alimentation des Bovins, Ovins et Caprins: Besoins des Animaux, Valeurs des Aliments: Tables Inra 2007, Mise à Jour 2010; Editions Quae: Versailles, France, 2010; p. 17. [Google Scholar]
- Lui, S.M.; Master, D.G. Potential impact of nematode parasitism on nutrient partitioning for wool production, growth and reproduction in sheep. Aust. J. Exp. Agric. 2003, 43, 1409–1417. [Google Scholar] [CrossRef]
- Méndez-Ortíz, F.A.; Sandoval-Castro, C.A.; Vargas-Magaña, J.J.; Sarmiento-Franco, L.; Torres-Acosta, J.F.J.; Ventura-Cordero, J. Impact of gastrointestinal parasitism on dry matter intake and live weight gain of lambs: A meta-analysis to estimate the metabolic cost of gastrointestinal nematodes. Vet. Parasitol. 2019, 265, 1–6. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production-Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Chartier, C.; Itard, J.; Morel, P.; Troncy, P. Précis de Parasitologie Vétérinaire Tropicale; Editions Tec et Doc: Paris, France, 2000; pp. 42–43. [Google Scholar]
- Zajac, A.M. Gastrointestinal nematodes of small ruminants: Life cycle, anthelmintics, and diagnosis. Vet. Clin. N. Am. Food. Anim. Pract. 2006, 22, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Zeller, W.E. Activity, purification, and analysis of condensed tannins: Current state of affairs and future endeavors. Crop Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- Cenci, F.B.; Louvandini, H.; McManus, C.M.; DelľPorto, A.; Costa, D.M.; Araújo, S.C.; Minho, A.P.; Abdalla, A.L. Effects of Condensed Tannin from Acacia mearnsii on Sheep Infected Naturally with Gastrointestinal Helminthes. Vet. Parasitol. 2007, 144, 132–137. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sarwar, M.; Jabbar, A.; Ahmed, S.; Nisa, M.; Sajid, M.S.; Khan, M.N.; Mufti, K.A.; Yaseen, M. Direct and indirect anthelmintic effects of condensed tannins in sheep. Vet. Parasitol. 2007, 144, 125–131. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: In vitro and in vivo studies. Vet. Parasitol. 2001, 99, 205–219. [Google Scholar] [CrossRef]



| Parameters | Group | p-Value | |
|---|---|---|---|
| G0 | G1 | ||
| Age (year) | 1.6 ± 0.6 | 1.6 ± 0.6 | 1 |
| Body weight (kg) | 28.4 ± 0.5 | 28.4 ± 1.1 | 1 |
| BCS (/5) | 2.8 ± 0.4 | 2.9 ± 0.4 | 0.8 |
| Gastrointestinal strongyle-“type” eggs (EPG) | 202 ± 10 | 277 ± 35 | 0.7 |
| Eimeria spp. oocysts (OPG) | 430 ± 24 | 493 ± 17 | 0.7 |
| Strongyloides papillosus eggs (EPG) | 93 ± 11 | 132 ± 25 | 0.7 |
| Parameters | G. ulmifolia (foliage) | C. nlemfuensis (hay) | ||
|---|---|---|---|---|
| Average | SD | Average | SD | |
| Dry matter (% Fresh matter) | 38.7 | 0.8 | 83.0 | 0.9 |
| Crude ash (% DM) | 10.7 | 1.3 | 10.5 | 2.7 |
| Crude protein (% DM) | 15.4 | 2.3 | 6.9 | 2.3 |
| Crude fiber (% DM) | 22.9 | 2.4 | 34.5 | 4.6 |
| NDF (% DM) | 62.7 | 3.8 | 68.5 | 8.2 |
| Total tannins (% DM) | 0.79 | 0.05 | / | |
| Condensed tannins (% DM) | 1.05 | 0.04 | / | |
| Calcium (g/kg DM) | 19.0 | 5.0 | 4.2 | 0.5 |
| Phosphorus (g/kg DM) | 3.1 | 0.4 | 1.7 | 0.9 |
| Potassium (g/kg DM) | 18.6 | 2.3 | 14.9 | 0.5 |
| Sodium (g/kg DM) | 0.1 | 0.1 | 1.1 | 1.1 |
| Magnesium (g/kg DM) | 4.4 | 0.7 | 1.7 | 0.3 |
| Copper (mg/kg DM) | 30.2 | 18.5 | 7.3 | 5.0 |
| Iron (mg/kg DM) | 86.5 | 23.8 | 368.5 | 261.9 |
| Manganese (mg/kg DM) | 49.9 | 5.5 | 79.4 | 8.4 |
| Zinc (mg/kg DM) | 31.0 | 14.4 | 32.7 | 6.4 |
| Net energy (kJ/kg DM) | 5693 | 72 | 4726 | 106 |
| IDP (% DM) | 9.0 | 0.5 | 6.2 | 0.8 |
| RDNB (g) | 66.5 | 21.9 | −9.8 | 7.9 |
| Parameters/Parasites | Group | p-Value | |
|---|---|---|---|
| G0 | G1 | ||
| Body weight (kg) | 27.8 ± 2.1 | 28.6 ± 1.9 | 0.77 |
| BCS (/5) | 2.6 ± 0.2 | 2.8± 0.2 | 0.32 |
| Gastrointestinal strongyle-“type” eggs (EPG) | 562 ± 241 | 791 ± 448 | 0.57 |
| Eimeria spp. oocysts (OPG) | 338 ± 123 | 353 ± 130 | 0.91 |
| Strongyloides papillosus eggs (EPG) | 141 ± 80 | 113 ± 55 | 0.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Bodo, E.; Hornick, J.-L.; Moula, N.; Zuñiga, S.A.; Martínez-Alfaro, J.C. Assessment of Gastrointestinal Parasites and Productive Parameters on Sheep Fed on a Ration Supplemented with Guazuma ulmifolia Leaves in Southern Mexico. Animals 2020, 10, 1617. https://doi.org/10.3390/ani10091617
Le Bodo E, Hornick J-L, Moula N, Zuñiga SA, Martínez-Alfaro JC. Assessment of Gastrointestinal Parasites and Productive Parameters on Sheep Fed on a Ration Supplemented with Guazuma ulmifolia Leaves in Southern Mexico. Animals. 2020; 10(9):1617. https://doi.org/10.3390/ani10091617
Chicago/Turabian StyleLe Bodo, Emelyne, Jean-Luc Hornick, Nassim Moula, Serrano Aracely Zuñiga, and Juan Carlos Martínez-Alfaro. 2020. "Assessment of Gastrointestinal Parasites and Productive Parameters on Sheep Fed on a Ration Supplemented with Guazuma ulmifolia Leaves in Southern Mexico" Animals 10, no. 9: 1617. https://doi.org/10.3390/ani10091617
APA StyleLe Bodo, E., Hornick, J.-L., Moula, N., Zuñiga, S. A., & Martínez-Alfaro, J. C. (2020). Assessment of Gastrointestinal Parasites and Productive Parameters on Sheep Fed on a Ration Supplemented with Guazuma ulmifolia Leaves in Southern Mexico. Animals, 10(9), 1617. https://doi.org/10.3390/ani10091617





