Plasmatic Profile of Pregnancy-Associated Glycoprotein (PAG) during Gestation and Postpartum in Sarda and Lacaune Sheep Determined with Two Radioimmunoassay Systems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Samples
2.2. Experimental Design
2.3. Progesterone Assay
2.4. Pregnancy-Associated Glycoprotein Assays
2.5. Pregnancy Diagnosis
2.6. Statistical Analysis
3. Results
3.1. Progesterone Concentrations
3.2. Profiles of RIA-706 and RIA-srPool during Pregnancy and the Postpartum Period in Sarda and Lacaune Ewes
3.3. Effects of Single Versus Multiple Pregnancies and Gender on PAG Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wallace, R.M.; Pohler, K.J.; Smith, M.F.; Green, J.A. Placental PAGs: Gene origins, expression patterns, and use as markers of pregnancy. Reproduction 2015, 149, R115–R126. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Low, B.G.; Nagel, R.J.; Kramer, K.K.; Anthony, R.V.; Zoli, A.P.; Beckers, J.F.; Roberts, R.M. Identification of the major pregnancy-specific antigens of cattle and sheep as inactive members of the aspartic proteinase family. Proc. Natl. Acad. Sci. USA 1991, 88, 10247–10251. [Google Scholar] [CrossRef]
- Willard, J.M.; White, D.R.; Wesson, C.A.R.; Stellflug, J.; Sasser, R.G. Detection of fetal twins in sheep using a radioimmunoassay for pregnancy-specific protein B. J. Anim. Sci. 1995, 73, 960–966. [Google Scholar] [CrossRef] [PubMed]
- El Amiri, B.; Remy, B.; Sousa, N.M.; Joris, B.; Otthiers, N.G.; Perény, Z.; Banga-Mboko, H.; Beckers, J.F. Isolation and partial characterization of three pregnancy-associated glycoproteins from the ewe placenta. Mol. Reprod. Dev. 2003, 64, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gogolin-Ewens, K.J.; Lee, C.S.; Mercer, W.R.; Moseby, A.M.; Brandon, M.R. Characterization of a sheep trophoblast-derived antigen first appearing at implantation. Placenta 1986, 7, 243–255. [Google Scholar] [CrossRef]
- Xie, S.; Green, J.A.; Bao, B.; Beckers, J.F.; Valdez, K.E.; Hakami, L.; Roberts, R.M. Multiple pregnancy-associated glycoproteins are secreted by day 100 ovine placental tissue. Biol. Reprod. 1997, 57, 1384–1393. [Google Scholar] [CrossRef]
- Xie, S.; Green, J.; Bixby, J.B.; Szafranska, B.; De Martini, J.C.; Hecht, S.; Roberts, R.M. The diversity and evolutionary relationships of the pregnancy-associated glycoproteins, an aspartic proteinase subfamily consisting of many trophoblast-expressed genes. Proc. Natl. Acad. Sci. USA 1997, 94, 12809–12816. [Google Scholar] [CrossRef]
- Green, J.A.; Xie, S.; Quan, X.; Bao, B.; Gan, X.; Mathialagan, N.; Roberts, R.M. Pregnancy-Associated glycoproteins exhibit spatially and temporally distinct expression patterns during pregnancy. Biol. Reprod. 2000, 62, 1624–1632. [Google Scholar] [CrossRef]
- Garbayo, J.M.; Green, J.A.; Manikkam, M.; Beckers, J.F.; Kiesling, D.O.; Ealy, A.D.; Roberts, R.M. Caprine pregnancy-associated glycoproteins (PAG): Their cloning, expression, and evolutionary relationship to other PAG. Mol. Reprod. Dev. 2000, 57, 311–322. [Google Scholar] [CrossRef]
- Touzard, E.; Reinaud, P.; Dubois, O.; Guyader-Joly, C.; Humblot, P.; Ponsart, C.; Charpigny, J. Specific expression patterns and cell distribution of ancient and modern PAG in bovine placenta during pregnancy. Reproduction 2013, 146, 347–362. [Google Scholar] [CrossRef]
- Ranilla, M.J.; Sulon, J.; Carro, M.D.; Mantecon, A.R.; Beckers, J.F. Plasmatic profiles of pregnancy–associated glycoprotein and progesterone levels during gestation in Churra and Merino sheep. Theriogenology 1994, 42, 537–545. [Google Scholar] [CrossRef]
- Barbato, O.; Sousa, N.M.; Debenedetti, A.; Canali, C.; Todini, L.; Beckers, J.F. Validation of a new pregnancy-associated glycoprotein radioimmunoassay method for the detection of early pregnancy in ewes. Theriogenology 2009, 72, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Vandaele, L.; Verberckmoes, S.; El Amiri, B.; Sulon, J.; Duchateau, L.; Van Soom, A.; Beckers, J.F.; de Kruift, A. Use of homolougous radioimmunoassay (RIA) to evaluate the effect of maternal and foetal parameters on pregnancy-associated glycoprotein (PAG) concentrations in sheep. Theriogenology 2005, 63, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Ledezma-Torres, R.A.; Beckers, J.F.; Holtz, W. Assessment of plasma profile of pregnancy-associated glycoprotein (PAG) in sheep with a heterologous (anti-caPAG22 + 59) RIA and its potential for diagnosing pregnancy. Theriogenology 2006, 66, 906–912. [Google Scholar] [CrossRef]
- El Amiri, B.; Sulon, J.; Karen, A.; Alvarez, A.V.; Cogniè, Y.; Sousa, N.M.; Szenci, O.; Beckers, J.F. Measurement of ovine pregnancy-associated glycoprotein (PAG) during early pregnancy in Lacaune sheep. Reprod. Domest. Anim. 2007, 42, 257–262. [Google Scholar] [CrossRef] [PubMed]
- El Amiri, B.; Sousa, N.M.; Alvarez Oxiley, A.; Hadarbach, D.; Beckers, J.F. Pregnancy-Associated glycoprotein (PAG) concentration in plasma and milk samples for early pregnancy diagnosis in Lacaune dairy sheep. Res. Vet. Sci. 2015, 99, 30–36. [Google Scholar] [CrossRef]
- Rovani, M.T.; Skrebsky Cezar, A.; Lazzari Rigo, M.; Garziera Gasperin, B.; da Nobrega, J.E.; Dias Torres, F.; Bayard Dias Goncalves, P.; Ferreira, R. Evaluation of a bovine pregnancy glycoprotein enzyme-linked immunosorbent assay kit for serological diagnosis of pregnancy in sheep. Cienc. Rural 2016, 46, 362–367. [Google Scholar] [CrossRef]
- De Miranda e Silva Chaves, C.; da Costa, R.L.D.; Duarte, K.M.R.; Manchado, M.C.; de Paz, C.C.P.; Beltrame, R.T. Visual ELISA for detection of pregnancy-associated glycoproteins (PAGs) in ewes serum. Reprod. Domest. Anim. 2017, 97, 78–82. [Google Scholar] [CrossRef]
- Steckeler, P.; Weber, F.; Zerbe, H.; Rieger, A.; Voigt, K. Evaluation of a bovine visual pregnancy test for the detection of pregnancy-associated glycoproteins in sheep. Reprod. Domest. Anim. 2019, 54, 280–288. [Google Scholar] [CrossRef]
- Barbato, O.; Melo de Sousa, N.; Barile, V.L.; Canali, C.; Beckers, J.F. Purification of pregnancy associated glycoproteins from late pregnancy Bubalus bubalis placentas and development of a radioimmunoassay for pregnancy diagnosis in water buffalo females. BMC Vet. Res. 2013, 9, 89. [Google Scholar] [CrossRef]
- Barbato, O.; Menchetti, L.; Sousa, N.M.; Malfatti, A.; Brecchia, G.; Canali, C.; Beckers, J.F.; Barile, V.L. Pregnancy-Associated glycoproteins (PAGs) concentrations in water buffaloes (Bubalus bubalis) during gestation and the postpartum period. Theriogenology 2017, 97, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Beriot, M.; Tchimbou, A.F.; Barbato, O.; Beckers, J.F.; de Sousa, N.M. Identification of pregnancy-associated glycoproteins and alpha protein in fallow deer (Dama dama) placenta. Acta Vet. Scandinava 2014, 56, 4. [Google Scholar] [CrossRef] [PubMed]
- Mialon, M.M.; Camous, S.; Renand, G.; Martal, J.; Ménissier, F. Peripheral gestation of a 60_kDa pregnancy serum protein during gestation and after calving and in relationship to embryonic mortality in cattle. Reprod. Nutr. Dev. 1993, 33, 269–282. [Google Scholar] [CrossRef]
- Ranilla, M.J.; Sulon, J.; Mantecon, A.R.; Beckers, J.F.; Carro, M.F. Plasma pregnancy-associated glycoprotein and progesterone concentrations in pregnant Assaf ewes carrying single and twin lambs. Small Rumin. Res. 1997, 24, 125–131. [Google Scholar] [CrossRef]
- Karen, A.; El Amiri, B.; Beckers, J.F.; Sulon, J.; Taverne, M.A.M.; Szenzi, O. Comparison of accuracy of transabdominal ultrasonography, progesterone and pregnancy-associated glycoproteins tests for discrimination between single or multiple pregnancy in sheep. Theriogenology 2006, 66, 314–322. [Google Scholar] [CrossRef]
- Mercadante, P.M.; Waters, K.M.; Mercadante, V.G.R.; Lamb, G.C.; Elzo, M.A.; Johnson, M.E.; Rae, D.O.; Yelich, J.V.; Ealy, A.D. Subspecies differences in early fetal development and plasma pregnancy-associated glycoprotein concentrations in cattle. J. Anim. Sci. 2013, 91, 3693–3701. [Google Scholar] [CrossRef]
- Lobago, F.; Bekana, M.; Gustafsson, H.; Beckers, J.F.; Yohannes, G.; Aster, Y.; Kindahl, H. Serum Profiles of Pregnancy-Associated Glycoprotein, Oestrone Sulphate and Progesterone During Gestation and Some Factors Influencing the Profiles in Ethiopian Borana and Crossbred Cattle. Reprod. Domest. Anim. 2009, 44, 685–692. [Google Scholar] [CrossRef]
- Patel, O.; Sulon, J.; Beckers, J.F.; Takahashi, T.; Hirako, M.; Sasaki, N.; Domeki, I. Plasma bovine pregnancy-associated glycoprotein concentrations throughout gestation in relationship to fetal number in the cow. Eur. J. Endocrinol. 1997, 137, 423–428. [Google Scholar] [CrossRef]
- Wallace, J.M.; Aitken, R.P.; Cheyne, M.A.; Humblot, P. Pregnancy-Specific protein B and progesterone concentrations in relation to nutritional regimen, placental mass and pregnancy outcome in growing adolescent ewes carrying singleton fetuses. J. Reprod. Fertil. 1997, 109, 53–58. [Google Scholar] [CrossRef][Green Version]
- Zoli, A.P.; Guilbault, L.A.; Delahaut, P.; Ortiz, W.B.; Beckers, J.F. Radioimmunoassay of a bovine pregnancy-associated glycoprotein in serum: Its application for pregnancy diagnosis. Biol. Reprod. 1992, 46, 83–92. [Google Scholar] [CrossRef]
- Macciotta, N.P.P.; Mele, M.; Cappio-Borlino, A.; Secchiari, P. Issues and perspectives in dairy sheep breeding. Ital. J. Anim. Sci. 2005, 4, 5–23. [Google Scholar] [CrossRef][Green Version]
- Carta, A.; Casu, S.; Salaris, S. Invited review: Current state of genetic improvement in dairy sheep. J. Dairy Sci. 2009, 92, 5814–5833. [Google Scholar] [CrossRef] [PubMed]
- Sitzia, M.; Bonanno, A.; Todaro, M.; Cannas, A.; Atzori, A.S.; Francesconi, A.H.D.; Trabalza-Marinucci, M. Feeding and management techniques to favour summer sheep milk and cheese production in the Mediterranean environment. Small Rumin. Res. 2015, 126, 43–58. [Google Scholar] [CrossRef]
- Todaro, M.; Dattena, M.; Acciaioli, A.; Bonanno, A.; Bruni, G.; Caroprese, M.; Mele, M.; Sevi, A.; Trabalza-Marinucci, M. Aseasonal sheep and goat milk production in the Mediterranean area: Physiological and technical insights. Small Rumin. Res. 2015, 126, 59–66. [Google Scholar] [CrossRef]
- Barillet, F.; Marie, C.; Jacquin, M.; Lagriffoul, G.; Astruc, J.M. The French Lacaune dairy sheep breed: Use in France and abroad in the last 40 years. Livest. Prod. Sci. 2001, 71, 17–29. [Google Scholar] [CrossRef]
- Karen, A.; Beckers, J.F.; Sulon, J.; El amiri, B.; Szabados, S.; Ismail, S.; Reiczigel, J.; Szenci, O. Evalaution of false transrectal ultrasonographic pregnancy diagnoses in sheep by measuring tha plasma level of pregnancy-associated glycoproteins. Reprod. Nutr. Dev. 2003, 43, 577–586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cannas, A. Feeding of lactating ewes. In Dairy Sheep Nutrition; Pulina, G., Ed.; CABI: Bologna, Italy, 2004; pp. 79–108. [Google Scholar]
- Todini, L.; Malfatti, A.; Barbato, O.; Costarelli, S.; Debenedetti, A. Progesterone plus PMSG priming in seasonally anovulatory lactating Sarda ewes exposed to the ram effect. J. Reprod. Dev. 2007, 53, 437–441. [Google Scholar] [CrossRef][Green Version]
- Todini, L.; Marinucci, M.T.; Malfatti, A.; Barbato, O.; Cavallucci, C.; Debenedetti, A. Pre and post-feed ind plasma gastrin-17 and insulin concentrations and feed-intake in female goats during different physiological stages. Small Rumin. Res. 2007, 71, 38–47. [Google Scholar] [CrossRef]
- Perenyi, Z.; Szenci, O.; Sulon, J.; Drion, P.V.; Beckers, J.F. Comparison of the ability of three radioimmunoassays to detect pregnancy-associated glycoproteins in bovine plasma. Reprod. Domest. Anim. 2002, 37, 100–104. [Google Scholar] [CrossRef]
- Perenyi, Z.; Szenci, O.; Drion, P.V.; Banga-Mboko, H.; Sousa, N.M.; El Amiri, B.; Beckers, J.F. Aspartic proteinase members secreted by the ruminant placenta: Specificity of three radioimmunoassay systems for the measurement of pregnancy-associated glycoproteins. Reprod. Domest. Anim. 2002, 37, 324–329. [Google Scholar] [CrossRef]
- Zoli, A.P.; Beckers, J.F.; Wouters-Ballman, P.; Closset, J.; Falmagne, P.; Ectors, F. Purification and characterization of a bovine pregnancy-associated glycoproteins. Biol. Reprod. 1991, 45, 1–10. [Google Scholar] [CrossRef]
- Greenwood, F.C.; Hunter, W.M.; Glover, J.S. The preparation of 131-I labeled human growth hormone of high specific radioactivity. Biochemistry 1963, 89, 114–123. [Google Scholar]
- Skelley, D.S.; Brown, L.P.; Besch, P.K. Radioimmunoassay. Clin. Chem. 1973, 2, 146–186. [Google Scholar] [CrossRef]
- Statistical Analysis Software [SAS]. SAS/STAT User Guide, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Gajewski, Z.; Beckers, J.F.; Sousa, N.M.; Thun, R.; Sulon, J.; Foundez, R. Determination of pregnancy associated glycoprotein concentration in sheep. A retrospective study. Adv. Cell Biol. 1999, 2, 89–96. [Google Scholar]
- Sousa, N.M.; Garbayo, J.M.; Figueiredo, J.R.; Sulon, J.; Goncalvesa, P.B.D.; Beckers, J.F. Pregnancy-Associated glycoprotein and progesterone profiles during pregnancy and postpartum in native goats from the north-east of Brazil. Small Rumin. Res. 1999, 32, 137–147. [Google Scholar] [CrossRef]
- Gonzalez, F.; Sulon, J.; Garbayo, J.M.; Batista, M.; Cabrera, F.; Calero, P.O.; Gracia, A.; Beckers, J.F. Secretory profiles of pregnancy associated glycoproteins at different stages. Reprod. Domest. Anim. 2000, 35, 79–82. [Google Scholar] [CrossRef]
- Barbato, O.; Menchetti, L.; Sousa, N.M.; Brecchia, G.; Malfatti, A.; Canali, C.; Beckers, J.F.; Barile, V.L. Correlation of two radioimmunoassay systems for measuring pregnancy-associated glycoproteins plasma concentration during early pregnancy and post-partum period in water buffalo. Reprod. Domest. Anim. 2018, 53, 1483–1490. [Google Scholar] [CrossRef]
- Ayad, A.; Sousa, N.M.; Sulon, J.; Iguer-Ouada, M.; Beckers, J.F. Comparison of five radioimmunoassay systems for PAG measurement: Ability to detect early pregnancy in cows. Reprod. Domest. Anim. 2007, 4, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Sulon, J.; Garbayo, J.M.; Batista, M.; Cabrera, F.; Calero, P.O.; Gracia, A.; Beckers, J.F. Early pregnancy diagnosis in goats of pregnancy associated glycoproteins in plasma samples. Theriogenology. 1999, 52, 717–725. [Google Scholar] [CrossRef]
- Ropstad, E.; Veiberg, V.; Sakkinen, H.; Dahl, A.; Kindahl, H.; Holand, O.; Beckers, J.F.; Eloranta, E. Endocrinology of pregnancy and early pregnancy detection by reproductive hormones in reindeer (Rangifer tarandus tarandus). Theriogenology 2005, 63, 1775–1788. [Google Scholar] [CrossRef]
- Osborn, A.; Beckers, J.F.; Sulon, J.; Gassett, J.W.; Muller, L.I.; Murphy, B.P.; Miller, K.V.; Marchinton, L.R. Use of glycoprotein assays for pregnancy diagnois in White-Tailed Deer. J. Wildl. Manag. 1996, 60, 388–393. [Google Scholar] [CrossRef]
- Haugejorden, G.; Wage, S.; Dahl, E.; Karlberg, K.; Beckers, J.F.; Ropstad, E. Pregnancy associated glycoproteins (PAG) in postpartum cows, ewes, goats and their offspring. Theriogenology 2006, 66, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Sasser, R.G.; Ruder, C.A.; Kristen, A.I.; Butler, J.E.; Hamilton, W.C. Detection of pregnancy by novel pregnancy-specific protein in serum of cows and a profile of serum concentration during gestation. Biol. Reprod. 1986, 35, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Kirakofe, G.H.; Wright, J.M.; Schalles, R.R.; Ruder, C.A.; Parish, S.; Sasser, R.G. Pregnancy-Specific protein B in serum of postpartum beef cows. J. Anim. Sci. 1997, 71, 2199–2205. [Google Scholar] [CrossRef]
- Sousa, N.M.; Zongo, M.; Pitala, W.; Boly, H.; Sawadogo, L.; Sanon, M.; Figuereido, J.R.; Concalves, P.B.D.; El Amiri, B.; Prenényi, Z.; et al. Pregnancy-Associated glycoprotein concentrations during pregnancy and post partum period in Azawak Zebu Cattle. Theriogenology 2003, 59, 1131–1142. [Google Scholar] [CrossRef]
- Klisch, K.; Boos, A.; Friedrich, M.; Herzog, K.; Feldmann, M.; Sousa, N.M.; Beckers, J.F.; Leiser, R.; Schuler, G. The glycosylation of pregnancy-associated glycoproteinsand prolactin-related protein-I in bovine binucleate trophoblastgiant cells changes before parturition. Reproduction 2006, 132, 791–798. [Google Scholar] [CrossRef]
- Wooding, F.B.P. Current topic: The synepitheliochorial placenta of ruminants: Binucleate cell fusions and hormone production. Placenta 1992, 12, 101–103. [Google Scholar] [CrossRef]
- Batalha, E.S.; Sulon, J.; Figueiredo, E.S.; Beckers, J.F.; Espechit, C.J.B.; Martins, R.; Silva, L.D.M. Plasma profile of pregnancy associated glycoprotein (PAG) in alpine goats using two radioimmunoassay (RIA) systems. Small Rumin. Res. 2001, 42, 111–118. [Google Scholar] [CrossRef]
- Shahin, M.; Friedrich, M.; Gauly, M.; Holtz, W. Pregnancy-Associated glycoprotein (PAG) profile of Holstein–Friesian cows as compared to dual-purpose and beef cows. Reprod. Domest. Anim. 2014, 49, 618–620. [Google Scholar] [CrossRef]
- Greenwood, P.L.; Slepetis, R.M.; Bell, A.W. Influences on fetal and placental weights during mid to late gestation in prolific ewes well nourished throughout pregnancy. Reprod. Fertil. Dev. 2000, 12, 149–156. [Google Scholar] [CrossRef]
- Kaulfusb, K.H.; Schramm, D.; Berttram, M. Effects of genotype, dams age, litter size, birth weight and ram on morphological parameters of the placenta sheep. DTW 2000, 107, 269–275. [Google Scholar]
- Guilbault, L.A.; Roy, G.L.; Beckers, J.F.; Dufour, J.S. Influence of breed of fetus periparturient endocrine responses and subsequent milk production of Ayrshire dams. J. Dairy Sci. 1990, 73, 2766–2773. [Google Scholar] [CrossRef]
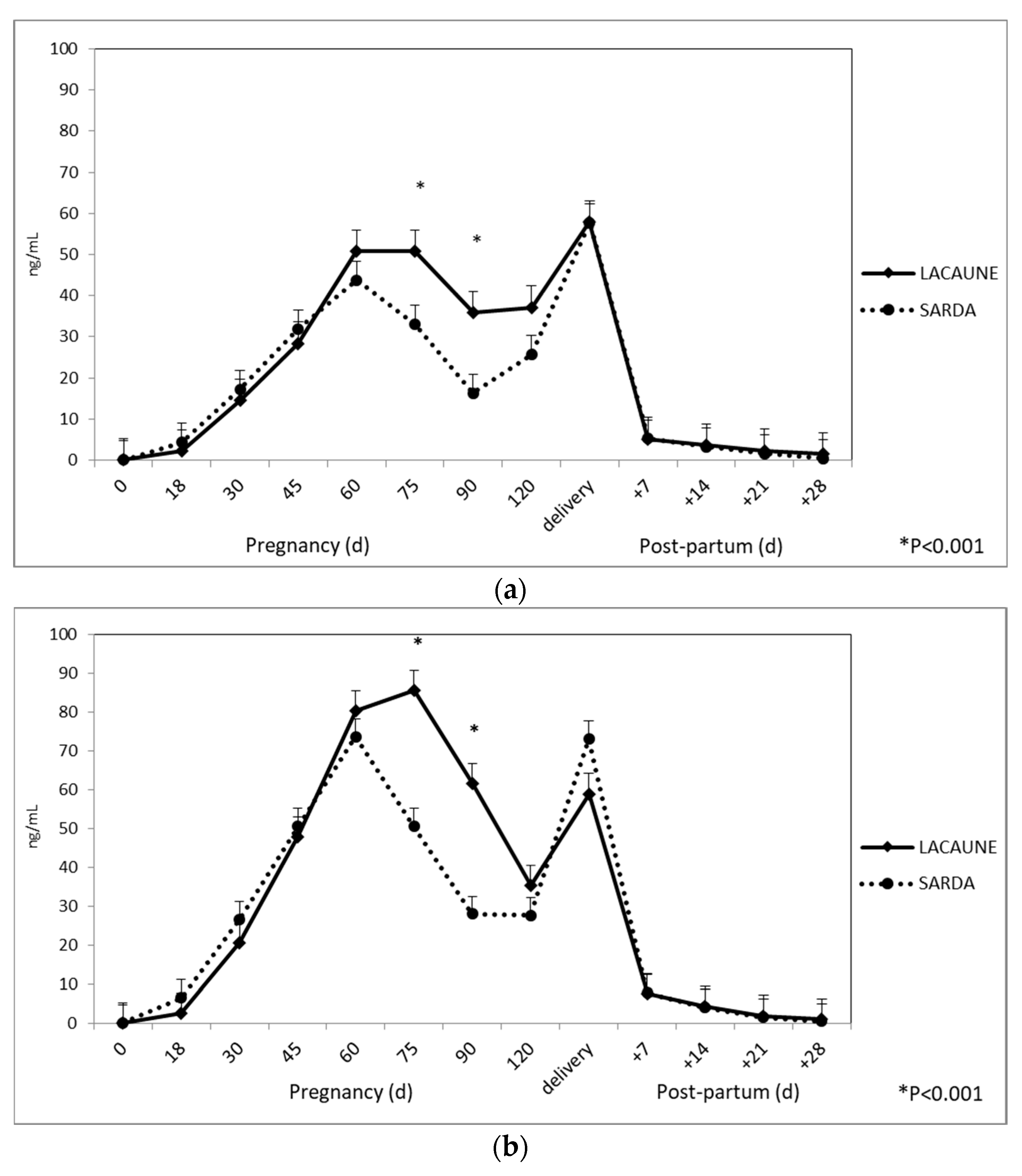


| Days | RIA-706 | RIA-srPool | p-Value |
|---|---|---|---|
| Mating | 0.148 | 0.059 | 0.23 |
| 18 | 3.735 | 4.775 | 0.69 |
| 30 | 16.907 | 24.034 | 0.59 |
| 45 | 31.298 | 50.189 | <0.001 |
| 60 | 48.709 | 78.444 | <0.001 |
| 75 | 44.836 | 69.821 | <0.001 |
| 90 | 29.183 | 46.975 | <0.001 |
| 120 | 33.852 | 34.171 | 0.33 |
| Delivery | 62.753 | 71.873 | 0.78 |
| 7 | 5.573 | 8.035 | 0.55 |
| 14 | 3.576 | 4.275 | 0.08 |
| 21 | 2.057 | 1.778 | 0.98 |
| 28 | 0.998 | 0.842 | 0.09 |
| Day of Pregnancy | Sarda | Lacaune | |||
|---|---|---|---|---|---|
| RIA-706 | RIA-srPool | RIA-706 | RIA-srPool | ||
| Day 18 | Nonpregnant | 1 | 4 | 2 | 5 |
| Pregnant | 25 | 22 | 18 | 15 | |
| Day 30 | Nonpregnant | 0 | 0 | 0 | 0 |
| Pregnant | 26 | 26 | 20 | 20 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Carolis, M.; Barbato, O.; Acuti, G.; Trabalza-Marinucci, M.; Melo de Sousa, N.; Canali, C.; Moscati, L. Plasmatic Profile of Pregnancy-Associated Glycoprotein (PAG) during Gestation and Postpartum in Sarda and Lacaune Sheep Determined with Two Radioimmunoassay Systems. Animals 2020, 10, 1502. https://doi.org/10.3390/ani10091502
De Carolis M, Barbato O, Acuti G, Trabalza-Marinucci M, Melo de Sousa N, Canali C, Moscati L. Plasmatic Profile of Pregnancy-Associated Glycoprotein (PAG) during Gestation and Postpartum in Sarda and Lacaune Sheep Determined with Two Radioimmunoassay Systems. Animals. 2020; 10(9):1502. https://doi.org/10.3390/ani10091502
Chicago/Turabian StyleDe Carolis, Martina, Olimpia Barbato, Gabriele Acuti, Massimo Trabalza-Marinucci, Noelita Melo de Sousa, Claudio Canali, and Livia Moscati. 2020. "Plasmatic Profile of Pregnancy-Associated Glycoprotein (PAG) during Gestation and Postpartum in Sarda and Lacaune Sheep Determined with Two Radioimmunoassay Systems" Animals 10, no. 9: 1502. https://doi.org/10.3390/ani10091502
APA StyleDe Carolis, M., Barbato, O., Acuti, G., Trabalza-Marinucci, M., Melo de Sousa, N., Canali, C., & Moscati, L. (2020). Plasmatic Profile of Pregnancy-Associated Glycoprotein (PAG) during Gestation and Postpartum in Sarda and Lacaune Sheep Determined with Two Radioimmunoassay Systems. Animals, 10(9), 1502. https://doi.org/10.3390/ani10091502





