Relationship between the Quality of Colostrum and the Formation of Microflora in the Digestive Tract of Calves
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbiological Assay
2.2. Chemical Analyses of Colostrum
2.3. Statistical Analyses
- 1st class colostrum density 1.070 g/cm3: 23 calves
- 2nd class colostrum density 1.057–1.070 g/cm3: 25 calves
- 3rd class colostrum density 1.044–1.056 g/cm3: 27 calves
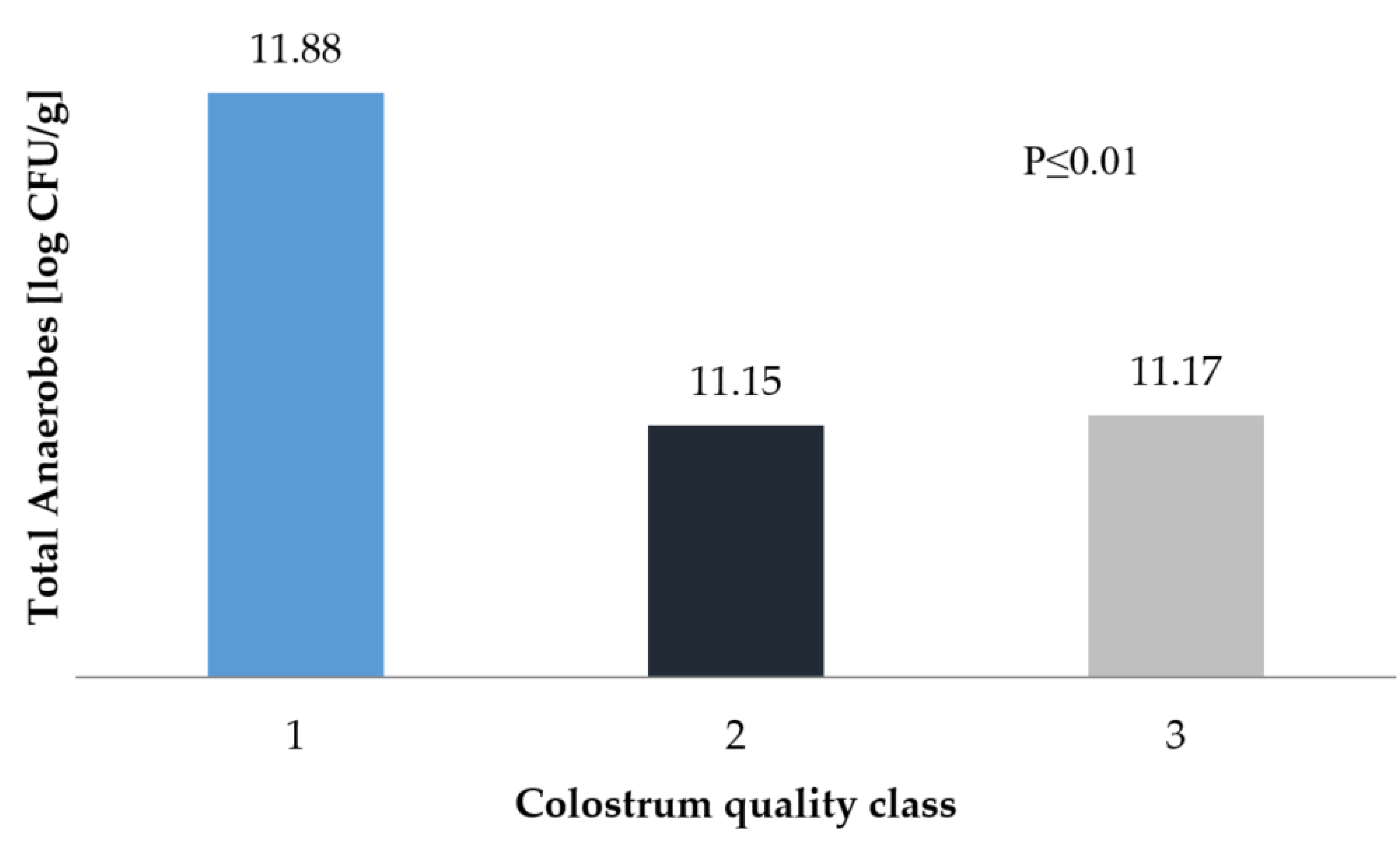
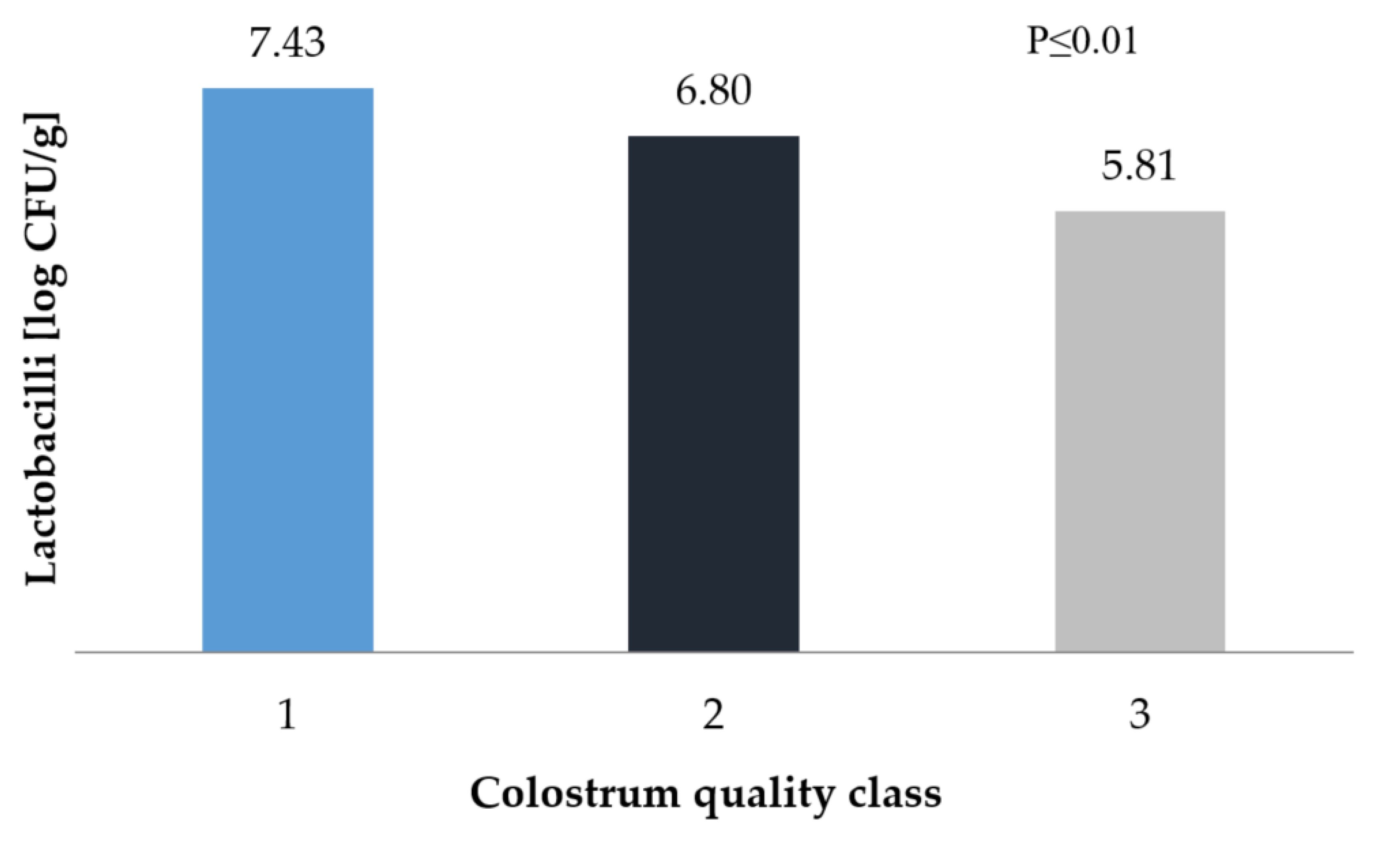
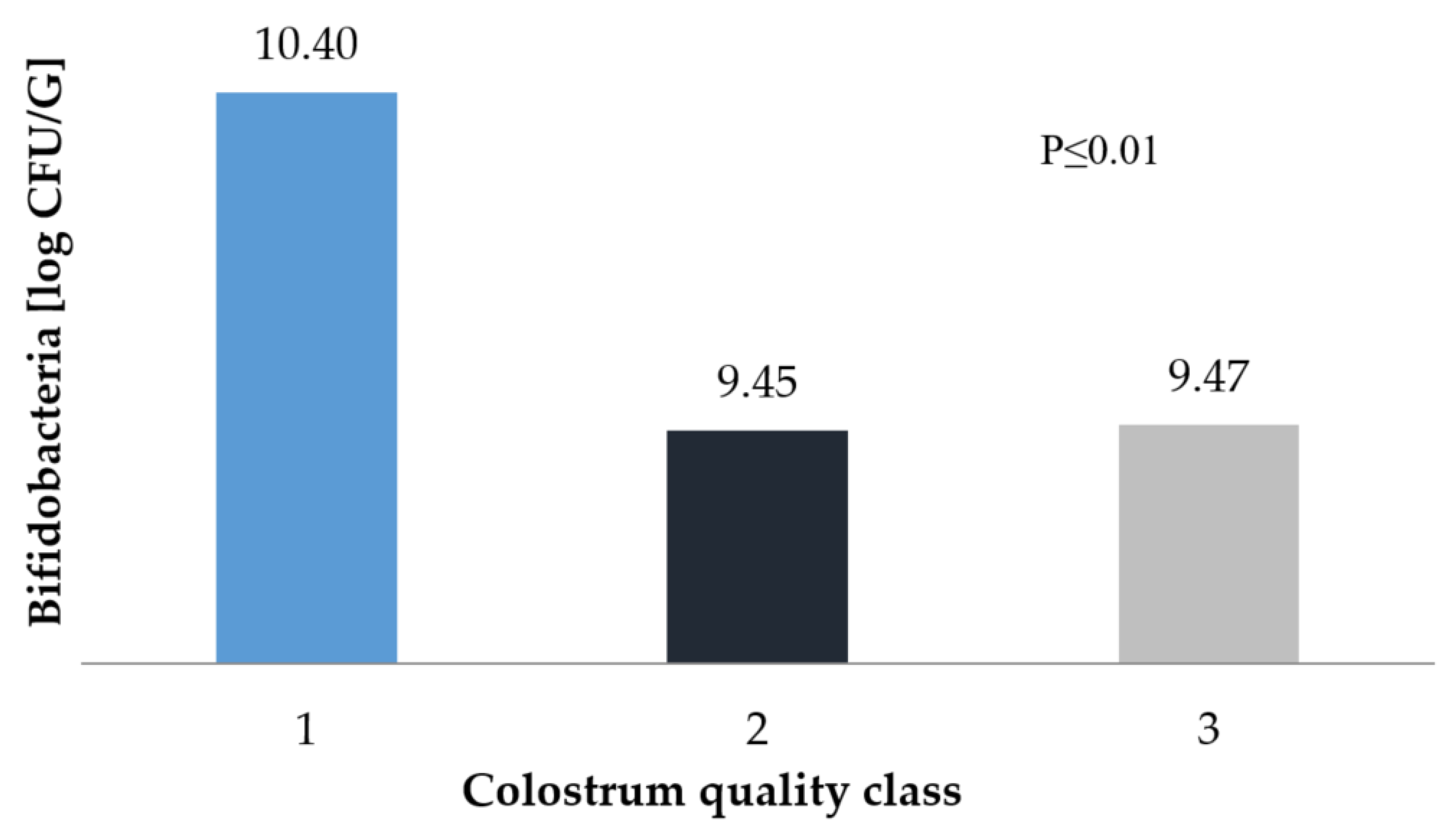
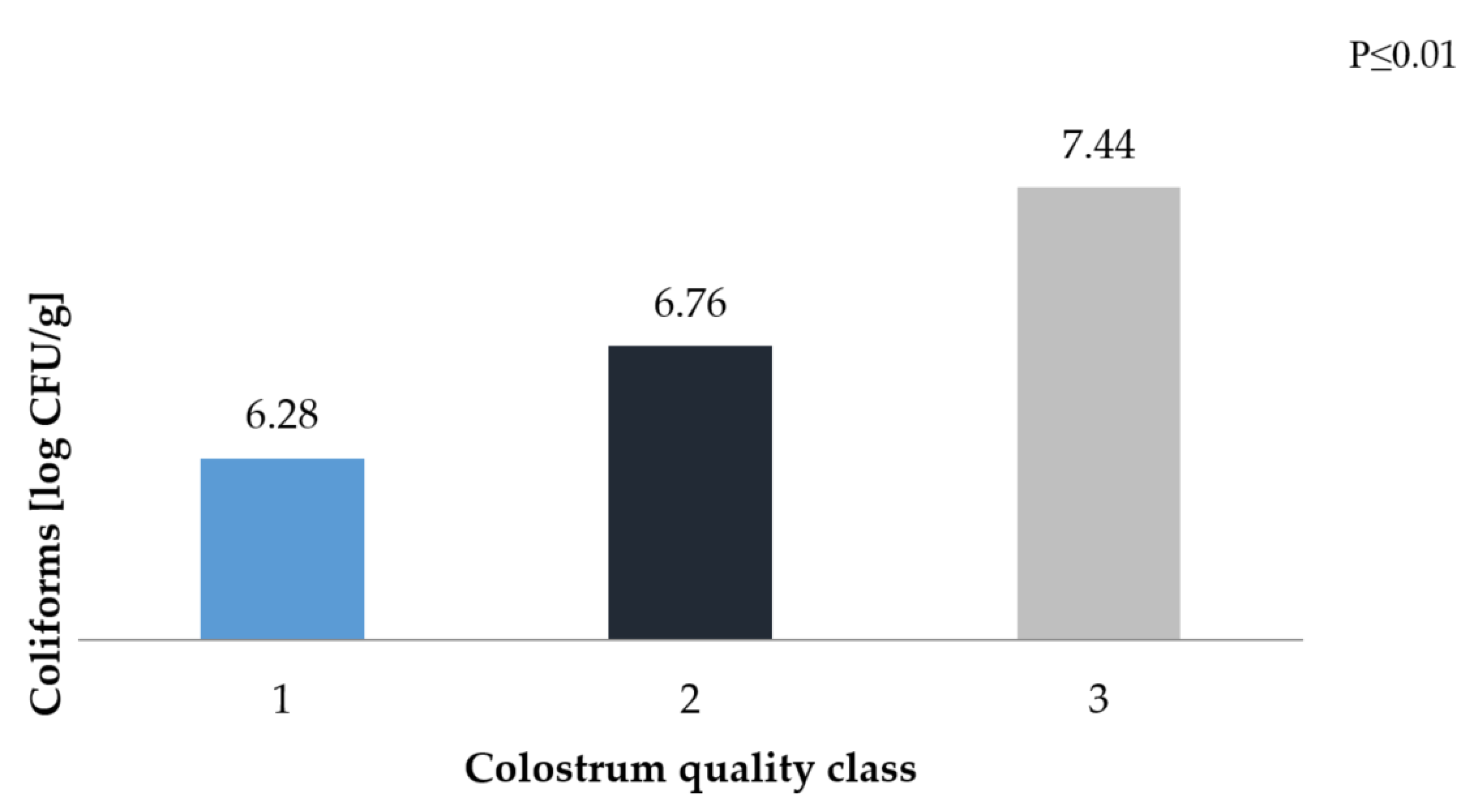
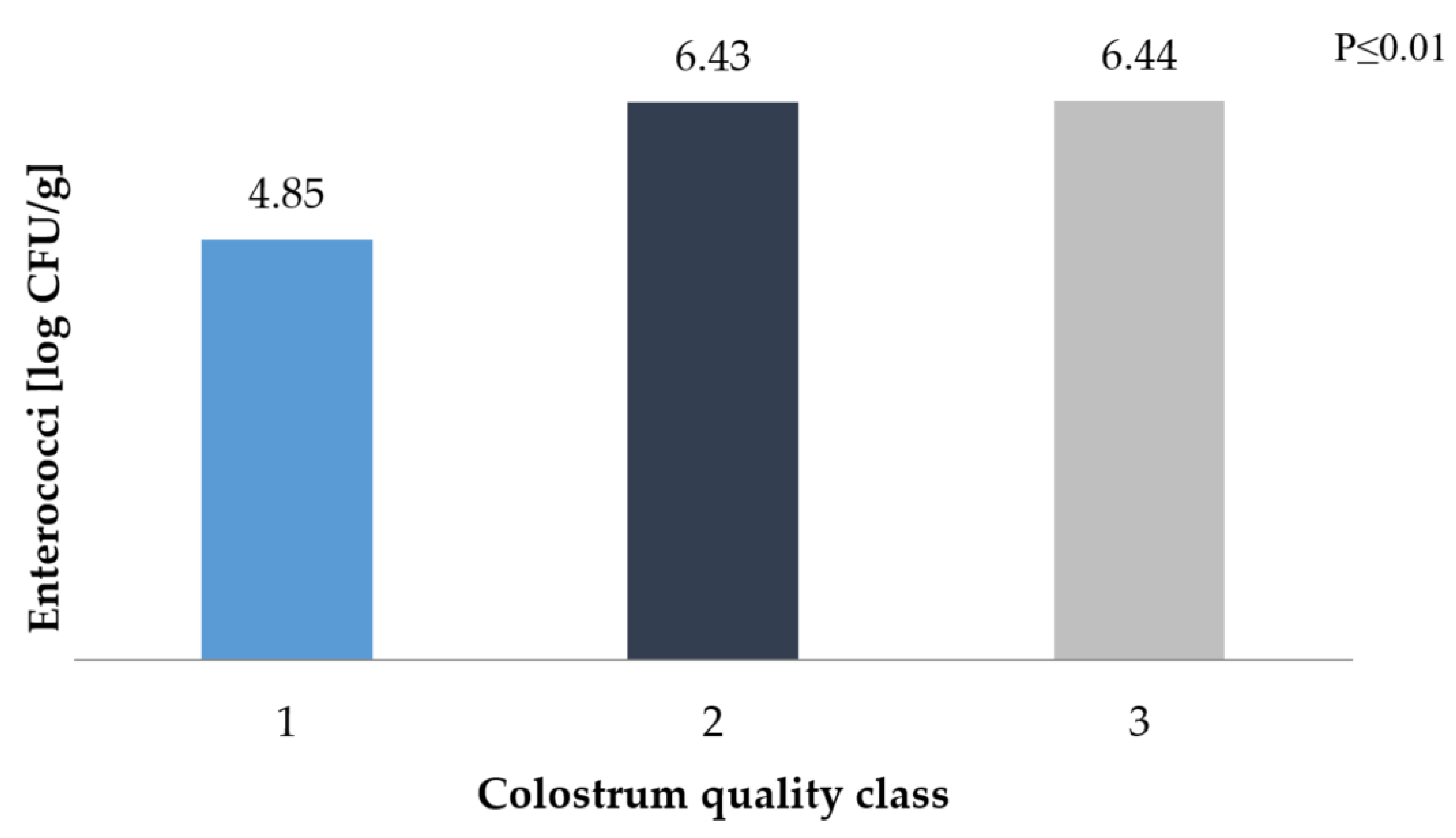
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maunsell, F.P.; Morin, D.E.; Constable, P.D.; Hurley, W.L.; McCoy, G.C.; Kakoma, I.; Isaacson, R.E. Effects of mastitis on the volume and composition of colostrum produced by Holstein cows. J. Dairy Sci. 1998, 81, 1291–1299. [Google Scholar] [CrossRef]
- Godden, S. Colostrum Managment for Dairy Calves. Vet. Clinic. Food Anim. Pract. 2008, 24, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Lacetera, N.; Bernabucci, U.; Ronchi, B. Composition of colostrum from dairy heifers exposed to high air temperatures during late pregnancy and the early postpartum period. J. Dairy Sci. 1997, 80, 838–844. [Google Scholar] [CrossRef]
- McGuirk, S.M.; Collins, M. Managing the production; storage and delivery of colostrum. Vet. Clinic. N. Am. Food Anim. Pract. 2004, 20, 593–603. [Google Scholar] [CrossRef]
- Georgiev, I.P. Differences in chemical composition between cow colostrum and milk. Bulg. J. Vet. Med. 2008, 11, 3–12. [Google Scholar]
- Bar, E.; Foris, I.; Miron, M.; Bojita, M.; Mihai, G. Determination of the Content of Immunoglobulin (IgG) and Lactoferrin. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Anim. Sci. Biotechnol. 2009, 66, 1–2. [Google Scholar]
- Artym, J.; Zimecki, M. Rola laktoferryny w prawidłowym rozwoju noworodka the role of lactoferrin in the proper development of newborns. Postepy Hig. Med. Doświad. 2005, 59, 421–432. [Google Scholar]
- Chaterton, D.E.; Smithers, G.; Roupas, P.; Brodkorb, A. Bioactivity of β-lactoglobulin and α-lactalbumin- Technological implications for processing. Int. Dairy J. 2006, 16, 1229–1240. [Google Scholar] [CrossRef]
- Fleming, K.; Thompson-Crispi, K.A.; Hodgins, D.C.; Miglior, F.; Corredig, M.; Mallard, B.A. Short communication: Variation of total immunoglobulin G and β-lactoglobulin concentrations in colostrum and milk from Canadian Holsteins classified as high; average; or low immune responders. J. Dairy Sci. 2016, 99, 2358–2363. [Google Scholar] [CrossRef]
- Wąsowska, E.; Puppel, K. Changes In the content of immunostimulating components of colostrum obtained from dairy cows at different levels of production. J. Sci. Food Agric. 2018, 98, 5062–5068. [Google Scholar] [CrossRef]
- Goel, K. Characterisation and Process Optimisation for the Production of Kharwas-a Colostrum Based Delicacy. Ph.D. Thesis, ICAR–National Dairy Research Institute, Karnal, India, 2015; pp. 6–32. [Google Scholar]
- Caffin, J.P.; Poutrel, B.; Rainard, P. Physiological and pathological factors influencing bovine α-lactalbumin and β-lactoglobulin concentrations in milk. J. Dairy Sci. 1985, 68, 1087–1094. [Google Scholar] [CrossRef]
- Król, J.; Litwińczuk, A.; Zarajczyk, A.; Litwińczuk, Z. Alfa-laktoalbumina i beta-laktoglobulina jako związki biologicznie czynne frakcji białkowej mleka. Med. Wet. 2008, 12, 1375–1378. [Google Scholar]
- Chaneton, L.; Sáez, J.P.; Bussmann, L.E. Antimicrobial activity of bovine β-lactoglobulin against mastitis-causing bacteria. J. Dairy Sci. 2011, 94, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Puppel, K.; Gołębiewski, M.; Grodkowski, G.; Slósarz, J.; Kunowska-Slósarz, M.; Solarczyk, P.; Łukasiewicz, M.; Balcerak, M.; Przysucha, T. Composition and Factors Affecting Quality of Bovine Colostrum: A Review. Animals 2019, 9, 1070. [Google Scholar] [CrossRef]
- Yeoman, C.J.; White, B.A. Gastrointestinal tract microbiota and probiotics in production animals. Ann. Rev. Anim. Biosci. 2014, 2, 469–486. [Google Scholar] [CrossRef]
- Francino, M.P. Early development of the gut microbiota and immune health. Pathogens 2014, 3, 769–790. [Google Scholar] [CrossRef]
- Nowacki, M.R. Cell proliferation in colonic crypts of germ-free and conventional mice-preliminary report. Folia Histochem. Cytobiol. 1993, 31, 77–81. [Google Scholar]
- Sommer, F.; Bäckhed, F. The gut microbiota- masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Nowak, A.; Slizewska, K.; Libudzisz, Z. Probiotyki-historia i mechanizmy działania. Żywność Nauka Technologia Jakość 2010, 17, 5–19. [Google Scholar]
- Gerdts, V.; Babiuk, L.A.; van Drunen Littel-van den Hurk, S.; Griebel, P.J. Fetal immunization by a DNA vaccine delivered orally into the amniotic fluid. Nat. Med. 2000, 6, 929–932. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Griebel, P.J.; Guan, L.L. The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front. Vet. Sci. 2015, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, G.; Teixeira, A.G.V.; Foditsch, C.; Bicalho, M.L.; Machado, V.S.; Bicalho, R.C. Fecal microbial diversity in pre-weaned dairy calves as described by pyrosequencing of metagenomic 16S rDNA. Associations of Faecalibacterium species with health and growth. PLoS ONE 2013, 8, e63157. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Różańska, H.; Lewtak-Piłat, A.; Osek, J. Enterokoki- bakterie o wielu obliczach. Życie Weterynaryjne 2013, 88, 562–564. [Google Scholar]
- Kłossowska, A.; Malinowski, E. Shigatoksyczne szczepy Escherichia coli w infekcjach gruczołu mlekowego u krów. Med. Wet. 2007, 63, 1108–1110. [Google Scholar]
- Frydendahl, K. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet. Microbiol. 2002, 85, 169–182. [Google Scholar] [CrossRef]
- Moxley, R.A.; Francis, D.H. Natural and experimental infection with an attaching and effacing strain of Escherichia coli in calves. Infect. Immun. 1986, 53, 339–346. [Google Scholar] [CrossRef]
- Smith, H.W. The development of the flora of the alimentary tract in young animals. J. Pathol. Bacteriol. 1965, 90, 495–513. [Google Scholar] [CrossRef]
- Tannock, G.W. Effect of dietary and environmental stress on the gastrointestinal microbiota. Hum. Intest. Microflora Health Dis. 1983, 517–539. [Google Scholar]
- Franczuk, A.; Jagusztyn-Krynicka, E.K. Rola mikroflory jelit w indukcji choroby Leśniowskiego-Crohna w świetle programu badań Human Microbiome Project. Postępy Mikrobiol. 2012, 51, 257–264. [Google Scholar]
- Hamouda, R.H.; Thannaa, K.H.; Nabih, A.M. Bacteriological and pathological studies on some aerobic and anaerobic bacteria causing diarrhoea in camel calves. Vet. Med. J. Giza 2010, 58, 177–197. [Google Scholar]
- Bashahun, G.M.; Amina, A. Colibacillosis in calves: A review of literature. J. Vet. Med. Sci. 2017, 2, 62–71. [Google Scholar] [CrossRef]
- Abe, F.; Ishibashi, N.; Shimamura, S. Effect of administration of Bifidobacteria and Lactic acid bacteria to newborn calves and piglets. J. Dairy Sci. 1995, 73, 2838–2846. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Fermisse, O.; Nion-Larmurier, I.; Beaugerie, L. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B. Gut Immune maturation depends on colonization with a host-specific microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef] [PubMed]
- Godden, S.M.; Smolenski, D.J.; Donahue, M.; Oakes, J.M.; Bey, R.; Wells, S.; Sreevatsan, S.; Stabel, J.; Fetrow, J. Heat-treated colostrum and reduced morbidity in preweaned dairy calves: Results of a randomized trial and examination of mechanisms of effectiveness. J. Dairy Sci. 2012, 95, 4029–4040. [Google Scholar] [CrossRef]
- Mohankumar, A.; Murugalatha, N. Characterization and antibacterial activity of bacteriocin producing Lactobacillus isolated from raw cattle milk sample. Int. J. Biol. 2011, 3, 128–143. [Google Scholar] [CrossRef]
- Ziarno, M.; Godlewska, A. Znaczenie i wykorzystani bakterii rodzaju Lactoccocus w mleczarstwie. Med. Wet. 2008, 64, 35–39. [Google Scholar]
- Puppel, K.; Bogusz, E.; Golebiewski, M.; Nalecz-Tarwacka, T.; Kuczynska, B.; Slosarz, J.; Budzinski, A.; Solarczyk, P.; Kunowska-Slosarz, M.; Przysucha, T. Effect of Dairy Cow Crossbreeding on Selected Performance Traits and Quality of Milk in First Generation Crossbreds. J. Food Sci. 2018, 83, 229–236. [Google Scholar] [CrossRef]
- IBM Crop. Released IBM SPSS for Windows, version 23.0; IBM Crop: Armonk, NY, USA, 2020. [Google Scholar]
- Demkowicz, M. Bioaktywne Preparaty z Siary Krów Zachowujące Parametry jej Wartości Biologicznej Oraz ich Przyswajalność u Jagniąt i Cieląt. Praca Doktorska. 2012, pp. 13–32. Available online: http://www.dbc.wroc.pl/dlibra/doccontent?id=20342 (accessed on 29 June 2020).
- Wroński, M.; Sosnowska, W. Relationships between the density and composition of colostrums and the performance of calves. Pol. J. Nat. Sci. 2008, 23, 573–582. [Google Scholar] [CrossRef]
- Pecka, E.; Zachwieja, A.; Zawadzki, W.; Kaszuba, J.; Tumanowicz, J. Wpływ stadium laktacji na wydajność i właściwości fizykochemiczne oraz skład podstawowy mleka krów pierwiastek. Acta Sci. Pol. Med. Vet. 2012, 11, 5–14. [Google Scholar]
- Hyrslova, I.; Krausova, G.; Bartova, J.; Kolesar, L.; Curda, L. Goat and bovine colostrum as a basis for new probiotic functional foods and dietary supplements. J. Microb. Biochem. Technol. 2016, 8, 56–59. [Google Scholar]
- Fonty, G.; Gouet, P.; Jouany, J.P.; Senaud, J. Establishment of the microflora and anaerobic fungi in the rumen of lambs. Microbiology 1987, 133, 1835–1843. [Google Scholar] [CrossRef]
- Minato, H.; Otsuka, M.; Shirasaka, S.; Itabashi, H.; Mitsumori, M. Colonization of microorganisms in the rumen of young calves. J. Gen. App. Microbiol. 1992, 38, 447–456. [Google Scholar] [CrossRef]
- Anderson, K.L.; Nagaraja, T.G.; Morrill, J.L.; Avery, T.B.; Galitzer, S.J.; Boyer, J.E. Ruminal microbial development in conventionally or early-weaned calves. J. Anim. Sci. 1987, 64, 1215–1226. [Google Scholar] [CrossRef]
- Teraguchi, S.; Shin, K.; Ozawa, K.; Nakamura, S.; Fukuwatari, Y.; Tsuyuki, S.; Namihira, H.; Shimamura, S. Bacteriostatic effect of orally administered bovine lactoferrin on proliferation of Clostridium species in the gut of mice fed bovine milk. Appl. Environ. Microbiol. 1995, 61, 501–506. [Google Scholar] [CrossRef]
- Flint, H.J.; Bayer, E.A. Plant cell wall breakdown by anaerobic microorganisms from the mammalian digestive tract. Ann. N. Y. Acad. Sci. 2008, 1125, 280–288. [Google Scholar] [CrossRef]
- Higginbotham, G.E.; Bath, D.L. Evaluation of Lactobacillus fermentation cultures in calf feeding systems. J. Dairy Sci. 1993, 76, 615–620. [Google Scholar] [CrossRef]
- Ellinger, D.K.; Muller, L.D.; Glantz, P.J. Influence of feeding fermented colostrum and Lactobacillus acidophilus on fecal flora of dairy calves. J. Dairy Sci. 1980, 63, 478–482. [Google Scholar] [CrossRef]
- Vlková, E.; Rada, V.; Trojanová, I.; Killer, J.; Šmehilová, M.; Molatová, Z. Occurrence of bifidobacteria in faeces of calves fed milk or a combined diet. Arch. Anim. Nutr. 2008, 62, 359–365. [Google Scholar] [CrossRef]
- Suo, C.; Yin, Y.; Wang, X.; Lou, X.; Song, D.; Wang, X.; Gu, Q. Effects of lactobacillus plantarumZJ316 on pig growth and pork quality. BMC Vet. Res. 2012, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Godden, S.; Molitor, T.; Ames, T.; Hagman, D. Effects of feeding heat-treated colostrum on passive transfer of immune and nutritional parameters in neonatal dairy calves. J. Dairy Sci. 2007, 90, 5189–5198. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, S.E.; Bruce, B.B.; Bush, L.J.; Stale, T.E. Comparisons of Two Strains of Lactobacillus acidophilus as Dietary Adjuncts for Young Calves. J. Dairy Sci. 1980, 63, 964–972. [Google Scholar] [CrossRef]
- Gregory, N.G.; Jacobson, L.H.; Nagle, T.A.; Muirhead, R.W.; Leroux, G.J. Effect of preslaughter feeding system on weight loss; gut bacteria; and the physico-chemical properties of digesta in cattle. N. Z. J. Agric. Res. 2000, 43, 351–361. [Google Scholar] [CrossRef]
| Specification | Dry Cow Groups | |
|---|---|---|
| I | III | |
| Assumptions | ||
| Cow weight (kg) | 640 | 675 |
| Pregnancy (days) | 220 | 270 |
| Maintenance requirements | ||
| NEL (Mcal/day) | 9.8 | 11.6 |
| Metabolic protein (g/day) | 463 | 653 |
| Ca (g/day) | 11 | 16.5 |
| P (g/day) | 12 | 16.3 |
| K (g/day) | 54 | 55 |
| Foetus requirements | ||
| NEL (Mcal/day) | 2.9 | 3 |
| Metabolic protein (g/day) | 239 | 245 |
| Ca (g/day) | 4 | 5 |
| P (g/day) | 3 | 4 |
| K (g/day) | 1 | 2 |
| Total requirements | ||
| NEL (Mcal/day) | 12.8 | 14.4 |
| Metabolic protein (g/day) | 700 | 901 |
| Ca (g/day) | 15 | 21.5 |
| P (g/day) | 15 | 20.3 |
| K (g/day) | 55 | 57 |
| Colostrum Class | Density [g/cm3] | Casein [%] | Protein [%] | Fat [%] |
|---|---|---|---|---|
| 1st | >1.070 | 9.60 aB | 28.42 AB | 7.29 AB |
| 2nd | 1.057–1.070 | 8.53 ac | 22.39 AC | 6.64 Ac |
| 3rd | 1.044–1.056 | 7.39 Bc | 18.22 BC | 5.12 Bc |
| Colostrum Class | LF [g/L] | α-LA [g/L] | β-LG [g/L] | IgG [g/L] |
|---|---|---|---|---|
| 1st | 4.40 AB | 7.52 AB | 8.04 AB | 92.00 AB |
| 2nd | 3.03 Ac | 4.55 AC | 6.16 AC | 74.60 AC |
| 3rd | 2.53 Bc | 3.47 BC | 4.8 BC | 53.50 BC |
| Colostrum Class | Weight [kg] | Daily Weight Gain [kg/day] | |
|---|---|---|---|
| Day 0 | Day 7 | ||
| 1st | 40.17 | 43.45 | 0.468 AB |
| 2nd | 41.67 | 43.23 | 0.222 A |
| 3rd | 42.17 | 43.50 | 0.190 B |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puppel, K.; Gołębiewski, M.; Konopka, K.; Kunowska-Slósarz, M.; Slósarz, J.; Grodkowski, G.; Przysucha, T.; Balcerak, M.; Madras-Majewska, B.; Sakowski, T. Relationship between the Quality of Colostrum and the Formation of Microflora in the Digestive Tract of Calves. Animals 2020, 10, 1293. https://doi.org/10.3390/ani10081293
Puppel K, Gołębiewski M, Konopka K, Kunowska-Slósarz M, Slósarz J, Grodkowski G, Przysucha T, Balcerak M, Madras-Majewska B, Sakowski T. Relationship between the Quality of Colostrum and the Formation of Microflora in the Digestive Tract of Calves. Animals. 2020; 10(8):1293. https://doi.org/10.3390/ani10081293
Chicago/Turabian StylePuppel, Kamila, Marcin Gołębiewski, Katarzyna Konopka, Małgorzata Kunowska-Slósarz, Jan Slósarz, Grzegorz Grodkowski, Tomasz Przysucha, Marek Balcerak, Beata Madras-Majewska, and Tomasz Sakowski. 2020. "Relationship between the Quality of Colostrum and the Formation of Microflora in the Digestive Tract of Calves" Animals 10, no. 8: 1293. https://doi.org/10.3390/ani10081293
APA StylePuppel, K., Gołębiewski, M., Konopka, K., Kunowska-Slósarz, M., Slósarz, J., Grodkowski, G., Przysucha, T., Balcerak, M., Madras-Majewska, B., & Sakowski, T. (2020). Relationship between the Quality of Colostrum and the Formation of Microflora in the Digestive Tract of Calves. Animals, 10(8), 1293. https://doi.org/10.3390/ani10081293






