In Vitro Characterization of Indigenous Probiotic Strains Isolated from Colombian Creole Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation
2.2. Morphological and Biochemical Tests
2.3. Bacterial Identification
2.4. Resistance under Gastrointestinal Conditions: Temperature, pH, and Sodium Chloride
2.5. Bile Salt Tolerance
2.6. Assessment of Antimicrobial Activity against Enteropathogenic Bacteria
2.7. Antibiotic Susceptibility Testing
2.8. Data and Statistical Analysis
3. Results
3.1. Morphological and Biochemical Tests of the Lactic Acid Bacteria Isolates
3.2. Physiological Characteristics of the Lactic Acid Bacteria Isolates
3.3. Assessment of Antimicrobial Activity of the Lactic Acid Bacteria Isolates
3.4. Evaluation of the Lactic Acid Bacteria Isolates on Antibiotic Sensitivity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sirichokchatchawan, W.; Pupa, P.; Praechansri, P.; Am-in, N.; Tanasupawat, S. Autochthonous lactic acid bacteria isolated from pig faeces in Thailand show probiotic properties and antibacterial activity against enteric pathogenic bacteria. Microb. Pathog. 2018, 119, 208–215. [Google Scholar] [CrossRef]
- Long, S.F.; Xu, Y.T.; Pan, L.; Wang, Q.; Wang, C.L.; Wu, J.Y.; Piao, X.S. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim. Feed Sci. Tech. 2018, 235, 23–32. [Google Scholar] [CrossRef]
- Zeng, X.; Lin, J. Factors influencing horizontal gene transfer in the intestine. Anim. Health Res. Rev. 2017, 18, 153–159. [Google Scholar] [CrossRef]
- Diraviyam, T.; Zhao, B.; Wang, Y.; Schade, R.; Michael, A.; Zhang, X. Effect of Chicken Egg Yolk Antibodies (IgY) against Diarrhea in Domesticated Animals: A Systematic Review and Meta-Analysis. PLoS ONE. 2014, 9, 97716. [Google Scholar] [CrossRef]
- Wu, Z. Balancing food security and AMR. A review of economic literature on antimicrobial use in food animal production. China Agric. Econ. Rev. 2017, 9, 14–31. [Google Scholar] [CrossRef]
- He, T.; Zhu, Y.H.; Yu, J.; Xia, B.; Liu, X. Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica Infantis. Vet. Microbiol. 2019, 230, 187–194. [Google Scholar] [CrossRef]
- Liu, G.; Aguilar, Y.M.; Zhang, L.; Ren, W.; Chen, S. Dietary supplementation with sanguinarine enhances serum metabolites and antibodies in growing pigs. J. Anim. Sci. 2016, 94, 75–78. [Google Scholar] [CrossRef]
- Unterweger, C.; Kahler, A.; Gerlach, G.F.; Viehmann, M.; Von Altrock, A. Administration of non-pathogenic isolates of Escherichia coli and Clostridium perfringens type A to piglets in a herd affected with a high incidence of neonatal diarrhoea. Animal 2017, 11, 670–676. [Google Scholar] [CrossRef]
- Unterweger, C.; Schwarz, L.; Viehmann, M.; von Altrock, A.; Gerlach, G.F. Treatment with probiotic bacteria does Not Diminish the impact of a Cystoisospora suis challenge in suckling piglets. Front. Vet. Sci. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- De Mille, C.M.; Curry, S.M.; Gabler, N.K.; Kerr, B.J. Effect of prebiotic, fatty acid and copper and zinc ingredients on nursery pig performance. J. Anim. Sci. 2018, 96, 156–157. [Google Scholar] [CrossRef]
- Chen, J.; Kang, B.; Zhao, Y.; Yao, K.; Fu, C. Effects of natural dietary supplementation with Macleaya cordata extract containing sanguinarine on growth performance and gut health of early-weaned piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Julio-Gonzalez, L.C.; Hernandez-Hernandez, O.; Moreno, F.J.; Olano, A.; Jimeno, M.L. Trans-β-galactosidase activity of pig enzymes embedded in the small intestinal brush border membrane vesicles. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.L.; Li, P.; Zeng, Z.K.; Tian, Q.Y.; Piao, X.S.; Kuang, E.Y.W. A comparison of the nutritional value of organic-acid preserved corn and heat-dried corn for pigs. Anim. Feed Sci. Tech. 2016, 214, 95–103. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Liu, J.B.; Cao, S.C.; Liu, J.; Xie, Y.N.; Zhang, H.F. Effect of probiotics and xylo-oligosaccharide supplementation on nutrient digestibility, intestinal health and noxious gas emission in weanling pigs. Asian Australas. J. Anim. Sci. 2018, 31, 1660–1669. [Google Scholar] [CrossRef]
- Liu, W.; Devi, S.; Park, J.; Kim, I. Effects of complex probiotic supplementation in growing pig diets with and without palm kernel expellers on growth performance, nutrient digestibility, blood parameters, fecal microbial shedding and noxious gas emission. Anim. Sci. J. 2018, 89, 552–560. [Google Scholar] [CrossRef]
- Patarapreecha, P.; Jaikan, W.; Juangsaman, A.; Khajarern, J. Effects of dietary Bacillus subtilis supplementation as probiotics on growth performance and nutrients digestibility in fattening pigs. Pak. J. Nutr. 2018, 17, 634–640. [Google Scholar]
- Shawk, D.J.; Feehan, B.J.; Harrison, O.L.; Woodworth, J.C.; Tokach, M.D. Effects of antimicrobial or probiotics on growth performance of 6 to 25 kg nursery pigs. J. Anim. Sci. 2018, 96, 114–115. [Google Scholar] [CrossRef]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Z.; Qiu, Y.; Wang, L. Effects of probiotic solid-state fermented complete feed on growth performance and pork quality of finishing pigs. J. Anim. Sci. 2018, 96, 232. [Google Scholar] [CrossRef]
- Patel, S.; Shukla, R.; Goyal, A. Probiotics in valorization of innate immunity across various animal models. J. Funct. Foods 2015, 14, 549–561. [Google Scholar] [CrossRef]
- Ma, T.; Suzuki, Y.; Guan, L.L. Dissect the mode of action of probiotics in affecting host-microbial interactions and immunity in food producing animals. Vet. Immunol. Immunopathol. 2018, 205, 35–48. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. Biomed. Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kleniewska, P.; Pawliczak, R. Influence of synbiotics on selected oxidative stress parameters. Oxidative Med. Cell. Longev. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Roselli, M.; Pieper, R.; Rogel-Gaillard, C.; de Vries, H.; Bailey, M. Immunomodulating effects of probiotics for microbiota modulation, gut health and disease resistance in pigs. Anim. Feed Sci. Technol. 2017, 233, 104–119. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P. Efficacy of species-specific probiotic Pediococcus acidilactici FT28 on blood biochemical profile, carcass traits and physicochemical properties of meat in fattening pigs. Res. Vet. Sci. 2018, 117, 60–64. [Google Scholar] [CrossRef]
- Chiang, M.L.; Chen, H.C.; Chen, K.N.; Lin, Y.C.; Lin, Y.T. Optimizing production of two potential probiotic Lactobacilli strains isolated from piglet feces as feed additives for weaned piglets. Asian Australas. J. Anim. 2015, 28, 1163–1170. [Google Scholar] [CrossRef]
- Qiao, J.; Li, H.; Wang, Z. Effects of Lactobacillus acidophilus dietary supplementation on the performance, intestinal barrier function, rectal microflora and serum immune function in weaned piglets challenged with Escherichia coli lipopolysaccharide. Antonie van Leeuwenhoek 2015, 107, 883–891. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Latorre, J.D.; Hernandez-Velasco, X.; Kuttappan, V.A.; Wolfenden, R.E.; Vicente, J.L. Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and Clostridium perfringens proliferation using an In vitro digestive model in different poultry diets. Front. Vet. Sci. 2015, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility Test Methods: Dilution and Disk Diffusion Methods. In Manual of Clinical Microbiology, 11th ed.; American Society of Microbiology: Washington, DC, USA, 2015; pp. 1253–1273. [Google Scholar]
- Vlková, E.; Rada, V.; Popelářová, P.; Trojanová, I.; Killer, J. Antimicrobial susceptibility of bifidobacteria isolated from gastrointestinal tract of calves. Livest. Sci. 2006, 105, 253–259. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, M.T.; Huang, L.; Chu, W.S. The dnaJ gene as a molecular discriminator to differentiate among species and strain within the Lactobacillus casei group. Mol. Cell. Probes 2015, 29, 479–484. [Google Scholar] [CrossRef]
- Brandt, K.; Barrangou, R. Using glycolysis enzyme sequences to inform Lactobacillus phylogeny. Microb. Genom. 2018, 4, 1–13. [Google Scholar] [CrossRef]
- Ayala, D.I.; Chen, J.C.; Bugarel, M.; Loneragan, G.H.; den Bakker, H.C. Molecular detection and quantification of viable probiotic strains in animal feedstuffs using the commercial direct fed microbial Lactobacillus animalis NP51 as a model. J. Microbiol. Methods 2018, 149, 36–43. [Google Scholar] [CrossRef]
- Kubota, H.; Senda, S.; Tokuda, H.; Uchiyama, H.; Nomura, N. Stress resistance of biofilm and planktonic Lactobacillus plantarum subsp. plantarum JCM 1149. Food Microbiol. 2009, 26, 592–597. [Google Scholar]
- Ito, T.; Miyamoto, H.; Kumagai, Y.; Udagawa, M.; Shinmyo, T. Thermophile-fermented compost extract as a possible feed additive to enhance fecundity in the laying hen and pig: Modulation of gut metabolism. J. Biosci. Bioeng. 2016, 121, 659–664. [Google Scholar] [CrossRef]
- Sayan, H.; Assavacheep, P.; Angkanaporn, K.; Assavacheep, A. Effect of Lactobacillus salivarius on growth performance, diarrhea incidence, fecal bacterial population and intestinal morphology of suckling pigs challenged with F4+ enterotoxigenic Escherichia coli. Asian Australas. J. Anim. Sci. 2018, 31, 1308–1314. [Google Scholar] [CrossRef]
- Secher, T.; Kassem, S.; Benamar, M.; Bernard, I.; Boury, M. Oral administration of the probiotic strain Escherichia coli Nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front. Immunol. 2017, 8, 1–10. [Google Scholar]
- Hu, P.L.; Yuan, Y.H.; Yue, T.L.; Guo, C.F. Bile acid patterns in commercially available oxgall powders used for the evaluation of the bile tolerance ability of potential probiotics. PLoS ONE. 2018, 13, e0192964. [Google Scholar] [CrossRef]
- Gancel, F.; Dzierszinski, F.; Tailliez, R. Identification and characterization of Lactobacillus species isolated from fillets of vacuum-packed smoked and salted herring (Clupea harengus). J. Appl. Microbiol. 1997, 82, 722–728. [Google Scholar] [CrossRef]
- Önal, D.D.; Sönmez, Ş.; Beyatli, Y. The effects of inulin as a prebiotic supplement and the synbiotic interactions of probiotics to improve oxalate degrading activity. Int. J. Food Sci. Technol. 2019, 54, 121–131. [Google Scholar] [CrossRef]
- De Filippis, F.; Troise, A.D.; Vitaglione, P.; Ercolini, D. Different temperatures select distinctive acetic acid bacteria species and promotes organic acids production during Kombucha tea fermentation. Food Microbiol. 2018, 73, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Laine, M.; von Wright, A.; Vuopio-Varkila, l.; Korhonen, T.; Mattila-Sandholm, T. Development of selection criteria for probiotic strains to assess their potential in functional foods: A nordic and european approach. Biosci. Microflora 1996, 15, 61–70. [Google Scholar] [CrossRef]
- De Keyser, K.; Dierick, N.; Kanto, U.; Hongsapak, T.; Buyens, G. Medium chain glycerides affect gut morphology, immune and goblet cells in post-weaning piglets: In vitro fatty acid screening with Escherichia coli and in vivo consolidation with LPS challenge. J. Anim. Physiol. Anim. Nutr. 2019, 103, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Vera, R.; Ormaza, J.; Muñoz, J.; Arteaga, F. Sánchez, Lactobacillus plantarum strains with probiotic potentials isolated from creole pigs. Rev. Salud. Anim. 2018, 40, 1–12. [Google Scholar]
- Menconi, A.; Kallapura, G.; Latorre, J.D.; Morgan, M.J.; Pumford, N.R. Effect of prebiotic, fatty acid and copper and zinc ingredients on nursery pig performance. Biosci. Microb. Food. 2014, H33, 25–30. [Google Scholar]
- Jurado, G.H.; Aguirre, F.D.; Ramírez, T.C. Characterization of isolated probiotic bacteria of the large intestine of pigs as alternative to using antibiotics. Rev. MVZ Cordoba 2009, 14, 1723–1735. [Google Scholar]
- Shazali, N.; Foo, H.L.; Loh, T.C.; Choe, D.W.; Abdul, R.R. Prevalence of antibiotic resistance in lactic acid bacteria isolated from the faeces of broiler chicken in Malaysia. Gut Pathog. 2014, 6, 1. [Google Scholar] [CrossRef]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic resistance among commercially available probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]
- Brilhante, M.; Perreten, V.; Donà, V. Multidrug resistance and multivirulence plasmids in enterotoxigenic and hybrid Shiga toxin-producing/enterotoxigenic Escherichia coli isolated from diarrheic pigs in Switzerland. Vet. J. 2019, 244, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Gama, J.A.; Zilhão, R.; Dionisio, F. Impact of plasmid interactions with the chromosome and other plasmids on the spread of antibiotic resistance. Plasmid 2018, 99, 82–88. [Google Scholar] [CrossRef] [PubMed]
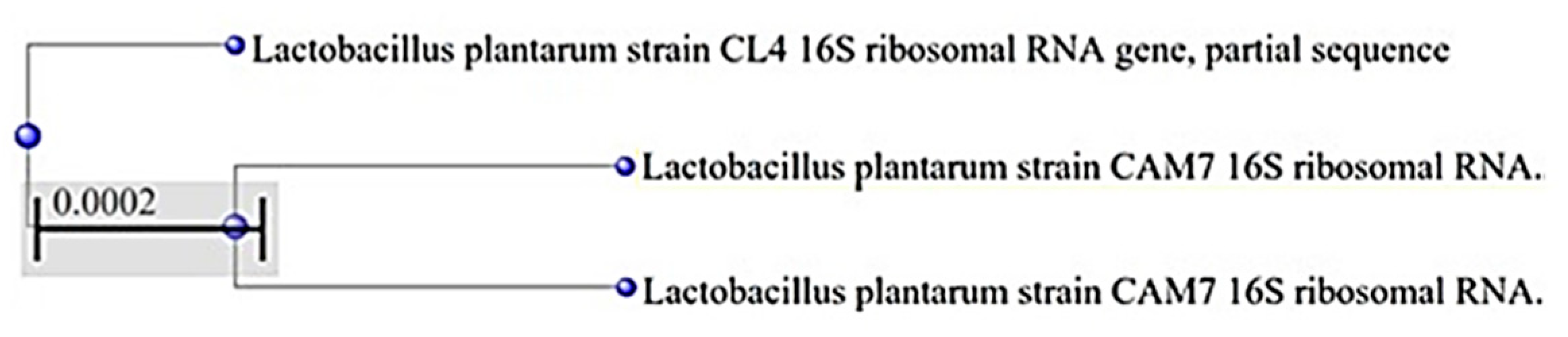
| Bacterial Strains | API 50 CH | GenBank Accession and URL |
|---|---|---|
| CAM6 | Lactobacillus plantarum | MK523644.1; URL: https://bit.ly/2t4cHC9 |
| CAM7 | Lactobacillus brevis | MK537353.1; URL: https://bit.ly/2MPnwzd |
| CL4 | Lactobacillus acidophilus | MK523645.1; URL: https://bit.ly/2tXJSYC |
| Items | Growth (Optical Density) of Lactobacillus Species | SEM ± | p Value | ||
|---|---|---|---|---|---|
| CAM6 | CAM7 | CL4 | |||
| pH | |||||
| 3 | 3.86 | 3.61 | 3.46 | 0.15 | 0.256 |
| 4 | 3.71 | 3.45 | 3.64 | 0.13 | 0.403 |
| 5.6 | 3.42 a | 3.04 b | 3.29 ab | 0.09 | 0.006 |
| 7 | 2.71 a | 2.45 ab | 2.14 b | 0.09 | 0.014 |
| Bile salts (%) | |||||
| 0.05 | 3.61 | 3.37 | 3.44 | 0.12 | 0.422 |
| 0.10 | 2.75 b | 3.06 ab | 3.28 a | 0.09 | 0.019 |
| 0.15 | 3.24 | 3.45 | 3.21 | 0.08 | 0.177 |
| 0.30 | 2.81 a | 2.71 a | 2.44 b | 0.05 | 0.006 |
| NaCl (%) | |||||
| 2 | 3.21 | 3.44 | 3.33 | 0.17 | 0.670 |
| 4 | 2.90 b | 3.35 a | 3.31 a | 0.08 | 0.015 |
| 7 | 2.54 b | 3.23 a | 2.12 c | 0.10 | 0.001 |
| 10 | 1.72 a | 1.12 b | 0.85 b | 0.12 | 0.006 |
| Temperature (°C) | |||||
| 30 | 3.70 | 3.38 | 3.52 | 0.18 | 0.506 |
| 37 | 3.57 | 3.48 | 3.51 | 0.17 | 0.935 |
| 42 | 2.47 | 2.89 | 2.86 | 0.14 | 0.136 |
| Pathogenic Bacteria | Inhibition Halo (mm) | ||
|---|---|---|---|
| CAM6 | CAM7 | CL4 | |
| E. coli strain NBRC 102203 | 19.7 | 0 | 6.9 |
| S. enterica serovar Typhimurium 4.5.12 | 7.1 | 6.2 | 7.9 |
| Klebsiella pneumoniae ATCC BAA-1705D-5 | 8.2 | 6.3 | 0 |
| Pseudomonas aeruginosa ATCC 15442 | 9.9 | 9.7 | 7.3 |
| Strains | Antibiotics | ||||
|---|---|---|---|---|---|
| Ciprofloxacin | Trimethoprim/Sulfamethoxazole | Amoxicillin | Tetracycline | Doxycycline | |
| CAM6 | 8.1 (R) | 19.5 (I) | 24.3 (S) | 8.2 (R) | 8.2 (R) |
| CAM7 | 8.4 (R) | 12.9 (R) | 25.6 (S) | 9.3 (R) | 9.0 (R) |
| CL4 | 8.0 (R) | 9.8 (R) | 26.7 (S) | 6.8 (R) | 8.1 (R) |
| E. coli strain NBRC 102203 | 26.1 (S) | 18.2 (I) | 7.0 (R) | 12.6 (R) | 7.3 (R) |
| S. enterica serovar Typhimurium 4.5.12: i:- | 28.7 (S) | 22.7 (S) | 8.5 (R) | 8.0 (R) | 0 (R) |
| K. pneumonia ATCC BAA-1705D-5 | 0 (R) | 8.8 (R) | 0 (R) | 0 (R) | 0 (R) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betancur, C.; Martínez, Y.; Tellez-Isaias, G.; Avellaneda, M.C.; Velázquez-Martí, B. In Vitro Characterization of Indigenous Probiotic Strains Isolated from Colombian Creole Pigs. Animals 2020, 10, 1204. https://doi.org/10.3390/ani10071204
Betancur C, Martínez Y, Tellez-Isaias G, Avellaneda MC, Velázquez-Martí B. In Vitro Characterization of Indigenous Probiotic Strains Isolated from Colombian Creole Pigs. Animals. 2020; 10(7):1204. https://doi.org/10.3390/ani10071204
Chicago/Turabian StyleBetancur, César, Yordan Martínez, Guillermo Tellez-Isaias, Mavir Carolina Avellaneda, and Borja Velázquez-Martí. 2020. "In Vitro Characterization of Indigenous Probiotic Strains Isolated from Colombian Creole Pigs" Animals 10, no. 7: 1204. https://doi.org/10.3390/ani10071204
APA StyleBetancur, C., Martínez, Y., Tellez-Isaias, G., Avellaneda, M. C., & Velázquez-Martí, B. (2020). In Vitro Characterization of Indigenous Probiotic Strains Isolated from Colombian Creole Pigs. Animals, 10(7), 1204. https://doi.org/10.3390/ani10071204







