Association of GHR Polymorphisms with Milk Production in Buffaloes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Samples Preparation
2.2. Sample Preparation
2.3. SNPs Detection and Genotyping
2.4. Real-Time PCR
2.5. Western Blot
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Detected SNPs
3.2. Analysis of Genotype and Haplotype Frequencies
3.3. Association of Genotypes and Haplotypes with Milk Yield and Composition
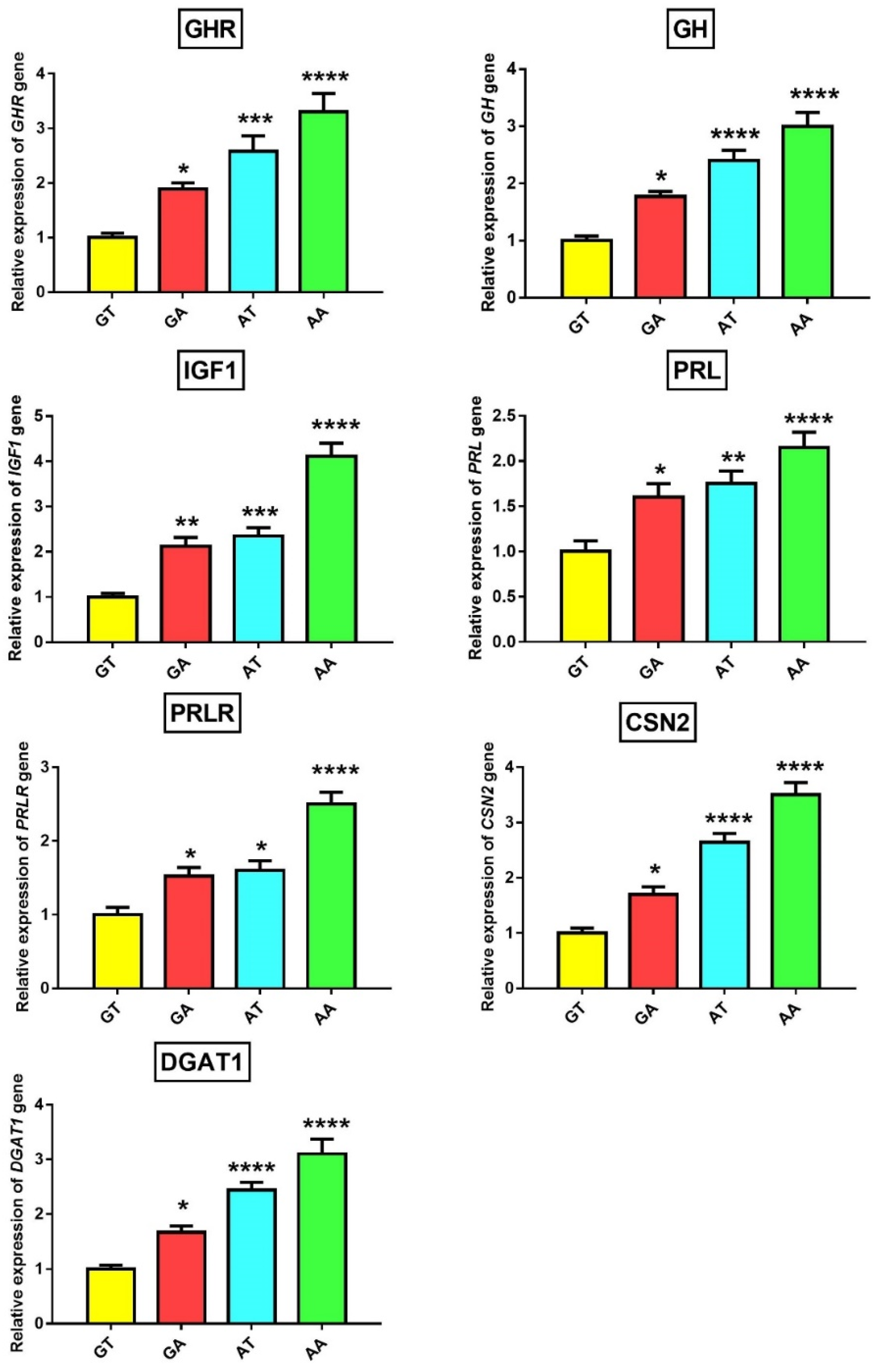
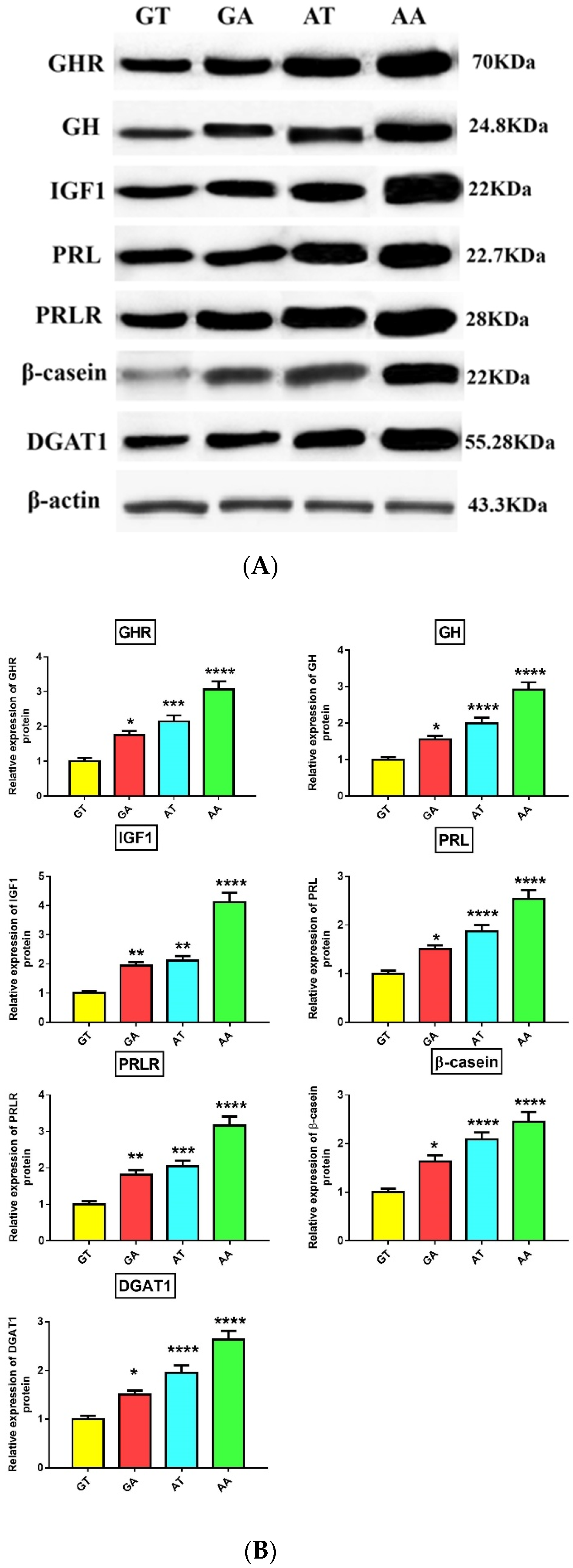
3.4. Association of GHR Polymorphisms with Gene and Protein Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gebreyesus, G.; Lund, M.S.; Janss, L.; Poulsen, N.A.; Larsen, L.B.; Bovenhuis, H.; Buitenhuis, A.J. Short communication: Multi-trait estimation of genetic parameters for milk protein composition in the danish holstein. J. Dairy Sci. 2016, 99, 2863–2866. [Google Scholar] [CrossRef]
- Jiang, H.; Lucy, M.C. Variants of the 5’-untranslated region of the bovine growth hormone receptor mrna: Isolation, expression and effects on translational efficiency. Gene 2001, 265, 45–53. [Google Scholar] [CrossRef]
- Maj, A.; Oprzadek, J.; Oprzadek, A.; Dymnicki, E.; Zwierzchowski, L. Polymorphism in the 5’-noncoding region of the bovine growth hormone receptor gene and its association with meat, production traits in cattle. Anim. Res. 2004, 53, 503–514. [Google Scholar] [CrossRef]
- Maj, A.; Zwierzchowski, L. Molecular evolution of coding and non-coding sequences of the growth hormone receptor (ghr) gene in the family bovidae. Folia Biol. (Krakow) 2006, 54, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Blott, S.; Kim, J.J.; Moisio, S.; Schmidt-Kuntzel, A.; Cornet, A.; Berzi, P.; Cambisano, N.; Ford, C.; Grisart, B.; Johnson, D.; et al. Molecular dissection of a quantitative trait locus: A phenylalanine-to-tyrosine substitution in the transmembrane domain of the bovine growth hormone receptor is associated with a major effect on milk yield and composition. Genetics 2003, 163, 253–266. [Google Scholar] [PubMed]
- Rahmatalla, S.A.; Muller, U.; Strucken, E.M.; Reissmann, M.; Brockmann, G.A. The f279y polymorphism of the ghr gene and its relation to milk production and somatic cell score in german holstein dairy cattle. J. Appl. Genet. 2011, 52, 459–465. [Google Scholar] [CrossRef]
- Sanchez, M.P.; Govignon-Gion, A.; Ferrand, M.; Gele, M.; Pourchet, D.; Amigues, Y.; Fritz, S.; Boussaha, M.; Capitan, A.; Rocha, D.; et al. Whole-genome scan to detect quantitative trait loci associated with milk protein composition in 3 french dairy cattle breeds. J. Dairy Sci. 2016, 99, 8203–8215. [Google Scholar] [CrossRef]
- Viitala, S.; Szyda, J.; Blott, S.; Schulman, N.; Lidauer, M.; Maki-Tanila, A.; Georges, M.; Vilkki, J. The role of the bovine growth hormone receptor and prolactin receptor genes in milk, fat and protein production in finnish ayrshire dairy cattle. Genetics 2006, 173, 2151–2164. [Google Scholar] [CrossRef]
- Waters, S.M.; McCabe, M.S.; Howard, D.J.; Giblin, L.; Magee, D.A.; MacHugh, D.E.; Berry, D.P. Associations between newly discovered polymorphisms in the bos taurus growth hormone receptor gene and performance traits in holstein-friesian dairy cattle. Anim. Genet. 2011, 42, 39–49. [Google Scholar] [CrossRef]
- Banos, G.; Woolliams, J.A.; Woodward, B.W.; Forbes, A.B.; Coffey, M.P. Impact of single nucleotide polymorphisms in leptin, leptin receptor, growth hormone receptor, and diacylglycerol acyltransferase (dgat1) gene loci on milk production, feed, and body energy traits of UK dairy cows. J. Dairy Sci. 2008, 91, 3190–3200. [Google Scholar] [CrossRef]
- Viale, E.; Tiezzi, F.; Maretto, F.; De Marchi, M.; Penasa, M.; Cassandro, M. Association of candidate gene polymorphisms with milk technological traits, yield, composition, and somatic cell score in Italian holstein-friesian sires. J. Dairy Sci. 2017, 100, 7271–7281. [Google Scholar] [CrossRef] [PubMed]
- Surya, T.; Vineeth, M.R.; Sivalingam, J.; Tantia, M.S.; Dixit, S.P.; Niranjan, S.K.; Gupta, I.D. Genomewide identification and annotation of snps in bubalus bubalis. Genomics 2019, 111, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.S.; Wang, J.; Yang, Y.; Lu, F.H.; Li, X.P.; Liu, Q.Y. Dgat1, gh, ghr, prl and prlr polymorphism in water buffalo (Bubalus bubalis). Reprod. Domest. Anim. Zuchthyg. 2012, 47, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Do, D.N.; Janss, L.L.; Jensen, J.; Kadarmideen, H.N. Snp annotation-based whole genomic prediction and selection: An application to feed efficiency and its component traits in pigs. J. Anim. Sci. 2015, 93, 2056–2063. [Google Scholar] [CrossRef]
- El-Bayomi, K.M.; Saleh, A.A.; Awad, A.; El-Tarabany, M.S.; El-Qaliouby, H.S.; Afifi, M.; El-Komy, S.; Essawi, W.M.; Almadaly, E.A.; El-Magd, M.A. Association of cyp19a1 gene polymorphisms with anoestrus in water buffaloes. Reprod. Fertil. Dev. 2018, 30, 487–497. [Google Scholar] [CrossRef]
- EL-Magd, M.A.; Saleh, A.A.; Nafeaa, A.A.; EL-Komy, S.M.; Afifi, M.A. Polymorphisms of the igf1 gene and their association with growth traits, serum concentration and expression rate of igf1 and igf1r in buffalo. J. Zhejiang Univ. Sci. B 2017, 18, 1064–1074. [Google Scholar] [CrossRef]
- Elmasry, E.; Omar, H.A.; Abdel Razek, F.A.; El-Magd, M.A. Preliminary studies on habitat and diversity of some sea urchin species (echinodermata: Echinoidea) on the southern levantine basin of egypt. Egypt. J. Aquat. Res. 2013, 39, 303–311. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Abo-Al-Ela, H.G.; El-Nahas, A.; Saleh, A.A.; Mansour, A.A. Effects of a novel snp of igf2r gene on growth traits and expression rate of igf2r and igf2 genes in gluteus medius muscle of egyptian buffalo. Gene 2014, 540, 133–139. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Saleh, A.A.; Abdel-Hamid, T.M.; Saleh, R.M.; Afifi, M.A. Is really endogenous ghrelin a hunger signal in chickens? Association of ghsr snps with increase appetite, growth traits, expression and serum level of ghrl, and gh. Gen. Comp. Endocrinol. 2016, 237, 131–139. [Google Scholar] [CrossRef]
- Boutinaud, M.; Rulquin, H.; Keisler, D.H.; Djiane, J.; Jammes, H. Use of somatic cells from goat milk for dynamic studies of gene expression in the mammary gland1. J. Anim. Sci. 2002, 80, 1258–1269. [Google Scholar] [CrossRef]
- Yakan, A.; Ozkan, H.; Eraslan, A.; Ünal, N.; Özbeyaz, C. Gene expression levels in some candidate genes for mastitis resistance, milk yield, and milk quality of goats reared under different feeding systems. Turk. J. Vet. Anim. Sci. 2018, 42. [Google Scholar] [CrossRef]
- El-Adawy, M.; El-Aziz, M.A.; El-Shazly, K.; Ali, N.G.; El-Magd, M.A. Dietary propionic acid enhances antibacterial and immunomodulatory effects of oxytetracycline on nile tilapia, oreochromis niloticus. Environ. Sci. Pollut. Res. 2018, 25, 34200–34211. [Google Scholar] [CrossRef] [PubMed]
- Sharawy, Z.Z.; Thiele, R.; Abbas, E.M.; El-Magd, M.A.; Hassaan, M.S.; Peter, C.; Schmidt, J.; Saborowski, R.; Goda, A.M.A.-S.; Slater, M.J. Antioxidant response, body composition of whiteleg shrimp litopenaeus vannamei co-cultured with nile tilapia oreochromis niloticus in recirculating aquaculture. Aquac. Environ. Interact. 2017, 9, 257–268. [Google Scholar] [CrossRef]
- Abo-Al-Ela, H.G.; El-Magd, M.A.; El-Nahas, A.F.; Mansour, A.A. Association of a novel snp in exon 10 of the igf2 gene with growth traits in egyptian water buffalo (Bubalus bubalis). Trop. Anim. Health Prod. 2014, 46, 947–952. [Google Scholar] [CrossRef] [PubMed]
- El-Magd, M.A.; Khalifa, S.F.; Alzahrani, F.A.A.; Badawy, A.A.; El-Shetry, E.S.; Dawood, L.M.; Alruwaili, M.M.; Alrawaili, H.A.; Risha, E.F.; El-Taweel, F.M.; et al. Incensole acetate prevents beta-amyloid-induced neurotoxicity in human olfactory bulb neural stem cells. Biomed. Pharmacother. 2018, 105, 813–823. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Abbas, H.E.; El-kattawy, A.M.; Mokhbatly, A. Novel polymorphisms of the igf1r gene and their association with average daily gain in egyptian buffalo (Bubalus bubalis). Domest. Anim. Endocrinol. 2013, 45, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cole, J.W.; Grond-Ginsbach, C. Departure from hardy weinberg equilibrium and genotyping error. Front. Genet. 2017, 8, 167. [Google Scholar] [CrossRef]
- Do, D.N.; Bissonnette, N.; Lacasse, P.; Miglior, F.; Zhao, X.; Ibeagha-Awemu, E.M. A targeted genotyping approach to enhance the identification of variants for lactation persistency in dairy cows. J. Anim. Sci. 2019, 97, 4066–4075. [Google Scholar] [CrossRef]
- Fontanesi, L.; Scotti, E.; Tazzoli, M.; Beretti, F.; Dall’Olio, S.; Davoli, R.; Russo, V. Investigation of allele frequencies of the growth hormone receptor (ghr) f279y mutation in dairy and dual purpose cattle breeds. Ital. J. Anim. Sci. 2007, 6, 415–420. [Google Scholar] [CrossRef]
- Fallin, D.; Cohen, A.; Essioux, L.; Chumakov, I.; Blumenfeld, M.; Cohen, D.; Schork, N.J. Genetic analysis of case/control data using estimated haplotype frequencies: Application to apoe locus variation and alzheimer’s disease. Genome Res. 2001, 11, 143–151. [Google Scholar] [CrossRef]
- Wyszynska-Koko, J.; Pierzchala, M.; Flisikowski, K.; Kamyczek, M.; Rozycki, M.; Kuryl, J. Polymorphisms in coding and regulatory regions of the porcine myf6 and myog genes and expression of the myf6 gene in m. Longissimus dorsi versus productive traits in pigs. J. Appl. Genet. 2006, 47, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Lin, J.; Ye, L.; Zhang, Q.; Chen, J.; Yang, Q.; Yu, Q. Modulation of mammary gland development and milk production by growth hormone expression in gh transgenic goats. Front. Physiol. 2016, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.C.; Verkerk, G.A.; Whyte, B.E.; Macdonald, K.A.; Burton, L.; Cursons, R.T.; Roche, J.R.; Holmes, C.W. Somatotropic axis components and nutrient partitioning in genetically diverse dairy cows managed under different feed allowances in a pasture system. J. Dairy Sci. 2009, 92, 526–539. [Google Scholar] [CrossRef]
- Oleński, K.; Suchocki, T.; Kamiński, S. Inconsistency of associations between growth hormone receptor gene polymorphism and milk performance traits in polish holstein-friesian cows and bulls. Anim. Sci. Pap. Rep. 2010, 28, 229–234. [Google Scholar]
- Maj, A.; Gajewska, A.; Pierzchala, M.; Kochman, K.; Zwierzchowski, L. Single base substitution in growth hormone receptor gene influences the receptor density in bovine liver. Neuroendocr. Lett. 2007, 28, 401–405. [Google Scholar]
- Etherton, T.D. Somatotropic function: The somatomedin hypothesis revisited. J. Anim. Sci. 2004, 82 (E-Suppl.), E239–E244. [Google Scholar] [PubMed]
- Cosenza, G.; Iannaccone, M.; Auzino, B.; Macciotta, N.P.P.; Kovitvadhi, A.; Nicolae, I.; Pauciullo, A. Remarkable genetic diversity detected at river buffalo prolactin receptor (prlr) gene and association studies with milk fatty acid composition. Anim. Genet. 2018, 49, 159–168. [Google Scholar] [CrossRef]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (prl) and its receptor: Actions, signal transduction pathways and phenotypes observed in prl receptor knockout mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, H.-M.; Moon, S.-J.; Kang, M.-J. Cloning and molecular characterization of porcine β-casein gene (cns2). Asian-Australas. J. Anim. Sci. 2012, 25, 421–427. [Google Scholar] [CrossRef][Green Version]
- Gu, M.; Cosenza, G.; Nicolae, I.; Bota, A.; Guo, Y.; Di Stasio, L.; Pauciullo, A. Transcript analysis at dgat1 reveals different mrna profiles in river buffaloes with extreme phenotypes for milk fat. J. Dairy Sci. 2017, 100, 8265–8276. [Google Scholar] [CrossRef]
- Pegolo, S.; Cecchinato, A.; Mele, M.; Conte, G.; Schiavon, S.; Bittante, G. Effects of candidate gene polymorphisms on the detailed fatty acids profile determined by gas chromatography in bovine milk. J. Dairy Sci. 2016, 99, 4558–4573. [Google Scholar] [CrossRef]
- Li, C.; Sun, D.; Zhang, S.; Wang, S.; Wu, X.; Zhang, Q.; Liu, L.; Li, Y.; Qiao, L. Genome wide association study identifies 20 novel promising genes associated with milk fatty acid traits in Chinese holstein. PLoS ONE 2014, 9, e96186. [Google Scholar] [CrossRef] [PubMed]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function—An update. Anim. Feed Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Anim. Int. J. Anim. Biosci. 2013, 7 (Suppl. 1), 132–162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, L.; Prakapenka, D.; VanRaden, P.M.; Cole, J.B.; Da, Y. A large-scale genome-wide association study in U.S. Holstein cattle. Front. Genet. 2019, 10, 412. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer | Reverse Primer | Ta (°C) | Localization * | Size (bp) | Experiment |
|---|---|---|---|---|---|---|
| GHR.E4 | AGGACCATCCATTACCCTCCTGATTT | TCCATTCCCATCACTGCATGAC | 56 | Exon4 (E4), introns(I) 2, 3 | 265 | SNPs detection |
| GHR.E5 | AGGAGCTGGCACCTTATATGCAGT | CCCCGCTTATGTAATCTAAAGCCATGT | E5, I4, I5 | 472 | ||
| GHR.E6 | ACTGATTCTCTGCTGAAATGCACAGT | CCATTTTCCACTGGGTCTCATTCAGT | E6, I5 | 205 | ||
| GHR.E8 | CTTTGGAATACTTGGGCTAG | CACTTCACTCAGGATTCAC | E8, I8 | 166 | ||
| GHR | CCAGCTTTCCTTGTCAGAGCA | TGTGATTAGCCCCATCTGTCC | 60 | E2 and E3 | 148 | Relative expression by qRT-PCR |
| PRLR | AACCATTGAGACTGGCAGGG | AAGGGGGTTTTGTCTTGGGG | E10 | 114 | ||
| PRL | GCATGCTTGGCTCTAATGGG | TGTCAGTTTCTGCTATTTGTGAC | Coding sequences | 186 | ||
| GH | CAGCCATCTGTTGTTTGCCC | CCCCCAGAATAGAATGACACC | 130 | |||
| IGF1 | TTGGTGGATGCTCTCCAGTTC | AGCAGCACTCATCCACGATTC | 218 | |||
| β-casein | AATCTGCACCTTCCTCTGCC | ACTGAGAAAGGGACAGCACG | 109 | |||
| DGAT1 | GGTCCGGGACACAGACAAG | CTGCTGAAGCCACTGTCAGA | 111 | |||
| β-actin | CGACAACGGCTCCGGCATGT | CTCCTCAGGGGCCACACGGA | 211 |
| SNP | Genotype Frequency (Number) | Allele Frequency | χ2 (p-Value) | He | Ne | MAF | PIC | |||
|---|---|---|---|---|---|---|---|---|---|---|
| c.380G>A | GG | GA | AA | G | A | 5.16 (0.023) | 0.38 | 1.61 | 0.255 | 0.31 |
| 0.585 (234) | 0.32 (128) | 0.095 (38) | 0.745 | 0.255 | ||||||
| c.387C>T | CC | CT | TT | C | A | 5.09 (0.024) | 0.49 | 1.99 | 0.47 | 0.37 |
| 0.32 (128) | 0.42 (168) | 0.26 (104) | 0.53 | 0.47 | ||||||
| c.435A>G | AA | AG | GG | A | G | 6.75 (0.009) | 0.49 | 1.97 | 0.445 | 0.37 |
| 0.35 (140) | 0.41 (164) | 0.24 (96) | 0.555 | 0.445 | ||||||
| c.836T>A | TT | TA | AA | T | A | 0.012 (0.914) | 0.30 | 1.43 | 0.185 | 0.26 |
| 0.665 (266) | 0.30 (120) | 0.035 (14) | 0.815 | 0.185 | ||||||
| Traits | GG (n = 234) | GA (n = 128) | AA (n = 38) |
|---|---|---|---|
| Milk yield (kg, 305 day) | 2048.33 ± 46.28 b | 2052.04 ± 49.50 b | 2330.48 ± 48.27 a |
| Fat percentage | 6.12 ± 0.12 Bb | 6.45 ± 0.13 Bb | 7.26 ± 0.14 Aa |
| 305 day-fat yield (kg) | 130.35 ± 2.74 Bb | 132.41 ± 2.20 Bb | 151.19 ± 5.07 Aa |
| Protein percentage | 4.05 ± 0.10 Bb | 4.32 ± 0.09 Bb | 4.81 ± 0.12 Aa |
| 305 day-protein yield (kg) | 84.36 ± 2.19 Bb | 86.65 ± 2.34 Bb | 104.48 ± 4.60 Aa |
| Lactose percentage | 5.32 ± 0.17 | 5.14 ± 0.25 | 5.08 ± 0.19 |
| Total solid percentage | 16.42 ± 0.38 | 16.60 ± 0.42 | 17.05 ± 0.45 |
| Traits | TT (n = 266) | TA (n = 120) | AA (n = 14) |
|---|---|---|---|
| Milk yield (kg, 305 day) | 2032.33 ± 30.25 b | 2058.80 ± 33.22 b | 2369.25 ± 31.47 a |
| Fat percentage | 7.09 ± 0.10 Aa | 6.55 ± 0.09 Ab | 6.00 ± 0.08 Bc |
| 305 day-fat yield (kg) | 144.17 ± 0.68 Aa | 135.63 ± 0.59 Bb | 136.80 ± 0.62 b |
| Protein percentage | 4.92 ± 0.08 Aa | 4.39 ± 0.07 Ab | 4.15 ± 0.06 Bb |
| 305 day-protein yield (kg) | 102.67 ± 0.74 Aa | 90.83 ± 0.62 Bb | 95.01 ± 0.60 b |
| Lactose percentage | 5.49 ± 0.28 | 5.30 ± 0.21 | 5.11 ± 0.10 |
| Total solid percentage | 17.26 ± 0.34 | 17.10 ± 0.31 | 16.87 ± 0.49 |
| Traits | GT (n = 256) | GA (n = 48) | AT (n = 68) | AA (n = 28) |
|---|---|---|---|---|
| Milk yield (kg, 305 day) | 2090.48 ± 43.82 b | 2340.17 ± 40.80 a | 2315.48 ± 44.19 a | 2438.37 ± 43.05 a |
| Fat percentage | 6.03 ± 0.12 Bb | 6.78 ± 0.10 a | 7.04 ± 0.16 Aa | 7.38 ± 0.15 Aa |
| 305 day-fat yield (kg) | 126.16 ± 1.94 Cc | 158.35 ± 2.20 Bb | 163.20 ± 2.16 Bb | 179.38 ± 2.54 Aa |
| Protein percentage | 4.05 ± 0.08 Bb | 4.51 ± 0.07 a | 4.62 ± 0.09 Aa | 4.98 ± 0.11 Aa |
| 305 day-protein yield (kg) | 84.66 ± 1.04 Bc | 105.75 ± 1.13 Ab | 106.47 ± 1.15 Ab | 114.56 ± 1.37 Aa |
| Lactose percentage | 5.10 ± 0.14 | 5.30 ± 0.16 | 5.24 ± 0.23 | 5.51 ± 0.34 |
| Total solid percentage | 17.24 ± 0.39 | 16.85 ± 0.30 | 17.00 ± 0.34 | 17.37 ± 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Komy, S.M.; Saleh, A.A.; Abdel-Hamid, T.M.; El-Magd, M.A. Association of GHR Polymorphisms with Milk Production in Buffaloes. Animals 2020, 10, 1203. https://doi.org/10.3390/ani10071203
El-Komy SM, Saleh AA, Abdel-Hamid TM, El-Magd MA. Association of GHR Polymorphisms with Milk Production in Buffaloes. Animals. 2020; 10(7):1203. https://doi.org/10.3390/ani10071203
Chicago/Turabian StyleEl-Komy, Shymaa M., Ayman A. Saleh, Tamer M. Abdel-Hamid, and Mohammed A. El-Magd. 2020. "Association of GHR Polymorphisms with Milk Production in Buffaloes" Animals 10, no. 7: 1203. https://doi.org/10.3390/ani10071203
APA StyleEl-Komy, S. M., Saleh, A. A., Abdel-Hamid, T. M., & El-Magd, M. A. (2020). Association of GHR Polymorphisms with Milk Production in Buffaloes. Animals, 10(7), 1203. https://doi.org/10.3390/ani10071203







