Simple Summary
The objective of this study was to assess the effect of adding a mixture of plant extracts on the productive performance, blood constituents, carcass characteristics, percentage relation between organ weight and live weight, quality, and sensory analysis of pork produced under heat stress conditions. The study was performed during the summer in the state of Sonora, Mexico. The production of pork is affected by heat stress conditions. The addition of plant extracts can reduce the negative effects of heat stress, because it is known that they improve health, growth, and some parameters of meat quality. Results indicate that the addition of plant extracts to diets of growing pigs improves the productive performance and carcass weight, without modifying blood constituents, and the quality and sensory attributes of the meat.
Abstract
The effect of plant extracts (PE; artichoke, celery, beet, onion, garlic, spinach, avocado, oats, and parsley) in the diet of growing pigs under heat stress was investigated. Parameters included growth performance, blood constituents, carcass characteristics, organ percentage, quality and sensory appraisal of the pork. The study was performed during the Mexican summer, using 60 pigs. Treatments included the control, to which 0.1% PE, and 0.15% PE were added. The use of PE (0.1 and 0.15%) generated an increase in the average daily gain (ADG, by 10.0% for both treatments), and final live weight (LW, by 6.3% and 6.8%) (p < 0.05). The level of blood albumin at 95 kg was higher when supplementing with 0.1% PE (p < 0.05). At 120 kg LW, creatine kinase values showed a tendency to be different (p = 0.07). Carcass weight increased (p < 0.05) when adding PE. Supplementation with 0.1% PE decreased (p < 0.05) the red/green (a *) hue of the meat, whereas supplementation with 0.1% and 0.15% PE increased the yellow/blue (b *) hue (p < 0.05). The addition of PE improves pig growth performance, and carcass weight by reducing the negative effects of heat stress, without markedly modifying blood constituents, meat quality, and sensory attributes of the pork.
1. Introduction
Northwest Mexico is characterized by a hot climate, mainly during the summer months, when temperatures range between 26 °C and 48 °C in the shade, with an average of 33 °C. The thermoneutral zone for growing-finishing pigs is between 18 °C and 25 °C [1], and it is well known that temperatures above this thermoneutral zone induce heat stress [2], which has negative effects on feed intake, weight gain, feed conversion, and carcass characteristics [3,4]. Some plant extracts were shown to be beneficial in heat-stress poultry [5,6]. Research in pigs indicates that the use of plant extracts (PE) can improve productive performance [7,8], and digestibility of dry matter and protein [9,10]. PE possess anti-inflammatory effects [11], and antimicrobial effects for several pathogens [12]. Together with the improvements in pig health, improvements in the parameters of meat quality such as the oxidative stability, smell, and taste have been reported [13].
Some plant extracts individually induce positive effects on the animal. Celery (Apium graveolens) contains analgesic, and anti-inflammatory components [14], whereas garlic (Allium sativum) improves digestibility [15]. Aji et al. [16] indicate that onions (Allium cepa) can improve productive performance. Other extracts, like that of avocado (Persea americana) and parsley (Petroselinum crispun), have shown vasodilation [17] and antimicrobial [18] effects, respectively. Several plants extracts possess antioxidant properties including artichoke (Cynara scolymus) [19], oats (Avena sativa) [20], beet (Beta vulgaris) [14], and spinach (Spinacea olearace) [21].
Yang et al. [22] and Krotkiewski and Janiak [23] demonstrated that synergistic effects can be evoked when using mixtures of herbal components, both in vivo and in vitro. Currently, no information is available on the effect that the mixture of extracts of the aforementioned plants could have in supplementing pigs kept in commercial conditions during the summer. It is proposed that with the use of a mixture of different PE (celery, garlic, onion, avocado, parsley, artichoke, oat, beets, and spinach, contained in a commercial product, PROTORGAN®, Tlaquepaque, Jalisco, Mexico), benefits for the pig can be inferred.
The objective of the present study was to assess the effect of adding plant extracts to the grower and finisher diets of pigs on growth performance, blood constituents, carcass characteristics, organ weight as a percentage of live weight (LW), meat quality, and sensory analysis of pork meat in the growing and finishing stage, produced under hot climate conditions categorized as heat stress.
2. Materials and Methods
All procedures involving animal handling were conducted under the approved Mexican official guidelines for domestic animal care [24,25,26], and the study was approved by the University of Sonora Committee (Num.: USO313005360).
2.1. Animal and Housing Conditions
The study was performed in the porcine experimental unit of the Department of Agriculture and Livestock of the Universidad de Sonora. The study was performed in 60 pigs of commercial terminal crosses (Yorkshire × Landrace × Duroc), 30 males and 30 females, which were housed in an opened building similar to that used in commercial conditions in the growing to finishing periods, they were in individual pens, equipped with stainless steel feeders and nipple-type drinkers. Water and food were provided ad libitum. This study was performed in three phases of feeding: phase I (35 to 70 kg), phase II (70 to 95 kg) and phase III (95 to 120 kg live weight (LW). Pigs were individually identified with ear tags. Treatments were distributed based on weight and sex of the pigs, with 20 experimental units per treatment.
2.2. Source of the Plant Extracts (PE)
In this study, a commercial patented product (PROTORGAN®, from Guwlab, Tlaquepaque, Jalisco, Mexico) was used. The product contained extracts from the following plants: artichoke (Cynara sculymus), celery (Apium graveolens), beet (Beta vulgaris), onion (Allium cepa), garlic (Allium sativum), spinach (Spinacea olerace), avocado (Persea americana), oats (Avena sativa), and parsley (Petroselinum crispun).
2.3. Treatments
Pigs received one of three diets, provided as a meal: (1) control diet (CON), designed to satisfy the nutrients for high lean potential, high productivity, and used as a commercial ration in Hermosillo, Mexico during summer [27]; (2) 0.1% PE, which was the control diet +0.1% (as fed) of plant extracts, PROTORGAN®; and (3) 0.15% PE, which was the control diet +0.15% (as fed) of plant extracts, PROTORGAN®. Diets were formulated to be isonitrogenous and isocaloric (Table 1).

Table 1.
Composition of experimental diets for pigs in growing-finishing stages.
2.4. Growth Performance
The total weight of the feed provided, and the feed rejected in each pen was recorded daily during the study period. At the end of each phase, the average daily feed intake (ADFI) was calculated. Pigs were weighed individually at the same frequency as feed intake was measured, and these data were used to calculate the average daily gain (ADG), and feed conversion ratio (F:G) per phase. The experiment was finished once the pigs reached an average of 120 kg LW, when they were sent for slaughter.
2.5. Blood Metabolites
At the end of the last two phases, blood samples were taken from the 10 pigs in each treatment (approximately 7 mL of blood was collected via jugular venipuncture) using two Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). One tube contained ethylenediaminetetraacetic acid (EDTA), and the other contained no additive.
Blood from tubes containing EDTA was used for the hemogram blood test. This included the determination of red blood cells, hemoglobin, hematocrit, leukocytes, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), lymphocytes, monocytes, and platelets. This was performed using an automated Coulter Electronics × 10 system. The blood from tubes containing no additive was centrifuged at 10,000 rpm for 10 min, and the serum was separated and stored at −20 °C until it was assayed for blood parameters (glucose, total protein, albumin, creatine kinase [CK] and cortisol). Glucose, total proteins, albumin, and creatine kinase (CK) were determined using the appropriate laboratory kits, following the manufacturer’s instructions (RANDOX® Manual). Cortisol was determined using ELISA methodology (Sigma-Aldrich®, St. Louis, MO, USA).
2.6. Slaughtering and Carcass Traits
Pigs were sent for slaughter at 120 kg LW. Pigs were slaughtered in the abattoir of the Departamento de Agricultura y Ganadería, of the Universidad de Sonora by trained personnel. Electrical stunning (head to head) using conventionally methods was applied before sticking and exsanguination, complying with the corresponding official Mexican standards [24]. The carcasses were individually weighed to record the hot carcass weight (HCW, carcass weight included head, skin and legs, without viscera, internal organs, flare fat, kidneys, diaphragm, genitals and tail). Carcasses were then chilled for 24 h at 4 °C to obtain the cold carcass weight (CCW), carcass lengths, dressing percentage. Carcass shrinkage was determined (HCW–CCW as a percentage of original HCW), fat thickness, the longissimus thoracis muscle (LM) area, and marbling (24 h postmortem) was determined. Fat thickness and the LM area (cm2) were measured at the 10th and 12th ribs. Marbling was also evaluated according to the guidelines of the United States Department of Agriculture. Finally, cooling loss and dressing values were calculated. The percentage of lean yield was calculated from equations as indicated in the corresponding Mexican Norm [28].
2.7. Percentage Relation between the Weight of Organs and Live Weight
The liver, heart, lungs, stomach, spleen, and kidneys were extracted from each animal, and the weight was recorded to calculate the organ weight as a percentage of LW for each organ.
2.8. Dissection of the Longissimus Thoracis (LT) Muscle and Sampling
After evaluating the carcasses, the LT of the left side was extracted (4th to 12th intercostal space) from 10 pigs per treatment. The meat samples were marked for identification, vacuum packed, and transported under refrigeration to the facilities of the Centro de Investigación en Alimentación y Desarrollo (CIAD) in the city of Hermosillo, Sonora, for the corresponding analyses.
After arrival at the laboratory, the samples were kept frozen at −18 °C. Before analysis, samples were thawed for 24 h at 4 °C, and then sectioned to carry out chemical, physicochemical, and sensory measurements. Sectioning of the samples was consistently performed following the same protocol, and in the same order from the caudal end (the 12th-rib interface) to the cranial part of the LT muscle. The first cut (2.0 cm) was used to determine the contents of moisture, protein, and intramuscular fat. Four pairs of samples (2.54 cm each) were used for the Warner–Bratzler shear force (WBSF) test, cooking loss determination, and sensory analysis. A 2.5 cm slice was used to analyze color, pH, and water-holding capacity (WHC). All measurements were recorded immediately after the samples were sectioned.
2.9. Meat Quality
2.9.1. Chemical Composition
The moisture, intramuscular fat, and protein content of the meat was determined following AOAC methods [29] for moisture (method 950.46), fat (method 920.39), and protein (method 955.04). The results are expressed as a percentage of fresh weight.
2.9.2. Physical Analysis
To measure the color parameters in the meat cuts, a Hunter Lab colorimeter was used. Color determination included the parameters L *, a *, b *, hue angle (HUE) using the formula tan−1 (b/a), and chroma (color saturation) using the formula Chroma = (a * + b *) ½. For measurements, the illuminator D65 with 10° was used in the colorimeter. Color determinations were performed on the surface of the cold samples (4–6 °C) in five sites on the surface of the muscle [30].
The pH was determined in cold meat samples at 4–6 °C, using a portable digital HANNA (Hanna Instruments, Woonsocket, RI, USA) potentiometer with a penetrating electrode provided with a HANNA HI 99163 thermometer. Measurements were performed in triplicate.
Water Holding Capacity (WHC) was determined following the methodology of Sutton et al. [31]. The sample was placed in micro-nylon fabric, and introduced into a 50 mL propylene tube. The sample was centrifuged at 2800 g for 5 min at 4 °C. The WHC percentage was calculated according to the difference in weight of the sample before and after the centrifugation.
Texture (WBSF) measurements were made with a Texture Analyzer TAXT-Plus (Texture Technologies Corp., Scarsdale, NY, USA). To measure the cutting effort (CE) of the meat, 2.54 cm thick slices were cut, and then cooked in an electric fryer (Cook Master Ester, Model 3222-3) until reaching an internal temperature of 71 °C. Once cooked, samples were cooled to room temperature (25–30 °C) and then refrigerated at 4 °C for 24 h. To measure the cutting effort, the cooked sample was cut in pieces of 1 cm2 by 3 cm length along the direction of muscle fibers (10 times per cut). The CE was measured perpendicularly to the muscle fibers, using the Warner–Bratzler accessory cutter mounted on the TAXT-Plus texture meter. The WBSF values was expressed in kilogram-force.
Cooking loss was determined by calculating the difference in weight of the sample before and after cooking it at an internal temperature of 71 °C in an electric fryer (Cook Master Ester, Model 3222-3), following the AMSA [32] technique.
2.10. Sensory Analysis
Sensory assessment was performed by a trained panel of 10 members [33]. The training of the assessment panel was achieved using the AMSA [32] methodology. One day before performing the sensory analysis, the meat cuts were removed from the freezer, and thawed at 4 °C for 24 h. The meat cuts were cooked using the same procedure described for the assessment of texture (WBSF). Each portion of meat was cut to a thickness of 1.27 × 1.27 cm. The trained panel (using a dim red light) assessed the cooked samples in terms of odor intensity, taste intensity, fatty sensation, tenderness, juiciness, and amount of connective tissue, using a structured linear scale of 10 cm. The value anchored to the left (0 cm) of the linear scale refers to a descriptive term that represents the lowest intensity of odor, taste, fat, tenderness, juiciness, and amount of connective tissue. The right end (10 cm) refers to the highest degree for each sensory characteristic. Two characteristics were assessed visually (total color and total appearance) on raw samples, under white light and using the same type of scale.
2.11. Statistical Analysis
Each pig was considered as an experimental unit. All data were explored prior to statistical analysis to discard any possible outliers. For the analysis of variance (ANOVA) of growth performance, analysis of blood metabolites and carcass characteristics, a random complete blocks design was used [34], with initial weight as a blocking factor. Data of the quality and sensory analysis of the meat were analyzed by ANOVA with a completely randomized design, data normality was performed using the Shapiro–Wilk test. The experimental diet was included in the model as the main factor. When statistical differences (p < 0.05) were observed among treatments, then means were compared using Tukey’s multiple rank test. Effects were accepted as different at p < 0.05, and tended to be significant at p < 0.10. All data were processed with SAS (ver. 9.1. SAS Inst. Inc., Cary, NC, USA) statistical software [35].
3. Results
3.1. Climatic Conditions
The maxima, minimum and average temperature within the installation that housed the metabolic cages was recorded daily throughout the experimental period, utilizing a mercury thermometer (Figure 1). The thermometer was located in the installation at the height of the pigs. Temperatures ranged between 25.5 °C and 44.3 °C, average temperature of 32.4 °C. The relative humidity, was also recorded, and ranged between 19% and 56% with an average of 39%.
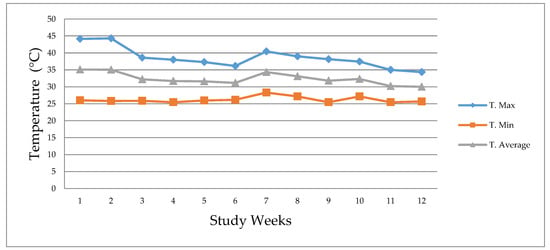
Figure 1.
Maximum temperature (T. Max), minimum temperature (T. Min), and average temperature (T. Average).
3.2. Growth Performance
Table 2 depicts the growth performance data in the three feeding phases. In Phase I (35 to 70 kg LW), there was only a difference in ADFI (p < 0.05), with a 15.0% increase when was comparing the 0.1% plant extract supplementation respect to the control diet. In the 70 to 95 kg stage there was a 14.3% increase in ADFI when supplementing with 0.1 plant extract as compared to the control diet, aside from a tendency in ADG to be different in PE-supplemented diets from the control diet (p < 0.10). Addition of 0.1% PE induced an increase (6.0%) in the final weight when compared to the control diet (p < 0.05). Regarding the last stage, 95 to 120 kg, addition of PE (0.1 and 0.15%) increased the ADFI in 17.2%, when compared to the control diet (p < 0.05). The ADG increased 18.2% when adding 0.15% of PE as compared to the control diet (p < 0.05). The final weight in this stage was higher in the animals whose diets was supplemented with 0.1% and 0.15% PE compared to the control diet, increases were of 6.3% and 6.8%, respectively (p < 0.05).

Table 2.
Growth performance according to phase.
Analysis of the whole experimental stage (35 to 120 kg LW) revealed an improvement in ADG with either level of PE (0.1 or 0.15%) when compared to the control diet; increases were of 10.0% for both treatments (p < 0.05), without affecting the F:G (p > 0.05). There was an increase in feed intake (12.0%) when adding 0.1% PE, compared to the control diet (p < 0.05).
3.3. Blood Metabolites
Table 3 depicts the results of the blood metabolite analyses. At 95 kg LW, there were no statistical differences in most blood constituents (p > 0.05). The level of albumin was higher in the 0.1% PE diet, compared to the 0.15% PE and control diet, (6.7% for both treatments, p < 0.05). Creatine kinase (CK) values were not different (p = 0.1051). At 120 kg LW, there were no significant differences in the variables. CK values tended to be different among treatments (p = 0.07).

Table 3.
Blood metabolites, sampled at 95 and 120 kg of average live weight.
3.4. Carcass Traits
Carcass weight was increased when using either PE level (0.1% or 0.15% PE) compared to the control, both in the hot carcass (5.7% and 6.9%, respectively, p < 0.05), and the cold carcass (6.4% and 7.1%, respectively, p < 0.05, Table 4). Dressing percentage was not difference between groups (p > 0.05), whereas a reduction in the percentage of carcass shrinkage (p < 0.05) was observed when using 0.1% PE, as compared to the control diet. Length of the carcass, dorsal fat, depth, and area of the ribeye were similar among treatments (p > 0.05). Lean yield presented a tendency to be different (p < 0.10).

Table 4.
Carcass characteristics from growing to finishing pigs.
3.5. Organ Weight as a Percentage of Live Weight
No differences were observed among treatments when using PE at any concentration (p > 0.05) (Table 5).

Table 5.
Organ weight as a percentage of live weight (%).
3.6. Meat Quality
3.6.1. Chemical Composition
Supplementation with either 0.1 or 0.15% PE did not induce changes in the content of moisture and protein as compared to the meat from non-supplemented animals (p > 0.05) (Table 6). Supplementation with 0.15% PE diminished intramuscular fat numerically as compared to the meat from non-supplemented animals (p > 0.05), but this was not significant.

Table 6.
Meat quality (chemical composition and physical analysis) of pigs supplemented with plant extracts (PE) during development and growing-finishing stages.
3.6.2. Physical Analysis
Physical parameters of the meat from pigs supplemented with PE are presented in Table 6. Addition of 0.1% and 0.15% PE induced a tendency to increase the values of L*, compared to the meat from non-supplemented animals (p < 0.10). Regarding parameter a* (red hue of the meat), the addition of 0.1% PE induced a close to 4.7% decrement in the values of the meat compared to the control group, and a 6.2% reduction compared with the 0.15% PE supplementation (p < 0.05). Addition of 0.1% and 0.15% PE increased parameter b* slightly (3.3% and 4.9%, respectively, p < 0.05) compared to the meat from control animals. The pH and WHC were not modified by the addition of PE (p > 0.05). Values of texture, a parameter assessed by measuring the cutting effort, were 9.9% higher in the meat from animals supplemented with 0.1% PE versus control animals, and 11.4% higher in animals supplemented with 0.15% of PE (p = 0.09).
3.7. Sensory Analysis
Table 7 depicts the sensory parameters of the meat from animals supplemented with PE. Addition of 0.15% PE produced meat cuts with higher visual coloration (p < 0.05). No changes were observed in overall color by adding PE (p > 0.05). The trained panel did not detect differences in the appearance of the meat cuts when sourced from animals supplemented with PE (p > 0.05). The meat cuts from animals supplemented with 0.15% PE had a better taste than those from control animals, and those supplemented with 0.1% of PE (p > 0.05). No differences were detected in the odor of meat supplemented with PE (p > 0.05). Addition of 0.1% of PE did not influence tenderness (p > 0.05). Juiciness of the assessed meat cuts was not different between groups (p > 0.05).

Table 7.
Sensory quality of meat from pigs supplemented with plant extracts (PE) during the growing-finishing stage.
4. Discussion
4.1. Climatic Conditions
Animals have an optimal growth rate within their thermoneutral zone (TZ), which is defined as the range of environmental temperatures at which the normal maintenance, and productive functions of an animal in non-stressing conditions compensates for the heat lost to the environment without requiring a change in its metabolic heat rate [36]. For pigs in the growing and finishing stages, the TZ is in the range of 18 °C to 21 °C [37]. De Oliveira et al. [1] report that the thermoneutral conditions for the same stages (growing-finishing) are 18 °C to 25 °C. It is also known that temperatures above the TZ induce heat stress [2]. In the present study, temperatures ranged between 25.5 °C and 44.3 °C, with an average of 32.4 °C. These climatic conditions indicate that the animals were housed in pens at environmental temperatures very much above the optimal production conditions generating heat stress.
4.2. Growth Performance
Addition of PE induced an improvement in the ADG, ADFI and final LW, under heat stress conditions during the growing to finishing period. These results agree with other studies in which the addition of some type of plant extract or mixture of plant extracts have been assessed [5,6,38]. Frankič et al. [39] indicate that the addition of PE can impact physiological functions, and intestinal health, for a positive effect on growth performance. Despite the existing reports indicating the positive effect of adding PE to the diet of grower-finisher animals, there are reports that do not observe a positive effect on the productive performance [13]. The differences reported in the diverse studies could be due to the types of plant extracts added to the diet, as well as to different conditions in which the tests were performed, such as doses, health status, stage, ages at slaughtering, environment, among others. Despite of heat stress climatic conditions during the trial period, the use of PE cause an increment in the growth performance, a very favorable result for pig production in desert climates.
4.3. Blood Metabolites
Some blood metabolites are changed when the animals are under stress [40]. At 95 kg LW, the content of albumin, a metabolite related with stress in animals, increased when adding 0.1% PE, whereas the CK values tended to be different, probably due to the body weight reached by the pigs supplemented with PE, because the higher the weight of the animals, more susceptible they will be to heat [41]. Some reports indicate that the addition of a combination of plant extracts (buckwheat, thyme, curcuma, black pepper, and ginger) induces an increase in red blood cells, white blood cells, and lymphocytes in growing pigs [38], and in weanling pigs [42]. Increases in red blood cells of pigs in the growing-finishing stage when supplemented with Coptis chinensis extract [43] have been reported. No changes in these blood metabolites were reported. No modifications occurred in leukocytes and lymphocytes in those studies, which is similar to the results of this study. Halas et al. [44] and Li et al. [45] indicated an increase in lymphocytes when supplementing essential oils. All these parameters provide evidence that the addition of PE does not have any negative effect on blood metabolites; furthermore, it is important to note that the expected change in stress variables with increasing final LW was not perceived.
4.4. Carcass Characteristics
The weight of the carcasses, both hot and cold, improved with the addition of PE at the two analyzed concentrations (0.1% and 0.15%), which is related directly to the final LW obtained. Hossain et al. [46], and Rossi et al. [47] found no differences in the carcasses of pigs supplemented with plant extracts. Cullen et al. [48] found no differences in carcass characteristics using garlic and rosemary extracts for a 56-day period. Korniewicz et al. [49] reported that the addition of different levels of a mixture of plant extracts containing thymol, carvacrol, capsaicin, cinnamon aldehyde, eugenol, flavonoids, and essential oils to the diet of pigs of 20 to 100 kg LW did not induce differences in carcass quality variables. The discrepancy between the previously mentioned studies and our results are possibly due to the time of administration of the PE and the body weight at which the pigs were slaughtered at the end of testing.
4.5. Organ Weight as a Percentage of Live Weight
The use of growth-promoting molecules or certain diseases can cause the growth of an organ, which can influence the final weight and the yield. In the current study, the organ to LW percentage was not modified with any of the treatments. This agrees with Mahmood et al. [50], who indicated that the use of 0.5% garlic does not affect the weight of the heart, liver, and spleen. In contrast to our study, Dorhoi et al. [51] reported that the addition of a garlic extract to the feed of birds increases the weight of the thymus and spleen, together with an increment in the proliferation of lymphocytes and white blood cells. The differences reported in these studies could be due to the plant extract, doses, or health status.
4.6. Meat Quality
4.6.1. Chemical Composition
Supplementation with PE have been reported to modify the quality of the meat of fattening animals, involving the fatty acid profile, and oxidative stability of the meat [52]. In the current study, supplementation with 0.15% PE numerically reduced the intramuscular fat content, whereas moisture and protein were modified only minimally, but these were non-significant. The moisture content ranged from 71.6% to 72.0%, whereas the protein content was close to 21.6% in all treatments, which are normal values in pork meat.
4.6.2. Physical Analysis
Supplementation with 0.1% PE induced a small decrease in the red coloring of the meat, but the sensory panel did not detect this variation. Janz et al. [53], and Simitzis et al. [54] reported that color parameters of meat from pigs supplemented with plant extracts did not change. The addition of 0.1% PE tended to generate meat cuts with a greater cutting effort, a texture that was slightly detected by the trained panel, but again, not significantly so. In general terms of meat quality (Chemical Composition and Physical Analysis), it is evident that the addition of PE had no major effects on the pigs in the growing-finishing phases.
4.7. Sensory Analysis
Sensorial qualities (overall color, appearance, taste, odor, tenderness, juiciness, fat sensation and connective tissue) were not affected by the addition of the two PE concentrations, except in terms of visual color, where the panelists observed a higher intensity in the 0.15% PE diet compared with the control and 0.1% PE. Panelists categorized all meat cuts as close to a pale pink, the characteristic color of fresh pork meat. Hanczakowska et al. [13] reported a favorable effect on odor and taste of the meat sourced from pigs supplemented with PE, an effect that was attributed to an improvement in oxidative stability promoted by the plant extracts. Despite the favorable effect promoted by the addition of plant extracts on the meat, reports like the ones of Janz et al. [53] and Simitzis et al. [54] indicate that the addition of plant extracts to the diet of pigs does not modify the sensory characteristics of the meat.
The differences among the diverse mentioned studies can be due to the ingredients used in the diet, the environmental conditions, the dose of the added extract, time of extract administration, the health status of the animals, and the stage at which they were applied. It is important to continue with the assessment of plant extracts in other conditions that will improve the production and reduce the use of antibiotics.
5. Conclusions
The addition of plant extracts to the diets of pigs in the growing-finishing stage under heat stress improved growth performance, increased the carcass weight, and can be used as a strategy to minimize the negative effects of the summer, without marked effects on blood metabolites, percentage relation between organ weight and live weight, nor on the quality and sensory characteristics of the meat.
Author Contributions
Conceptualization, methodology, M.A.B.-S.; methodology, J.L.D.-R. and H.G.-R.; software, H.C.-M.; formal analysis, J.S.-C.; investigation, L.L.M.-A., J.G.M.-C., A.D.G.-S.; writing—original draft preparation, J.L.D.-R., H.G.-R. and E.S.-V.; writing—review and editing, J.A.-I., J.L.D.-R. and E.S.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by the Departamento de Agricultura y Ganadería, UNISON; and Guwlab, Tlaquepaque, Jalisco, Mexico.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Oliveira, A.C.D.F.; Vanelli, K.; Sotomaior, C.S.; Weber, S.H.; Costa, L.B. Impacts on performance of growing-finishing pigs under heat stress conditions: A meta-analysis. Vet. Res. Commun. 2019, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Nyachoti, C.M.; Zijlstra, R.T.; De Lange, C.F.M.; Patience, J.F. Voluntary feed intake in growing-finishing pigs: A review of the main determining factors and potential approaches for accurate predictions. Can. J. Anim. Sci. 2004, 84, 549–566. [Google Scholar] [CrossRef]
- Song, R.; Foster, D.N.; Shurson, G.C. Effects of feeding diets containing bacitracin methylene disalicylate to heat-stressed finishing pigs. J. Anim. Sci. 2011, 89, 1830–1843. [Google Scholar] [CrossRef]
- Cerisuelo, A.; Torres, A.; Lainez, M.; Moset, V. Increasing energy and lysine in diets for growing-finishing pigs in hot environmental conditions: Consequences on performance, digestibility, slurry composition, and gas emission. J. Anim. Sci. 2012, 90, 1489–1498. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotech. 2016, 7, 37. [Google Scholar] [CrossRef]
- Kanani, P.B.; Daneshyar, M.; Aliakbarlu, J.; Hamian, F. Effect of dietary turmeric and cinnamon powders on meat quality and lipid peroxidation of broiler chicken under heat stress condition. Vet. Res. Forum. 2017, 8, 163–169. [Google Scholar]
- Isley, S.E.; Miller, H.M.; Greathead, H.M.R.; Kamel, C. Plant extracts as supplements for lactating sows: Effects on piglet performance sow food intake and diet digestibility. Anim. Sci. 2003, 77, 247–254. [Google Scholar] [CrossRef]
- Devi, S.M.; Park, J.W.; Kim, I.H. Effect of plant extracts on growth performance and insulin-like growth factor 1 secretion in growing pigs. Rev. Bras. Zootecn. 2015, 44, 355–360. [Google Scholar] [CrossRef]
- Nualart, H.; Kamel, C.; Gasa., J.; Baucells, F. Effects on faecal digestibility of the inclusion of a formulation of natural plant extracts on a post weaning pig diet from 5 to 15 kg. In Proceedings of the European Association for Animal Production, Hague, The Netherlands, 2000; p. 43. [Google Scholar]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef]
- Liu, Y.; Song, M.; Che, T.M.; Almeida, J.A.S.; Lee, J.J.; Bravo, D.; Pettigrew, J.E. Dietary plant extracts alleviate diarrhea and alter immune responses of weaned pigs experimentally infected with a pathogenic Escherichia coli. J. Anim. Sci. 2013, 91, 5294–5306. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Hanczakowska, E.; Świątkiewicz, M.; Grela, E.R. Effect of dietary inclusion of a herbal extract mixture and different oils on pig performance and meat quality. Meat Sci. 2015, 108, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.H.; Alkofahi, A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J. Ethnopharmacol. 1998, 60, 117–124. [Google Scholar] [CrossRef]
- Chen, Y.J.; Kim, I.H.; Cho, J.H.; Yoo, J.S.; Wang, Q.; Wang, Y.; Huang, Y. Evaluation of dietary l-carnitine or garlic powder on growth performance, dry matter and nitrogen digestibilities, blood profiles and meat quality in finishing pigs. Anim. Feed Sci. Technol. 2008, 141, 141–152. [Google Scholar] [CrossRef]
- Aji, S.B.; Ignatius, K.; Ado, A.A.Y.; Nuhu, J.B.; Abdulkarim, A.; Aliyu, U.; Gambo, M.B.; Ibrahim, M.A.; Abubakar, H.; Bukar, M.M.; et al. Effects of feeding onion (Allium cepa) and garlic (Allium sativum) on some performance characteristics of broiler chickens. Res. J. Poult. Sci. 2011, 4, 22–27. [Google Scholar]
- Ojewole, J.; Kamadyaapa, D.R.; Gondwe, M.M.; Moodley, K.; Musabayane, C.T. Cardiovascular effects of Persea americana Mill (Lauraceae) (avocado) aqueous leaf extract in experimental animals. Cardiovasc. J. Afr. 2007, 18, 69. [Google Scholar]
- Wong, P.Y.Y.; Kitts, D.D. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006, 97, 505–515. [Google Scholar] [CrossRef]
- Zapolska-Downar, D.; Zapolski-Downar, A.; Naruszewicz, M.; Siennicka, A.; Krasnode˛bska, B.; KolCodziej, B. Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci. 2002, 71, 2897–2908. [Google Scholar] [CrossRef]
- Peterson, D.M. Oat antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Yang, J.; Cui, Y.; Kolb, M. How useful is traditional herbal medicine for pulmonary fibrosis? Respirology 2009, 14, 1082–1091. [Google Scholar] [CrossRef]
- Krotkiewski, M.; Janiak, R. Comparison of the weight-decreasing effects of different herbs with a mixture of herbal extracts exerting a probable synergistic effect. Praca Oryginalna 2008, 4, 137–142. [Google Scholar]
- NOM-033-ZOO-1995. Norma Oficial Mexicana 033. Sacrificio Humanitario de los Animales Domésticos y Silvestres. SAGARPA. In Diario Oficial de la Federación; Secretariat of the Interior: México, D.F., México, 1995; p. 17. Available online: http://www.cuautitlan.unam.mx/descargas/cicuae/normas/Norma033.pdf (accessed on 6 April 2020).
- NOM-051-ZOO-1995. Norma Oficial Mexicana 051. Trato Humanitario en la Movilización de Animales. In Diario Oficial de la Federación; Secretariat of the Interior: Mexico City, México, 1995; p. 23. Available online: http://www.fmvz.unam.mx/fmvz/p_estudios/apuntes_bioet/051zoo_movilizacion.pdf (accessed on 6 April 2020).
- NOM-062-ZOO-1999. Norma Oficial Mexicana. Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio. In Diario Oficial de la Federación; Secretariat of the Interior: Mexico City, Mexico, 1999; pp. 1–44. [Google Scholar]
- NRC. Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2012; p. 400. [Google Scholar]
- NMX-FF-081-2003. Norma Mexicana de Carne de Ganado Porcina. In Diario Oficial de la Federación; Secretariat of the Interior: Mexico City, Mexico, 2003; pp. 1–12. Available online: https://www.colpos.mx/bancodenormas/nmexicanas/NMX-FF-081-2003.PDF (accessed on 5 April 2020).
- AOAC. Official Methods of Analysis; Assoc. Offic. Anal. Chem.: Washington, DC, USA, 1990. [Google Scholar]
- Cassens, R.G.; Demeyer, D.; Eikelenboom, G.; Honikel, K.O.; Johanson, G.; Nielsen, T. Recommendations of reference methods for assessment of meat colour. In Proceedings of the 41st International Congress of Meat Science and Technology, San Antonio, TX, USA, 20–25 August 1995. [Google Scholar]
- Sutton, D.S.; Ellis, M.; Lan, Y.; McKeith, F.K.; Wilson, E.R. Influence of slaughter weight and stress gene genotype on the water-holding capacity and protein gel characteristics of three porcine muscles. Meat Sci. 1997, 46, 173–180. [Google Scholar] [CrossRef]
- AMSA. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Fresh Meat; American Meat Science Association: Chicago, IL, USA, 1995. [Google Scholar]
- ISO 8586-1. Sensory Analysis Methodology. General Guidance for the Selection and Training and Monitoring of Assessors; International Standards Organization Publications: Geneva, Switzerland, 1993; Part1. Selected Assessors. [Google Scholar]
- Steel, R.D.G.; Torrie, J.H. Principles and Procedures of Statistics; Mc. Graw-Hill Book Co.: New York, NY, USA, 1980; p. 663. [Google Scholar]
- SAS. SAS Statistics User’s Guide. Statistical Analytical System, 5th ed.; SAS Institute Inc.: Cary, NC, USA, 2004. [Google Scholar]
- NRC. Effect of Environment on Nutrient Requirements of Domestic Animals; National Academy Press: Washington, DC, USA, 1981. [Google Scholar]
- Holmes, M.A.; Close, W.H. The Influence of Climatic Variables on Energy Metabolism and Associated Aspects of Productivity in the Pig. In Nutrition and the Climatic Environment; Haresign, W., Swan, H., Lewis, D., Eds.; Butterworths: London, UK, 1977; pp. 51–74. [Google Scholar]
- Yan, L.; Meng, Q.W.; Kim, I.H. Effect of an herb extract mixture on growth performance, nutrient digestibility, blood characteristics and fecal noxious gas content in growing pigs. Livest. Sci. 2011, 141, 143–147. [Google Scholar] [CrossRef]
- Frankič, T.; Voljč, M.; Salobir, J.; Rezar, V. Use of herbs and spices and their extracts in animal nutrition. Acta Agric. Slov. 2009, 94, 95–102. [Google Scholar]
- Fraser, A.F.; Broom, D.M. Farm Animal Behaviour and Welfare; Bailliere Tindall. P. VII: London, UK, 1990. [Google Scholar]
- Renaudeau, D.; Gourdine, J.L.; St-Pierre, N.R. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 2011, 89, 2220–2230. [Google Scholar] [CrossRef]
- Yan, L.; Meng, Q.W.; Kim, I.H. Effect of an herb extract mixture on growth performance, nutrient digestibility, blood characteristics and fecal microbial shedding in weanling pigs. Livest. Sci. 2012, 145, 189–195. [Google Scholar] [CrossRef]
- Zhou, T.X.; Zhang, Z.F.; Kim, I.H. Effects of dietary Coptis chinensis herb extract on growth performance, nutrient digestibility, blood characteristics and meat quality in growing-finishing pigs. Asian Australas. J. Anim. 2013, 26, 108–115. [Google Scholar] [CrossRef]
- Halas, V.; Nochta, I.; Pásti, Z.; Szabó, C.; Tóthi, R.; Tossenberger, J.; Babinszky, L. Cellular immune response of weaned pigs fed diet supplemented with an essential Oil. Agric. Conspec. Sci. 2011, 76, 279–282. [Google Scholar]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian Australas. J. Anim. 2012, 25, 1617. [Google Scholar] [CrossRef]
- Hossain, M.E.; Ko, S.Y.; Yang, C.J. Dietary supplementation of green tea by-products on growth performance, meat quality, blood parameters and immunity in finishing pigs. J. Med. Plant Res. 2012, 6, 2458–2467. [Google Scholar]
- Rossi, R.; Pastorelli, G.; Cannata, S.; Tavaniello, S.; Maiorano, G.; Corino, C. Effect of long term dietary supplementation with plant extract on carcass characteristics, meat quality and oxidative stability in pork. Meat Sci. 2013, 95, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Cullen, S.P.; Monahan, F.J.; Callan, J.J.; O’Doherty, J.V. The effect of dietary garlic and rosemary on grower-finisher pig performance and sensory characteristics of pork. Ir. J. Agric. Food Res. 2005, 44, 57–67. [Google Scholar]
- Korniewicz, D.; Różański, H.; Usydus, Z.; Dobrzański, Z.; Korniewicz, A.; Kaczmarek, P.; Frankiewicz, A.; Szulc, K. Efficiency of plant extracts (herbiplant cs) in pigs fattening. Pol. J. Food Nutr. Sci. 2007, 57, 309–315. [Google Scholar]
- Mahmood, S.; Mushtaq-Ul-Hassan, M.; Alam, M.; Ahmad, F. Comparative efficacy of Nigella sativa and Allium sativum as growth promoters in broilers. Int. J. Agric. Biol. 2010, 11, 775–778. [Google Scholar]
- Dorhoi, A.; Dobrean, V.; Zăhan, M.; Virag, P. Modulatory effects of several herbal extracts on avian peripheral blood cell immune responses. Phytother. Res. 2006, 20, 352–358. [Google Scholar] [CrossRef]
- Wenk, C. Herbs and botanicals as feed additives in monogastric animals. Asian Australas. J. Anim. 2003, 16, 282–289. [Google Scholar] [CrossRef]
- Janz, J.A.M.; Morel, P.C.H.; Wilkinson, B.H.P.; Purchas, R.W. Preliminary investigation of the effects of low-level dietary inclusion of fragrant essential oils and oleoresins on pig performance and pork quality. Meat Sci. 2007, 75, 350–355. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Symeon, G.K.; Charismiadou, M.A.; Bizelis, J.A.; Deligeorgis, S.G. The effects of dietary oregano oil supplementation on pig meat characteristics. Meat Sci. 2010, 84, 670–676. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).