A Comparative Study of Intramuscular Alfaxalone- or Ketamine-Based Anesthetic Mixtures in Gray Squirrels Undergoing Gonadectomy: Clinical and Physiologic Findings
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Procedures
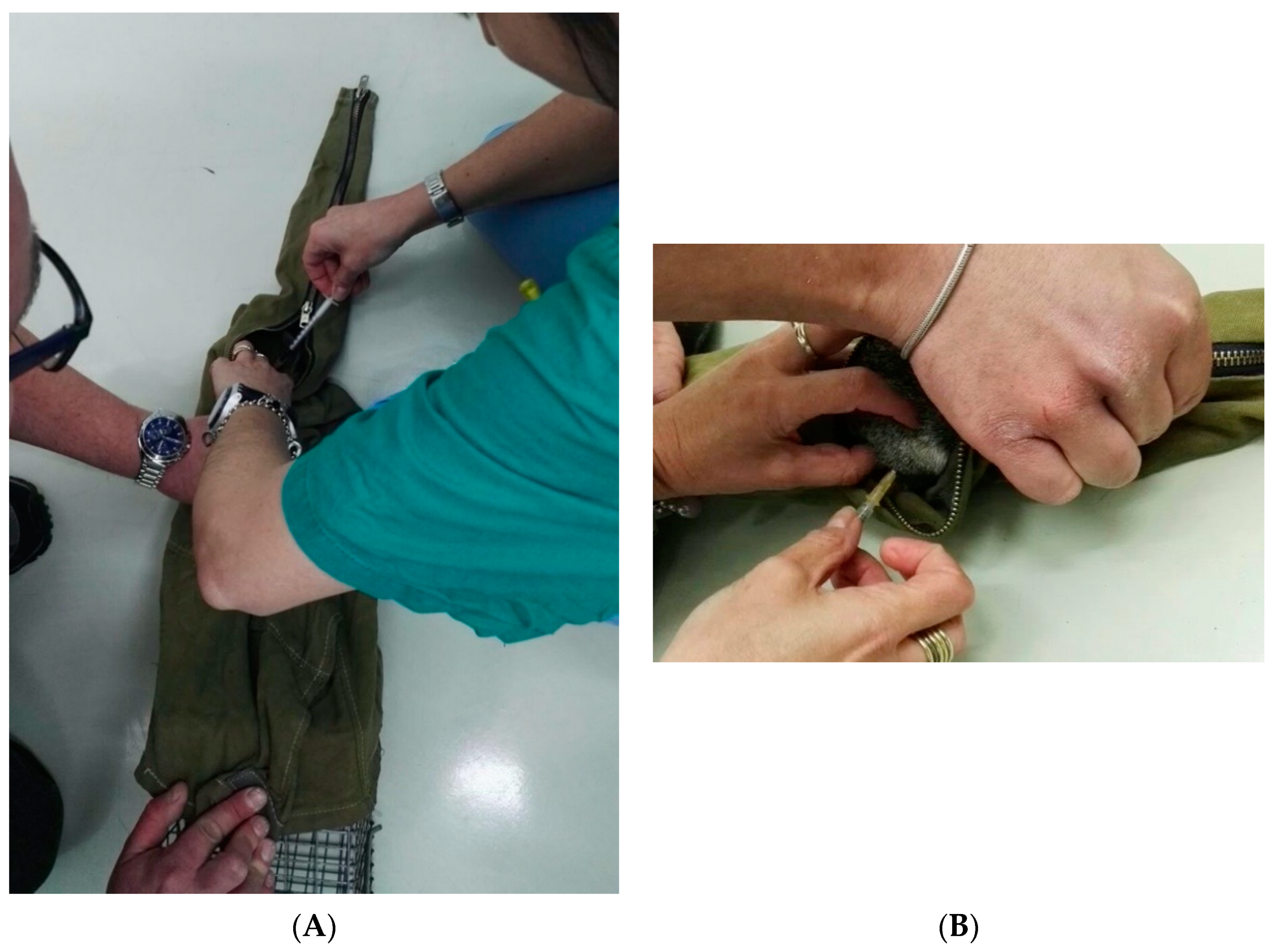
2.2.1. Animal Preparation
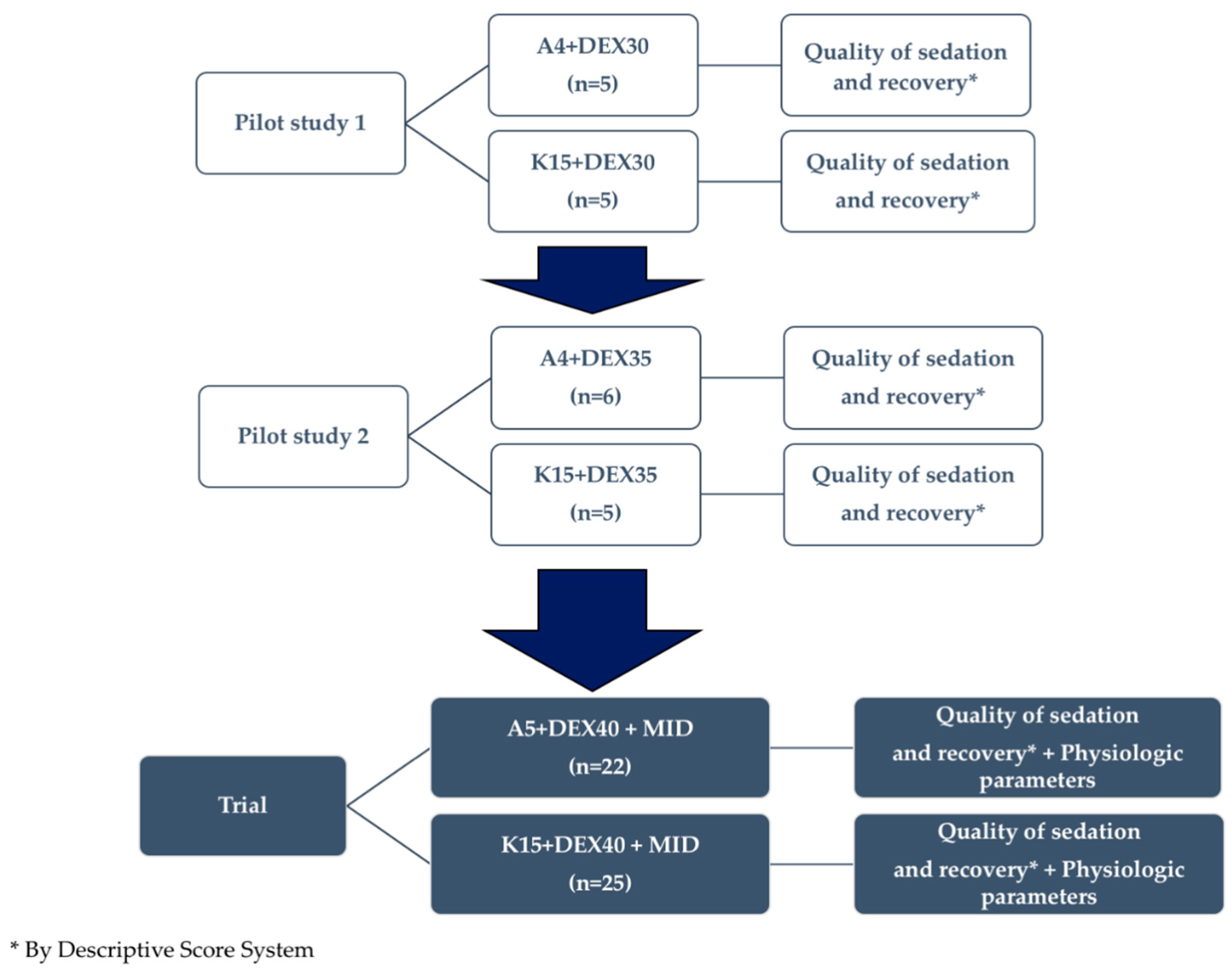
2.2.2. Pilot Studies to Define the Final Anesthetic Mixtures and Final Anesthetic Protocol
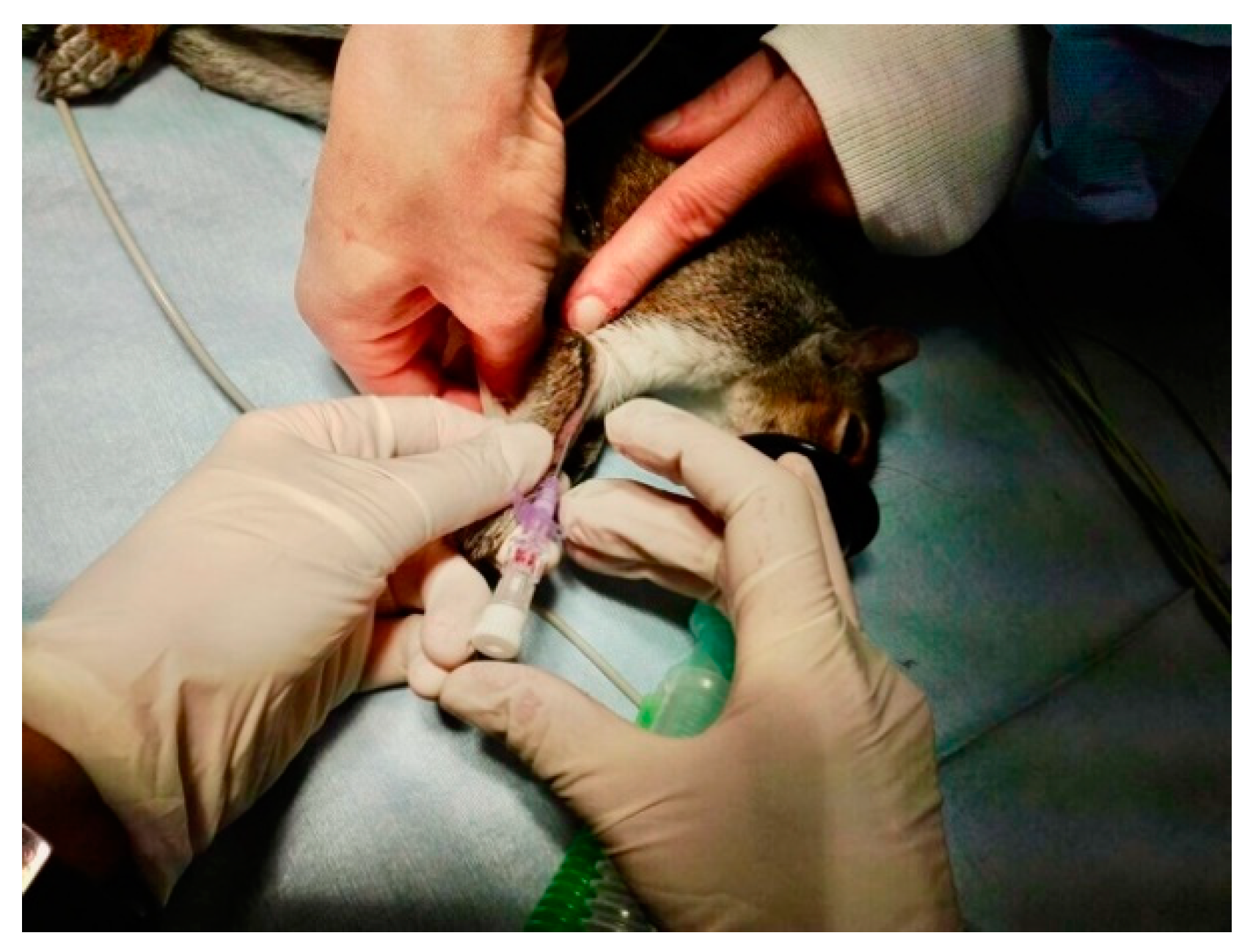
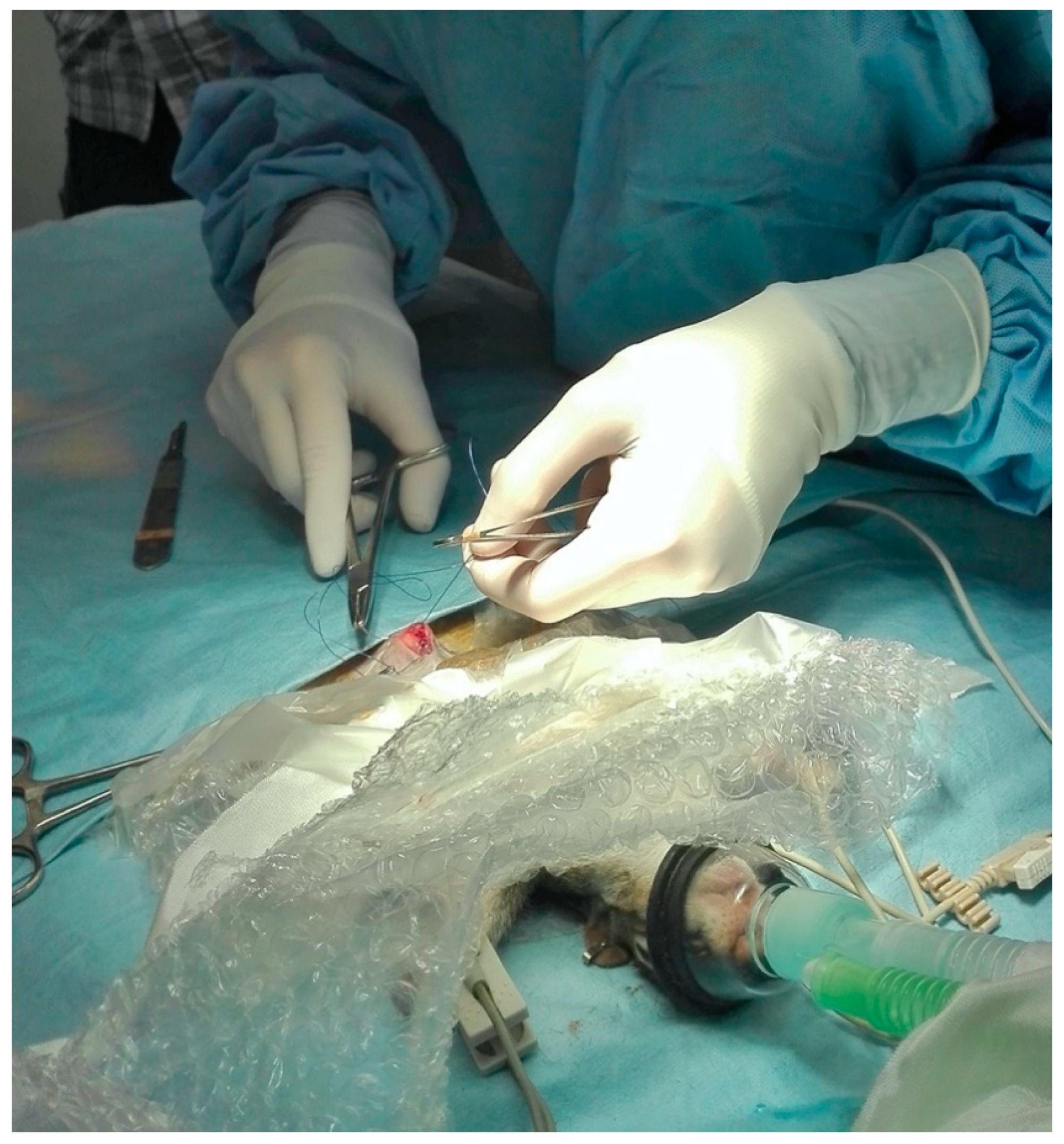
2.2.3. Surgical and Anesthetic Procedure
2.2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Emerton, L.; Howard, G. A Toolkit for the Economic Analysis of Invasive Species; Global Invasive Species Programme: Nairobi, Kenya, 2008. [Google Scholar]
- Vitousek, P.; Mooney, H.; Lubchenco, J.; Melillo, J. Human domination of Earth ecosystems. Science 1997, 277, 494–499. [Google Scholar] [CrossRef]
- Wittenberg, R.; Cock, M.J.W. Invasive Alien Species: A Toolkit of Best Prevention and Management Practices; CABI Publishing: Oxon, UK, 2001. [Google Scholar]
- Scalera, R.; Bevilacqua, G.; Carnevali, L.; Genovesi, P. Le Specie Esotiche Invasive: Andamenti, Impatti e Possibili Risposte; ISPRA: Rome, Italy, 2018; pp. 1–121.
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species a Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2000; pp. 1–12. [Google Scholar]
- Wauters, L.A.; Gurnell, J. The Mechanism of Replacement of Red Squirrels by Grey Squirrels: A Test of the Interference Competition Hypothesis. Ethology 1999, 105, 1053–1071. [Google Scholar] [CrossRef]
- Wauters, L.A.; Tosi, G.; Gurnell, J. A review of the competitive effects of alien grey squirrels on behaviour, activity and habitat use of red squirrels in mixed, deciduous woodland in Italy. Hystrix Ital. J. Mammal. 2005, 16, 27–40. [Google Scholar]
- Gurnell, J.; Pepper, H. A critical look at conserving the British red squirrel Sciurus vulgaris. Mammal. Rev. 1993, 23, 125–136. [Google Scholar] [CrossRef]
- Wauters, L.A.; Somers, L.; Dhondt, A.A. Settlement behaviour and population dynamics of reintroduced red squirrels Sciurus vulgaris in a park in Antwerp, Belgium. Biol. Conserv. 1997, 82, 101–107. [Google Scholar] [CrossRef]
- O’Teangana, D.; Reilly, S.; Montgomery, W.I.; Rochford, J. Distribution and status of the red squirrel (Sciurus vulgaris) and grey squirrel (Sciurus carolinensis) in Ireland. Mammal. Rev. 2000, 30, 45–56. [Google Scholar] [CrossRef]
- Lurz, P.W.W.; Hale, M.L.; Shirley, M.D.F.; Rushton, S.; Fuller, R.M.; Wolff, K. Impact of Landscape Management on the Genetic Structure of Red Squirrel Populations. Science 2001, 293, 2246–2248. [Google Scholar]
- Bertolino, S.; Genovesi, P. Spread and attempted eradication of the grey squirrel (Sciurus carolinensis) in Italy, and consequences for the red squirrel (Sciurus vulgaris) in Eurasia. Biol. Conserv. 2003, 109, 351–358. [Google Scholar] [CrossRef]
- Scapin, P.; Ulbano, M.; Ruggiero, C.; Balduzzi, A.; Marsan, A.; Ferrari, N.; Bertolino, S. Surgical sterilization of male and female grey squirrels (Sciurus carolinensis) of an urban population introduced in Italy. J. Vet. Med. Sci. 2019, 81, 641–645. [Google Scholar] [CrossRef]
- Rushton, S.P.; Lurz, P.W.W.; Gurnell, J.; Nettleton, P.; Bruemmer, C.; Shirley, M.D.F.; Sainsbury, A.W. Disease threats posed by alien species: The role of a poxvirus in the decline of the native red squirrel in Britain. Epidemiol. Infect. 2006, 134, 521–533. [Google Scholar] [CrossRef]
- Koprowski, J.L. Sciurus carolinensis. Mamm. Species 1994, 480, 1–9. [Google Scholar] [CrossRef]
- Clark, R.K.; Jessup, D.A. Wildlife Restraint Series; International Wildlife Veterinary Service: Salinas, CA, USA, 1992. [Google Scholar]
- Mantor, M.; Krause, S.; Hart, L.A. Trapping and handling squirrels: Trap modification and handling restraint to minimize injuries and stress. Wildl. Soc. Bull. 2014, 38, 152–159. [Google Scholar] [CrossRef]
- Barter, L.S.; Epstein, S.E. Cardiopulmonary effects of three concentrations of isoflurane with or without mechanical ventilation and supramaximal noxious stimulation in New Zealand white rabbits. Am. J. Vet. Res. 2013, 74, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Arenz, C.L. Handling fox squirrels: Ketamine-hydrochloride versus a simple restraint. Wildl. Soc. Bull. 1997, 25, 107–109. [Google Scholar]
- Belant, J.L. Field immobilization of raccoons (Procyon lotor) with telazol and xylazine. J. Wildl. Dis. 2004, 40, 787–790. [Google Scholar] [CrossRef]
- White, P.F.; Ham, J.; Way, W.L.; Trevor, A.J. Pharmacology of ketamine isomers in surgical patients. Anesthesiology 1980, 52, 231–239. [Google Scholar] [CrossRef]
- Green, C.J.; Knight, J.; Precious, S.; Simpkin, S. Ketamine alone and combined with diazepam or xylazine in laboratory animals: A 10-year experience. Lab. Anim. 1981, 15, 163–170. [Google Scholar] [CrossRef]
- Dupras, J.; Vachon, P.; Cuvelliez, S.; Blais, D. Anesthesie du lapin de Nouvelle-Zelande utilisant les combinaisons tiletamine-zolazepam et ketamine-midazolam avec ou sans xylazine. Can. Vet. J. 2001, 42, 455–460. [Google Scholar]
- Visser, S.A.; Smulders, C.J.; Reijers, B.P.; Van der Graaf, P.H.; Peletier, L.A.; Danhof, M. Mechanism-based pharmacokinetic–pharmacodynamic modeling of concentration-dependent hysteresis and biphasic electroencephalogram effects of alphaxalone in rats. J. Pharmacol. Exp. Ther. 2002, 302, 1158–1167. [Google Scholar] [CrossRef]
- Lau, C.; Ranasinghe, M.G.; Shiels, I. Plasma pharmacokinetics of alfaxalone after a single intraperitoneal or intravenous injection of Alfaxan® in rats. J. Vet. Pharmacol. Ther. 2013, 36, 516–520. [Google Scholar] [CrossRef]
- Tamura, J.; Ishizuka, T.; Fukui, S.; Oyama, N.; Kawase, K.; Miyoshi, K.; Sano, T.; Pasloske, K.; Yamashita, K. The pharmacological effects of the anesthetic alfaxalone after intramuscular administration to dogs. J. Vet. Med. Sci. 2015, 77, 289–296. [Google Scholar] [CrossRef]
- Khenissi, L.; Nikolayenkova-Topie, O.; Broussaud, S.; Touzot-Jourde, G. Comparison of intramuscular alfaxalone and ketamine combined with dexmedetomidine and butorphanol for castration in cats. J. Feline Med. Surg. 2017, 19, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Giral, M.; Garcia-Olmo, D.C.; Gomez-Juarez, M.; Gomez de Segura, I.A. Anaesthetic effects in the ferret of alfaxalone alone and in combination with medetomidine or tramadol: A pilot study. Lab. Anim. 2014, 48, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.; Poumeyrol, S.; Pignon, C.; Le Teuff, G.; Zilberstein, L. Intramuscular administration of alfaxalone for sedation in rabbits. Vet. Rec. 2015, 176, 255. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.E., 3rd; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar] [PubMed]
- Bufalari, A.; Maranesi, M.; Marenzoni, M.L.; Mercati, F.; Nannarone, S.; Dall’Aglio, C.; Bellocchi, F.; Zerani, M.; Petrucci, L.; Paoloni, D.; et al. Traditional gonadectomy vs. gonadectomy by Ligasure® in grey squirrels (Sciurus carolinensis): Comparing surgical techniques. Reprod. Domest. Anim. 2019, 54 (Suppl. S3), 137. [Google Scholar]
- Parker, W.T.; Muller, L.I.; Gerhardt, R.R.; O’rourke, D.P.; Ramsay, E.C. Field Use of Isoflurane for Safe Squirrel and Woodrat Anesthesia. J. Wildl. Manag. 2007, 72, 1262–1266. [Google Scholar] [CrossRef]
- Smith, E.N.; Johnson, C. Fear bradycardia in the eastern fox squirrel, Sciurus niger, and eastern grey squirrel, S. carolinesis. Comp. Biochem. Physiol. 1984, 78, 409–411. [Google Scholar] [CrossRef]
- Hoff, G.L.; Lassing, E.B.; Chan, M.S.; Bigler, W.J.; Doyle, T.J. Hematologic values for free-ranging urban gray squirrels (Sciurus carolinensis). Am. J. Vet. Res. 1976, 37, 99–101. [Google Scholar]
- Bolls, N.J.; Perfect, J.R. Summer Resting Metabolic Rate of the Gray Squirrel. Physiol. Zool. 1972, 45, 54–59. [Google Scholar] [CrossRef]
- Edling, T.M. Advances in anesthesia monitoring in birds, reptiles, and small mammals. Exotic DVM 2003, 5, 15–20. [Google Scholar]
- Fish, R.E.; Brown, M.J.; Danneman, P.J.; Karas, A.Z. Anesthesia and Analgesia in Laboratory Animals, 2nd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Pinelas, R.; Alibhai, H.I.; Mathis, A. Effects of different doses of dexmedetomidine on anaesthetic induction with alfaxalone—A clinical trial. Vet. Anaesth. Analg. 2014, 41, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Arenillas, M.; Gomez de Segura, I.A. Anaesthetic effects of alfaxalone administered intraperitoneally alone or combined with dexmedetomidine and fentanyl in the rat. Lab. Anim. 2018, 52, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Mans, C. Anesthetic and Postanesthetic Effects of Alfaxalone–Butorphanol Compared with Dexmedetomidine–Ketamine in Chinchillas (Chinchilla lanigera). J. Am. Assoc. Lab. Anim. Sci. 2017, 56, 290–295. [Google Scholar]
- Siriarchavatana, P.; Ayers, J.D.; Kendall, L.V. Anesthetic Activity of Alfaxalone Compared with Ketamine in Mice. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 426–430. [Google Scholar]
- Jones, K.L. Therapeutic review: Alfaxalone. J. Exot. Pet Med. 2012, 21, 347–353. [Google Scholar] [CrossRef]



| Score | Myorelaxation |
| 1 | Absent: Muscle tone in the pelvic and thoracic limbs, pedal reflex, head movement |
| 2 | Mild: Some muscle tone in thoracic limbs and pedal reflex |
| 3 | Excellent: No muscle tone, completely relaxed animal |
| Score | Depth of Anesthesia |
| 1 | Inadequate: Responsive to manipulation in the capture-sleeve (legs and paw pinch). Additional ½ dose is required |
| 2 | Mild: Purposeful response to manipulation (legs and paw pinch). Additional ½ dose is required |
| 3 | Profound: Relaxed and unresponsive to manipulation (legs and paw pinch). |
| Score | Recovery Time |
| 1 | Poor: Longer than 60 min from atipamezole |
| 2 | Good: From 10 to 60 min from atipamezole |
| 3 | Excellent: Within 10 min from atipamezole |
| Score | Recovery Quality |
| 1 | Poor: Long lasting lateral recumbency and diffuse tremors |
| 2 | Good: Lateral or sternal recumbency associated with head tossing and uncoordinated legs movements, minor tremors, or ataxia once standing |
| 3 | Excellent: Quickly standing and climbing, no tremors |
| Parameter | Group | ||
|---|---|---|---|
| K | A | ||
| Gender | Female (n, %) | 13 (52.0%) | 11 * (50.0%) |
| Male (n, %) | 12 (48.0%) | 11 (50.0%) | |
| Body weight (g; mean ± SD (range)) | 469 ± 73 (311–600) | 436 ± 78 (245–579) | |
| Age | Young | 8 (32.0%) | 8 (36.4%) |
| Adult | 17 (68.0%) | 14 (63.6%) | |
| HR (beats/min; mean ± SD) | 264 ± 52 | 250 ± 40 | |
| RR (breaths/min; mean ± SD) | 119 ± 55 | 82 ± 43 | |
| SpO2 (%; mean ± SD) | 96 ± 6 | 98 ± 1 | |
| Temperature (°C; mean ± SD) | 38 ± 1 | 38 ± 1 | |
| Parameter | Group | Significance | ||||
|---|---|---|---|---|---|---|
| K | A | Group | Gender | Capture Day # | ||
| Pre-existing injuries | No | 19 (76.0%) | 21 (95.5%) | 0.095 | 0.068 | 0.755 |
| Yes | 6 (24.0%) | 1 (4.5%) | ||||
| Additional anesthetics, 8 min from T0 | No | 25 (100.0%) | 18 (81.8%) | 0.999 | 0.683 | 0.999 |
| Yes | 0 (0.0%) | 4 (18.2%) | ||||
| Laparotomy | No | 10 (40.0%) | 9 (40.9%) | 1.000 | 0.998 | 0.999 |
| Yes | 15 (60.0%) | 13 (59.1%) | ||||
| Ligasure | No | 19 (76.0%) | 15 (75.0%) | 0.989 | 0.216 | 0.369 |
| Yes | 6 (24.0%) | 5 (25.0%) | ||||
| Sevoflurane administration (min tot) | 2 ± 1 | 3 ± 1 | 0.176 | 0.331 | 0.839 | |
| Anesthesia duration (min) | 34 ± 2 | 31 ± 2 | 0.278 | <0.001 * | 0.442 | |
| Surgery duration (min) | 14 ± 1 | 13 ± 1 | 0.309 | <0.001 † | 0.925 | |
| Time to achieve sternal recumbency (min from atipamezole) | 17 ± 13 | 81 ± 13 | <0.001 | 0.493 | 0.298 | |
| Time to stand up (min from atipamezole) | 17 ± 9 | 96 ± 9 | <0.001 | 0.482 | 0.693 | |
| Time to climb the cage (min from atipamezole) | 21 ± 12 | 137 ± 14 | <0.001 | 0.422 | 0.494 | |
| Parameter | Group | Baseline Value * | Significance | |||||
|---|---|---|---|---|---|---|---|---|
| K | A | Group | Gender | Capture Day # | Baseline Value | Time | ||
| HR (beats/min) | 219 ± 3 | 221 ± 3 | 256 | 0.646 | 0.272 | 0.043 † | <0.001 | <0.001 |
| RR (breaths/min) | 108 ± 4 | 84 ± 4 | 100 | <0.001 | 0.274 | 0.793 | <0.001 | 0.790 |
| SpO2 (%) | 98 ± 0 | 98 ± 0 | 97 | 0.932 | 0.400 | 0.935 | 0.422 | 0.669 |
| Temp. (°C) | 36.8 ± 0.1 | 37.1 ± 0.1 | 38.3 | 0.037 | 0.637 | 0.076 | <0.001 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nannarone, S.; Moretti, G.; Bellocchi, F.; Menchetti, L.; Bufalari, A. A Comparative Study of Intramuscular Alfaxalone- or Ketamine-Based Anesthetic Mixtures in Gray Squirrels Undergoing Gonadectomy: Clinical and Physiologic Findings. Animals 2020, 10, 1402. https://doi.org/10.3390/ani10081402
Nannarone S, Moretti G, Bellocchi F, Menchetti L, Bufalari A. A Comparative Study of Intramuscular Alfaxalone- or Ketamine-Based Anesthetic Mixtures in Gray Squirrels Undergoing Gonadectomy: Clinical and Physiologic Findings. Animals. 2020; 10(8):1402. https://doi.org/10.3390/ani10081402
Chicago/Turabian StyleNannarone, Sara, Giulia Moretti, Federica Bellocchi, Laura Menchetti, and Antonello Bufalari. 2020. "A Comparative Study of Intramuscular Alfaxalone- or Ketamine-Based Anesthetic Mixtures in Gray Squirrels Undergoing Gonadectomy: Clinical and Physiologic Findings" Animals 10, no. 8: 1402. https://doi.org/10.3390/ani10081402
APA StyleNannarone, S., Moretti, G., Bellocchi, F., Menchetti, L., & Bufalari, A. (2020). A Comparative Study of Intramuscular Alfaxalone- or Ketamine-Based Anesthetic Mixtures in Gray Squirrels Undergoing Gonadectomy: Clinical and Physiologic Findings. Animals, 10(8), 1402. https://doi.org/10.3390/ani10081402








