Nociceptive Threshold of Calves and Goat Kids Undergoing Injection of Clove Oil or Isoeugenol for Disbudding
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.1.1. Calves
2.1.2. Goats
2.2. Experimental Design and Procedures
2.2.1. Treatments
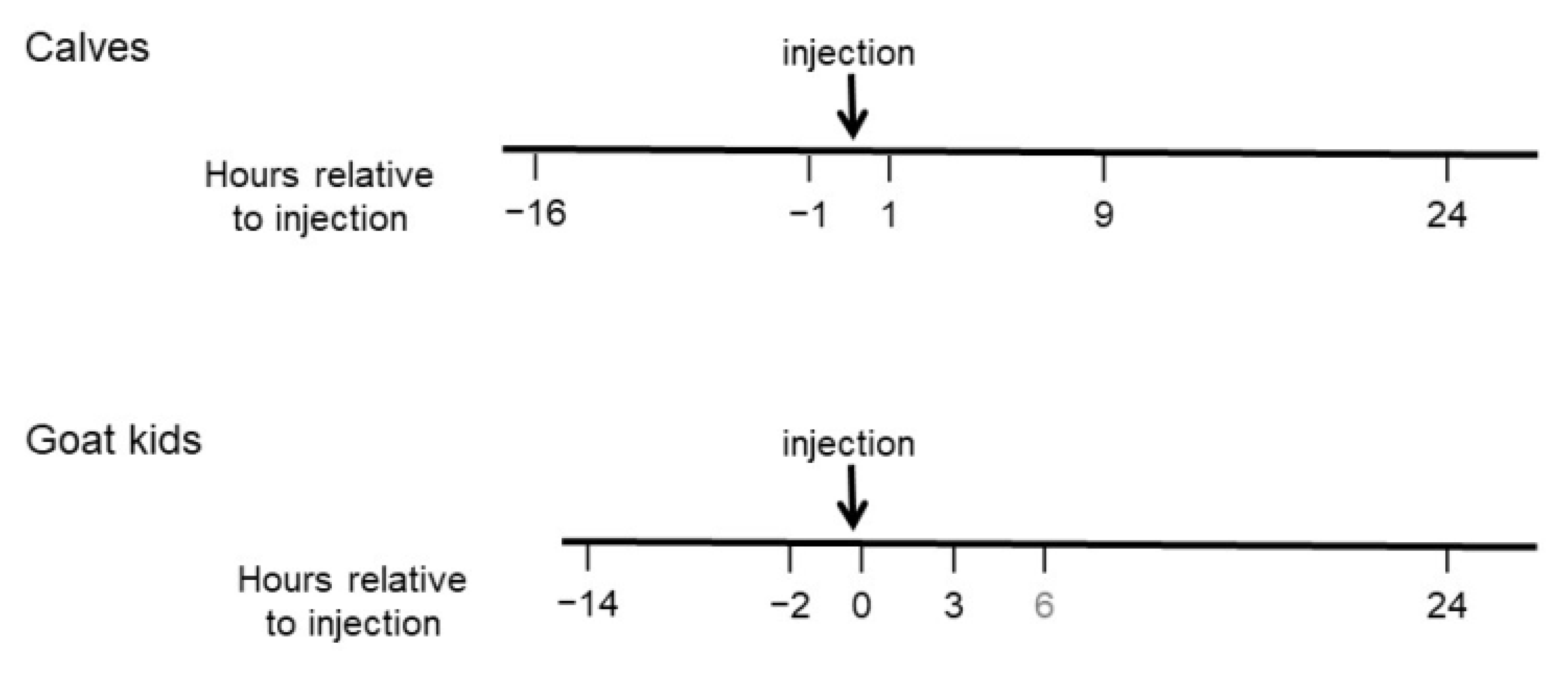
2.2.2. Time Points of MNT Measures
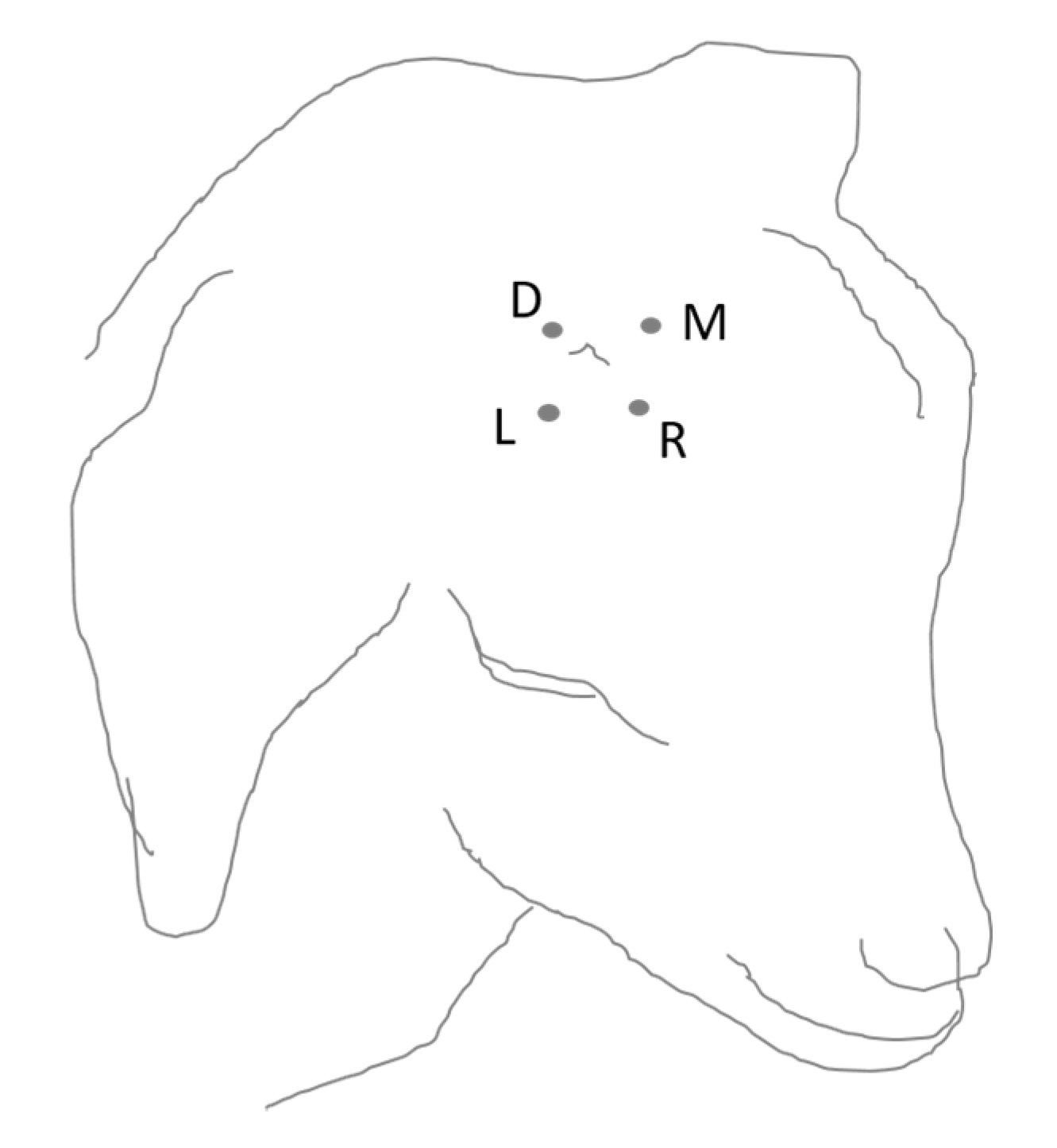
2.2.3. Procedure of MNT Measurement
Calves
Goats
2.3. Statistical Analysis
3. Results
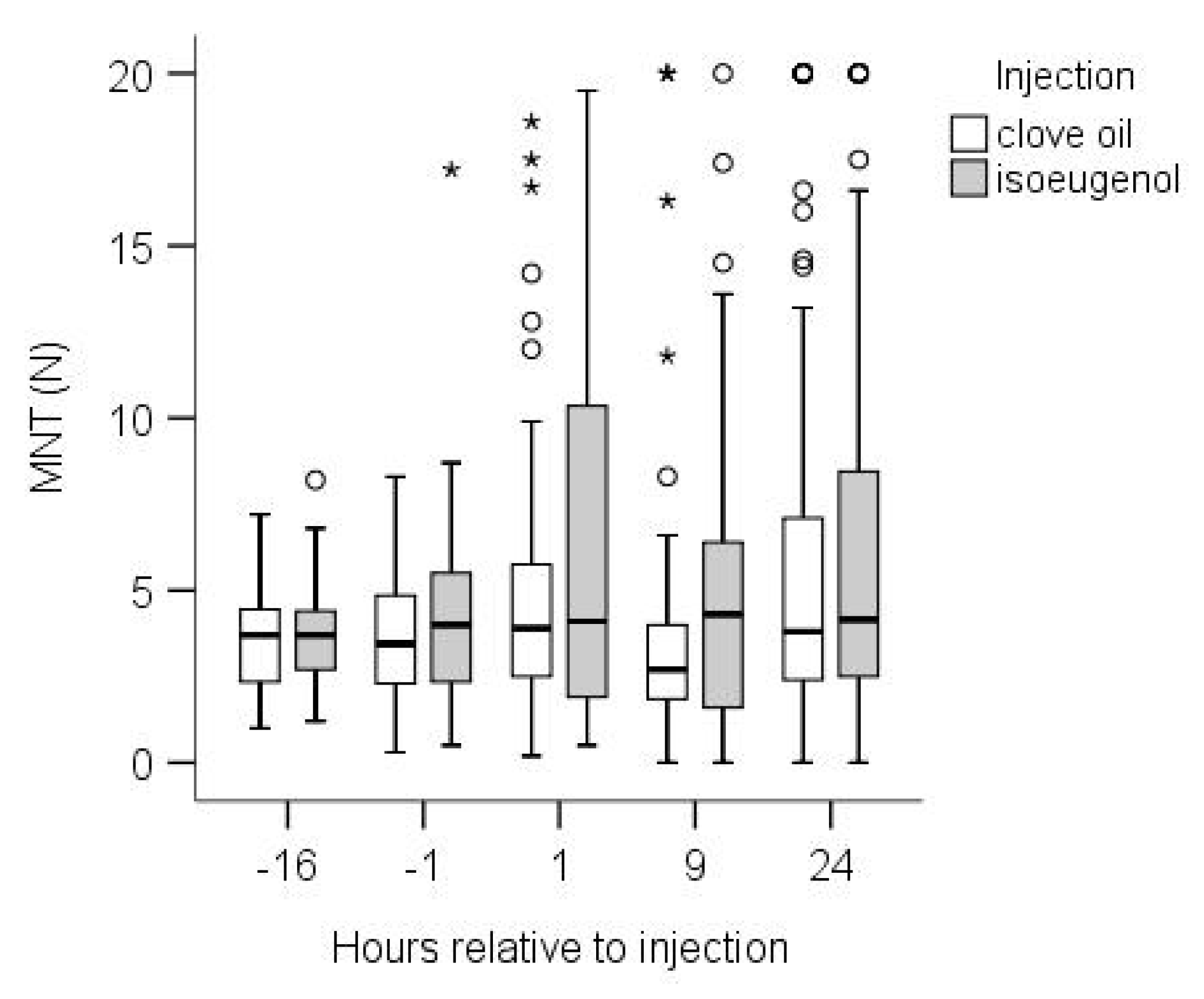
3.1. Calves
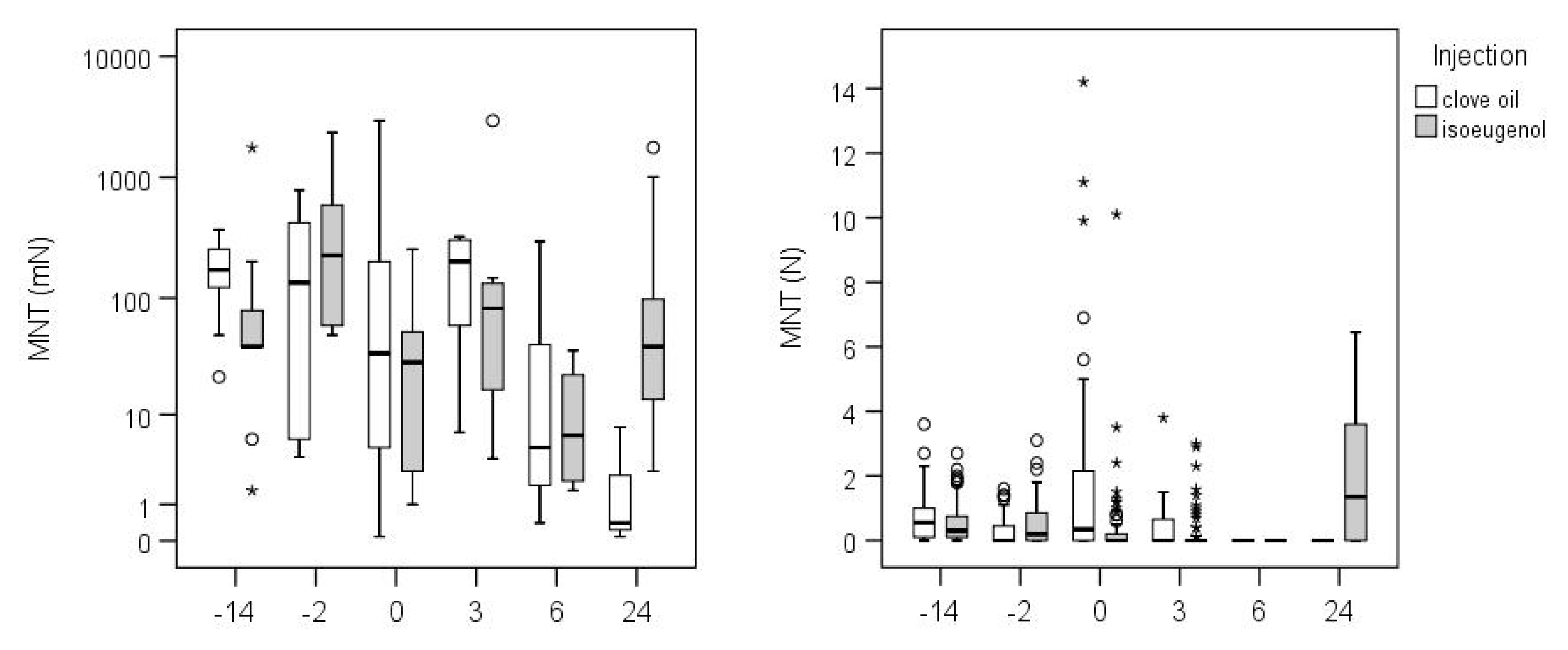
3.2. Goats
4. Discussion
4.1. Calves
4.2. Goats
4.3. General Discussion–Limitations of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grøndahl-Nielsen, C.; Simonsen, H.B.; Damkjer Lund, J.; Hesselholt, M. Behavioural, endocrine and cardiac responses in young calves undergoing dehorning without and with use of sedation and analgesia. Vet. J. 1999, 158, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.; Senn, M. Behavioural and physiological responses of calves to dehorning by heat cauterization with or without local anaesthesia. Appl. Anim. Behav. Sci. 1999, 62, 153–171. [Google Scholar] [CrossRef]
- Faulkner, P.M.; Weary, D.M. Reducing pain after dehorning in dairy calves. J. Dairy Sci. 2000, 83, 2037–2041. [Google Scholar] [CrossRef]
- Al-Sobayil, F.A. A new simple device for dehorning in small ruminants. Small Rumin. Res. 2005, 67, 232–234. [Google Scholar] [CrossRef]
- Cozzi, G.; Gottardo, F.; Brscic, M.; Contiero, B.; Irrgang, N.; Knierim, U.; Pentelescu, O.; Windig, J.J.; Mirabito, L.; Kling Eveilland, F.; et al. Dehorning of cattle in the EU member states: A quantitative survey of the current practices. Livest Sci. 2015, 179, 4–11. [Google Scholar] [CrossRef]
- Heinrich, A.; Duffield, T.F.; Lissemore, K.D.; Squires, E.J.; Millman, S.T. The impact of meloxicam on postsurgical stress associated with cautery dehorning. J. Dairy Sci. 2009, 92, 540–547. [Google Scholar] [CrossRef]
- Knierim, U.; Irrgang, N.; Roth, B.A. To be or not to be horned-consequences in cattle. Livest Sci. 2015, 179, 29–37. [Google Scholar] [CrossRef]
- Thompson, K.G.; Bateman, R.S.; Morris, P.J. Cerebral infarction and meningoencephalitis following hot-iron disbudding of goat kids. N. Z. Vet. J. 2005, 53, 368–370. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Ben Kahla-Nakbi, A.; Rouabhia, M.; Mahdouani, K.; Amina Bakhrouf, A. The chemical composition and biological activity of clove essential oil, eugenia caryophyllata (Syzigium aromaticum l. myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- PubChem. National Center for Biotechnology Information. U.S. National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Isoeugenol (accessed on 14 June 2020).
- Keene, J.L.; Noakes, D.L.G.; Moccia, R.D.; Soto, C.G. The efficacy of clove oil as an anaesthetic for rainbow trout, oncorhynchus mykiss (Walbaum). Aquac. Res. 1998, 29, 89–101. [Google Scholar] [CrossRef]
- Kahwa, D.; Rutaisire, J.; Kaiser, H. The use of clove oil to induce anaesthesia, and its effects on blood chemistry, in Lates niloticus from lake victoria, uganda. Afr. J. Aquat. Sci. 2015, 1–7. [Google Scholar] [CrossRef]
- Pattanasiri, T.; Taparhudee, W.; Suppakul, P. Acute toxicity and anaesthetic effect of clove oil and eugenol on siamese fighting fish, Betta splendens. Aquacult. Int. 2017, 25, 163–175. [Google Scholar] [CrossRef]
- Guenette, S.A.; Beaudry, F.; Marier, J.F.; Vachon, P. Pharmacokinetics and anesthetic activity of eugenol in male sprague–dawley rats. J. Vet. Pharmacol. Therap. 2006, 29, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Taher, Y.A.; Samud, A.M.; El-Taher, F.E.; ben-Hussin, G.; Elmezogi, J.S.; Al-Mehdawi, B.F.; Salem, H.A. Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice. Libyan J. Med. 2015, 10, 28685. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Supriyanto, E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules 2012, 17, 6290–6304. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef]
- Molaei, M.M.; Azari, O.; Esmaeilzadeh, S. Study of calves disbudding following injection of clove oil underhorn bud. J. Vet. Res. 2014, 69, 363–369. [Google Scholar]
- Molaei, M.M.; Mostafavi, A.; Kheirandish, R.; Azari, O.; Shaddel, M. Study of disbudding goat kids following injection of clove oil essence in horn bud region. Vet. Res. Forum. 2015, 6, 17–22. [Google Scholar]
- Sutherland, M.; Julian, A.; Huddart, F. Clove oil delays rather than prevents scur/horn growth in dairy cattle. Vet. Sci. 2019, 6, 102. [Google Scholar] [CrossRef]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Cave, V.M.; Turner, A.; Sutherland, M.A. The effectiveness of clove oil and two different cautery disbudding methods on preventing horn growth in dairy goat kids. PLoS ONE 2018, 13, e0198229. [Google Scholar] [CrossRef]
- Schoiswohl, J.; Stanitznig, A.; Waiblinger, S.; Frahm, S.; Krametter-Froetscher, R.; Wittek, T. Suppression of horn growth in cattle by clove oil and isoeugenol. J. Vet. Behav. 2020, 36, 1–3. [Google Scholar] [CrossRef]
- Heinrich, A.; Duffield, T.F.; Lissemore, K.D.; Squires, E.J.; Millman, S.T. The effect of meloxicam on behavior and pain sensitivity of dairy calves following cautery dehorning with a local anesthetic. J. Dairy Sci. 2010, 93, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.H.; Cramer, G.; Walter, J.J.; Millman, S.T.; Kelton, D.F. Randomized clinical trial of tetracycline hydrochloride bandage and paste treatments for resolution of lesions and pain associated with digital dermatitis in dairy cattle. J. Dairy Sci. 2013, 96, 7550–7557. [Google Scholar] [CrossRef]
- Stock, M.L.; Millman, S.T.; Barth, L.A.; Van Engen, N.K.; Hsu, W.H.; Wang, C.; Gehring, R.; Parsons, R.L.; Coetzee, J.F. The effects of firocoxib on cautery disbudding pain and stress responses in preweaned dairy calves. J. Dairy Sci. 2015, 98, 6058–6069. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.B.; Mintline, E.M.; Banuelos, J.; Walker, K.A.; Hoar, B.; Varga, A.; Drake, D.; Weary, D.M. Pain sensitivity and healing of hot-iron cattle brands. J. Anim. Sci. 2014, 92, 5674–5682. [Google Scholar] [CrossRef]
- Mintline, E.M.; Stewart, M.; Rogers, A.R.; Cox, N.R.; Verkerk, G.A.; Stookey, J.M.; Webster, J.R.; Tucker, C.B. Play behavior as an indicator of animal welfare: Disbudding in dairy calves. Appl. Anim. Behav. Sci. 2013, 144, 22–30. [Google Scholar] [CrossRef]
- Hempstead, M.N.; Waas, J.R.; Stewart, M.; Zobel, G.; Cave, V.M.; Julian, A.F.; Sutherland, M.A. Pain sensitivity and injury associated with three methods of disbudding goat kids: Cautery, cryosurgical and caustic paste. Vet. J. 2018, 239, 42–47. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Larive, J.; Cave, V.; Zobel, G. Behavioural and physiological responses to clove oil injected under the horn bud of calves. Appl. Anim. Behav. Sci. 2018, 204, 29–36. [Google Scholar] [CrossRef]
- Khalilzadeh, E.; Hazrati, R.; Saiah, G.V. Effects of topical and systemic administration of Eugenia caryophyllata buds essential oil on corneal anesthesia an d analgesia. RPS 2016, 11, 293–302. [Google Scholar] [CrossRef]
- Sarrami, N.; Pemberton, M.; Thornhill, M.; Theaker, E. Adverse reactions associated with the use of eugenol in dentistry. Br. Dent. J. 2002, 193, 257–259. [Google Scholar] [CrossRef]
- McMackin, M.Z.; Lewin, M.R.; Tabuena, D.R.; Arreola, E.; Moffatt, C.; Fuse, M. Use of von frey filaments to assess nociceptive sensitization in the hornworm, Manduca sexta. J. Neurosci. Methods. 2016, 257, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, C.; Galeotti, N.; Di Cesare Mannelli Mazzanti, G.; Bartolini, A. Local anaesthetic activity of -caryophyllene. Farmaco 2001, 56, 387–389. [Google Scholar] [CrossRef]
- Alqareer, A.; Alyaha, A.; Andersson, L. The effect of clove and benzocaine versus placebo as topical anesthetics. J. Dent. 2006, 34, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, B. Coping and coping strategies: A behavioural view. Appl. Anim. Behav. Sci. 1995, 43, 123–134. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; de Boer, S.F.; Coppens, C.M.; Buwalda, B. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front. Neuroendocrinol. 2010, 31, 307–321. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Van Reenen, C.G. Animal Behavior and well-being symposium: Interaction between coping style/personality, stress, and welfare: Relevance for domestic farm animals. J. Anim. Sci. 2016, 94, 2284–2296. [Google Scholar] [CrossRef]
- Ijichi, C.; Collins, L.M.; Elwood, R.W. Pain expression is linked to personality in horses. Appl. Anim. Behav. Sci. 2014, 152, 38–43. [Google Scholar] [CrossRef]
- van Amerongen, G.; de Boer, M.W.; Groeneveld, G.J.; Hay, J.L. A literature review on the pharmacological sensitivity of human evoked hyperalgesia pain models. Br. J. Clin. Pharmacol. 2016, 82, 903–922. [Google Scholar] [CrossRef]
- Stock, M.L.; Barth, L.A.; Van Engen, N.K.; Millman, S.T.; Gehring, R.; Wang, C.; Voris, E.A.; Wulf, L.W.; Labeur, L.; Hsu, W.H.; et al. Impact of carprofen administration on stress and nociception responses of calves to cautery dehorning. J. Anim. Sci. 2016, 94, 542–555. [Google Scholar] [CrossRef]
- Steagall, P.V.M.; Taylor, P.M.; Brondani, J.T.; Luna, S.P.L.; Dixon, M.J.; Ferreira, T.H. Effects of buprenorphine, carprofen and saline on thermal and mechanical nociceptive thresholds in cats. Vet. Anaesth. Analg. 2007, 34, 344–350. [Google Scholar] [CrossRef]
- Schnyder, P.; Schönecker, L.; Schüpbach-Regula, G.; Meylan, M. Effects of management practices, animal transport and barn climate on animal health and antimicrobial use in swiss veal calf operations. Prev. Vet. Med. 2019, 167, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Di Giminiani, P.; Stausholm, J.S.; Viitasaari, E.; Petersen, L.J.; Herskin, M.S. The effect of social isolation, gender and familiarity with the experimental procedure on tests of porcine nociceptive thresholds. Vet. Anaesth. Analg. 2015, 42, 648–656. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the human-animal relationship in farmed species: A critical review. Appl. Anim. Behav. Sci. 2006, 101, 185–242. [Google Scholar] [CrossRef]
- Krohn, C.; Jago, J.G.; Boivin, X. The effect of early handling on the socialisation of young calves to humans. Appl. Anim. Behav. Sci 2001, 74, 121–133. [Google Scholar] [CrossRef]
- Jago, J.G.; Krohn, C.C.; Matthews, L.R. The influence of feeding and handling on the development of the human-animal interactions in young cattle. Appl. Anim. Behav. Sci. 1999, 62, 137–151. [Google Scholar] [CrossRef]
- Lürzel, S.; Münsch, C.; Windschnurer, I.; Futschik, A.; Palme, R.; Waiblinger, S. The influence of gentle interactions on avoidance distance towards humans, weight gain and physiological parameters in group-housed dairy calves. Appl. Anim. Behav. Sci. 2015, 172, 9–16. [Google Scholar] [CrossRef]
| Time Point | Clove Oil | Isoeugenol | ||||
|---|---|---|---|---|---|---|
| N | EstM (mN) | 95% CI (mN) | N | EstM (mN) | 95% CI (mN) | |
| −14 h | 10 | 140.63 | 38.01–520.34 | 9 | 50.89 | 12.88–201.14 |
| −2 h | 10 | 72.73 | 19.65–269.10 | 10 | 222.96 | 60.26–824.98 |
| 0 h | 9 | 19.75 | 5.00–78.05 | 10 | 14.92 | 4.03–55.20 |
| 3 h | 9 | 105.51 | 26.68–417.30 | 10 | 59.76 | 16.15–221.11 |
| 6 h | 3 | 6.79 | 0.66–70.02 | 4 | 10.73 | 1.42–81.08 |
| 24 h | 3 | 0.80 | 0.08–8.21 | 9 | 47.58 | 12.04–188.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frahm, S.; Di Giminiani, P.; Stanitznig, A.; Schoiswohl, J.; Krametter-Frötscher, R.; Wittek, T.; Waiblinger, S. Nociceptive Threshold of Calves and Goat Kids Undergoing Injection of Clove Oil or Isoeugenol for Disbudding. Animals 2020, 10, 1228. https://doi.org/10.3390/ani10071228
Frahm S, Di Giminiani P, Stanitznig A, Schoiswohl J, Krametter-Frötscher R, Wittek T, Waiblinger S. Nociceptive Threshold of Calves and Goat Kids Undergoing Injection of Clove Oil or Isoeugenol for Disbudding. Animals. 2020; 10(7):1228. https://doi.org/10.3390/ani10071228
Chicago/Turabian StyleFrahm, Sandra, Pierpaolo Di Giminiani, Anna Stanitznig, Julia Schoiswohl, Reinhild Krametter-Frötscher, Thomas Wittek, and Susanne Waiblinger. 2020. "Nociceptive Threshold of Calves and Goat Kids Undergoing Injection of Clove Oil or Isoeugenol for Disbudding" Animals 10, no. 7: 1228. https://doi.org/10.3390/ani10071228
APA StyleFrahm, S., Di Giminiani, P., Stanitznig, A., Schoiswohl, J., Krametter-Frötscher, R., Wittek, T., & Waiblinger, S. (2020). Nociceptive Threshold of Calves and Goat Kids Undergoing Injection of Clove Oil or Isoeugenol for Disbudding. Animals, 10(7), 1228. https://doi.org/10.3390/ani10071228





