Breeding and Economic Aspects of Cytogenetic Screening Studies of Pigs Qualified for Reproduction
Abstract
Simple Summary
Abstract
1. Introduction
2. Pig Karyotype Abnormalities and Their Effect on Carrier Fertility
2.1. Reciprocal Translocations
2.2. Robertsonian Translocations and Tandem Fusions
2.3. Peri- and Paracentric Inversions
2.4. Sex Chromosome Aneuploidies and Leukocytic Chimerism
3. Cytomolecular Diagnostics
4. Cytogenetic Screening of the Pig Population
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Danielak-Czech, B.; Kozubska-Sobocińska, A.; Rejduch, B. Molecular cytogenetics in the diagnostics of balanced chromosome mutations in the pig (Sus scrofa)—A review. Ann. Anim. Sci. 2016, 16, 679–699. [Google Scholar] [CrossRef]
- Raudsepp, T.; Chowdharry, B.P. Cytogenetics and chromosome maps. In The Genetics of the Pig, 2nd ed.; Rothschild, M.P., Ruvinsky, A., Eds.; CAB International: Wallingford, UK, 2011; pp. 134–178. [Google Scholar]
- Szczerbal, I.; Nowacka-Woszuk, J.; Dzimira, S.; Alama, A.; Iskrzak, P.; Świtoński, M. Detection and quantification of leucocyte chimerism (XX/XY) using FISH and Digital droplet PCR (ddPCR) in the offspring of highly prolific sows. Comp. Cytogenet. 2018, 12, 353. [Google Scholar]
- Ducos, A.; Revay, T.; Kovacs, A.; Hidas, A.; Pinton, A.; Bonnet-Garnier, A.; Molteni, L.; Słota, E.; Świtoński, M.; Arruga, M.V.; et al. Cytogenetic screening of livestock populations in Europe: An overview. Cytogenet. Genome Res. 2008, 120, 26–41. [Google Scholar] [CrossRef]
- Babicz, M.; Danielak-Czech, B.; Kozubska-Sobocińska, A.; Łuszczewska-Sierakowska, I.; Wawrzyniak, A.; Grzebalska, A.M.; Kropiwiec-Domańska, K. Cytogenetic and molecular studies in conservation breeding of Pulawska breed pigs. Med. Weter. 2017, 73, 395–398. (In Polish) [Google Scholar] [CrossRef]
- Basrur, P.K.; Stranzinger, G. Veterinary cytogenetics: Past and perspective. Cytogenet. Genome Res. 2008, 120, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, L.; Di Berardino, D. Tools of the trade: Diagnostics and research in domestic animal cytogenetics. J. Appl. Genet. 2008, 49, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Rubes, J.; Pinton, A.; Bonnet-Garnier, A.; Fillon, V.; Musilova, P.; Michalova, K.; Kubickova, S.; Ducos, A.; Yerle, M. Fluorescence in situ hybridization applied to domestic animal cytogenetics. Cytogenet. Genome Res. 2009, 126, 34–48. [Google Scholar] [CrossRef]
- Kozubska-Sobocinska, A.; Smołucha, G.; Danielak-Czech, B. Early Diagnostics of freemartinism in Polish Holstein-Friesian female calves. Animals 2019, 9, 971. [Google Scholar] [CrossRef]
- Khare, V.; Khare, A. Modern approach in animal breeding by use of advanced molecular genetic techniques. Inter. J. Livestig. Res. 2017, 7, 1–22. [Google Scholar] [CrossRef]
- Villagomez, D.A.F.; Parma, P.; Radi, O.; Meo, G.D.; Pinton, A.; Iannuzzi, L.; King, W.A. Classical and Molecular Cytogenetics of Disorders of Sex Development in Domestic Animals. Cytogenet. Genome Res. 2009, 126, 110–131. [Google Scholar] [CrossRef]
- Danielak-Czech, B.; Słota, E. Karyotype control system of AI boars in Poland: The current survey. Ann. Anim. Sci. 2008, 8, 255–262. [Google Scholar]
- Ducos, A.; Calgaro, A.; Mouney-Bonnet, N.; Loustau, A.M.; Revel, C.; Barasc, H.; Mary, N.; Pinton, A. Contrôle chromosomique des populations porcines françaises Bilan de 20 années d’activités de la plateforme de cytogénétique ENVT-INRA. 2017. Available online: http://www.journees-recherche-porcine.com/texte/2017/genetique/G09.pdf (accessed on 15 July 2020).
- Kozubska-Sobocińska, A.; Danielak-Czech, B. Legitimacy of systematic karyotype evaluation of cattle qualified for reproduction. Med. Weter. 2017, 73, 451–455. (In Polish) [Google Scholar] [CrossRef][Green Version]
- Pinton, A.; Calgaro, A.; Bonnet, N.; Mary, N.; Dudez, A.M.; Barasc, H.; Plard, C.; Yerle, M.; Ducos, A. Chromosomal control of pig populations in France: 2007–2010 survey. Journées Rech. Porcine 2012, 44, 43–44. (In French) [Google Scholar]
- Quach, A.T.; Revay, T.; Villagomez, D.A.F.; Macedo, M.P.; Sullivan, A.; Maignel, L.; Wyss, S.; Sullivan, B.; King, W.A. Prevalence and consequences of chromosomal abnormalities in Canadian commercial swine herds. Genet. Sel. Evol. 2016, 48, 66. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, B.; Villagomez, D.A.F.; Revay, T.; Rezaei, S.; Kin, W.A. Non-random distribution of reciprocal translocation breakpoints in the pig genome. Genes 2019, 10, 769. [Google Scholar] [CrossRef]
- Danielak-Czech, B.; Słota, E.; Świtoński, M. Identification of the first reciprocal translocations in the pig population bred in Poland. In Proceedings of the 11th European Colloquium on Cytogenetic of Domestic Animals, Copenhagen, Denmark, 2–5 August 1994; pp. 20–24. [Google Scholar]
- Szczerbal, I.; Świtonski, M. Chromosome abnormalities in domestic animals as causes of disorders of sex development or impaired fertility. In Insights from Animal Reproduction; Careira, P.R., Ed.; InTechOpen: London, UK, 2016; pp. 207–225. [Google Scholar]
- Słota, E.; Danielak-Czech, B.; Pietraszewska, J.; Kozubska-Sobocińska, A. Preliminary identification of the fragile X in two crossbred cows. Vet. Med. 2000, 45, 308–310. [Google Scholar]
- Świtoński, M.; Danielak-Czech, B.; Słota, E.; Sysa, P. Lack of pairing loop formation in synaptonemal complex preparation of a boar carrying an inversion. Hereditas 1998, 128, 83–85. [Google Scholar] [CrossRef]
- Świtoński, M.; Stranzinger, G. Studies of synaptonemal complexes in farm mammals—A review. J. Hered. 1998, 89, 473–480. [Google Scholar] [CrossRef]
- Villagόmez, D.A.F.; Pinton, A. Chromosomal abnormalities, meiotic behaviour and fertility in domestic animals. Cytogenet. Genome Res. 2008, 120, 69–80. [Google Scholar]
- Yimer, N.; Rosnina, Y. Chromosomal anomalies and infertility in farm animals: A review. Pertanika J. Trop. Agric. Sci. 2014, 37, 1–18. [Google Scholar]
- Barasc, H.; Congras, A.; Mary, N.; Trouilh, L.; Marquet, V.; Ferchaud, S.; Raymond-Letron, I.; Calgaro, A.; Loustau-Dudez, A.M.; Mouney-Bonne, N.; et al. Meiotic pairing and gene expression disturbance in germ cells from an infertile boar with a balanced reciprocal autosome-autosome translocation. Chromosome Res. 2016, 24, 511–527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Danielak-Czech, B.; Słota, E. A new case of reciprocal translocation t(10;13)(q16;q21) diagnosed in an AI boar. J. Appl. Genet. 2007, 48, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Ducos, A.; Berland, H.M.; Bonnet, N.; Calgaro, A.; Billoux, S.; Mary, N.; Garnier-Bonnet, A.; Darré, R.; Pinton, A. Chromosomal control of pig population in France: 2002–2006 survey. Genet. Sel. Evol. 2007, 39, 583–597. [Google Scholar] [CrossRef]
- Ducos, A.; Berland, H.M.; Pinton, A.; Seguela, A.; Blanc, M.F.; Darre, A.; Sans, P.; Darre, R. Les translocations réciproques chez le porc: État des lieux et perspectives. J. Rech. Porcine 1997, 29, 375–382. [Google Scholar]
- Ducos, A.; Pinton, A.; Berland, H.M.; Sequela, A.; Yerle, M.; Sequela, A.; Brun-Barronat, C.; Bonnet, N.; Darre, R. Contrôle chromosomique des populations porcines an France: Bilan de cinq années d activité. J. Rech. Porcine 2002, 34, 269–275. [Google Scholar]
- Feve, K.; Foissac, S.; Pinton, A.; Mompart, F.; Esquerre, D.; Faraut, T.; Yerle, M.; Riquet, J. Identification of a t(3;4)(p1.3;q1.5) translocation breakpoint in pigs using somatic cell hybrid mapping and high-resolution mate-pair sequencing. PLoS ONE 2017, 12, e0187617. [Google Scholar] [CrossRef]
- O’Connor, R.E.; Fonseka, G.; Frodsham, R.; Archibald, A.L.; Lawrie, M.; Walling, G.A.; Griffin, D.K. Isolation of subtelomeric sequences of porcine chromosomes for translocation screening reveals errors in the pig genome assembly. Anim. Genet. 2017, 48, 395–403. [Google Scholar] [CrossRef]
- Pinton, A.; Calgaro, A.; Mary, N.; Barasc, H.; Bonnet, N.; Revel, C.; Ferchaud, S.; Letron, I.R.; Faraut, T.; Acloque, H.; et al. Meiotic and gene expression analyses in case of t(1;15) azoospermic boar. Comp. Cytogenet. 2018, 12, 343. [Google Scholar]
- Rodriquez, A.; Sanz, E.; De Mercado, E.; Gomez, E.; Martin, M.; Carrascosa, C.; Gomez-Fidalgo, E.; Villagomez, D.A.F.; Sanchez-Sanchez, R. Reproductive consequences of a reciprocal chromosomal translocation in two Duroc boars used to provide semen for artificial insemination. Theriogenology 2010, 74, 67–74. [Google Scholar] [CrossRef]
- Sanchez Sanchez, R.; De la Cruz Vigo, P.; Gomez Fidalgo, E.; Perez Garnelo, S.; Gonzales-Bulnes, A.; Martin-Lluch, M. Frequency of chromosomal rearrangements in breeding males from boar studs. Chromosome Res. 2016, 24 (Suppl. 1), S16. [Google Scholar]
- Villagomez, D.A.F.; Quach, A.T.; Revay, T.; St John, E.; Rezaei, S.; King, W.A. Prevalence and reproductive consequences of chromosomal abnormalities in Canadian swine herds. Chromosome Res. 2016, 24 (Suppl. 1), S17–S18. [Google Scholar]
- Danielak-Czech, B.; Świtoński, M.; Słota, E. First identification of reciprocal translocations in Polish pigs. J. Anim. Breed. Genet. 1997, 114, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Caputi-Jambrenghi, A.; Genualdo, V.; Giannico, F.; Castiglioni, B.; Pizzi, F.; Marletta, D.; Iannuzzi, A. Analysis of segregation and aneuploidy in a hybrid boar heterozygous carrier of a rob(15;17) by dual-colourSperm-FISH: Preliminary studies. Comp. Cytogenet. 2018, 12, 333–334. [Google Scholar]
- Danielak-Czech, B.; Kozubska-Sobocińska, A.; Rejduch, B. Diagnosis of tandem fusion translocation in the boar using FISH technique with human painting probes. Ann. Anim. Sci. 2010, 10, 361–366. [Google Scholar]
- Danielak-Czech, B.; Kozubska-Sobocińska, A.; Słota, E.; Rejduch, B.; Okularczyk, S. Decrease in pig fertility as result of reciprocal translocations and assisted economic effects on the basis of rcp(7;13)(q13;q46). J. Appl. Genet. 1996, 36, 373–384. [Google Scholar]
- Słota, E.; Kozubska-Sobocińska, A.; Danielak-Czech, B.; Rejduch, B.; Kowol, P.; Żyga, A. A note on cytogenetic monitoring of Polish Red cattle. J. Anim. Feed Sci. 2004, 13, 65–71. [Google Scholar] [CrossRef]
- Quach, T.A.; Villagómez, D.A.F.; Coppola, G.; Pinton, A.; Hart, E.J.; Reyes, E.R.; Basrur, P.K.; King, W.A. A cytogenetic study of breeding boars in Canada. Cytogenet Genome Res. 2009, 126, 271–280. [Google Scholar] [CrossRef]
- Rejduch, B.; Danielak-Czech, B.; Kozubska-Sobocińska, A. FISH-based comparative analysis of human and porcine chromosome region involving obesity-related genes. Ann. Anim. Sci. 2010, 10, 367–372. [Google Scholar]
- Rejduch, B.; Kozubska-Sobocińska, A.; Danielak-Czech, B. Use of human painting probes for identification of centric fusion in wild boar. Chromosome Res. 2010, 18, 727–728. [Google Scholar]
- Danielak-Czech, B.; Słota, E.; Kozubska-Sobocińska, A.; Rejduch, B. A unique chromosome mutation in pigs: Tandem fusion-translocation. Chromosome Res. 2010, 18, 715–716. [Google Scholar]
- Danielak-Czech, B.; Słota, E. Tandem fusion-translocation: A unique karyotype rearrangement in the domestic pig. Ann. Anim. Sci. 2008, 8, 343–348. [Google Scholar]
- Danielak-Czech, B.; Słota, E.; Bugno, M.; Pieńkowska-Schelling, A.; Schelling, C. Application of chromosome microdissection and chromosome painting techniques for reciprocal translocations diagnosis in pigs. Ann. Anim. Sci. 2006, 6, 219–224. [Google Scholar]
- Rejduch, B.; Słota, E.; Sysa, P.; Kwaczyńska, A.; Kozubska-Sobocińska, A.; Danielak-Czech, B. Diagnosis of a new reciprocal translocation rcp(9;14)(q14;q23) in infertile boar after the synaptonemal complex analysis. Ann. Anim. Sci. 2003, 3, 269–278. [Google Scholar]
- Sanchez Sanchez, R.; Martin-Lluch, M.; Gomez Fidalgo, E.; Perez Garnelo, S.; Gonzales-Bulnes, A.; De la Cruz Vigo, P. Several cases of homozygous pericentric inversion in a population of hyperprolific breeding sows. Chromosome Res. 2016, 24 (Suppl. 1), S15. [Google Scholar]
- Danielak-Czech, B.; Kozubska-Sobocińska, A.; Słota, E.; Rejduch, B.; Kwaczyńska, A. Preliminary identification of pair 1 chromosome rearrangement in the Polish Landrace sow. Cytogenet. Cell Genet. 1996, 74, 230. [Google Scholar]
- Hornak, M.; Oracova, E.; Hulinska, P.; Urbankova, L.; Rubes, J. Aneuploidy detection in pigs using comparative genomic hybridization: From the oocytes to blastocysts. PLoS ONE 2012, 7, e30335. [Google Scholar] [CrossRef]
- Pinton, A.; Barasc, H.; Raymond-Letron, I.; Bordedebat, M.; Mary, N.; Massip, K.; Bonnet, N.; Calgaro, A.; Dudez, A.M.; Feve, K.; et al. Meiotic studies of a 38,XY/39,XXY mosaic boar. Cytogenet. Genome Res. 2011, 133, 202–208. [Google Scholar] [CrossRef]
- Quilter, C.R.; Wood, D.; Southwood, O.I.; Griffin, D.K. X/XY/XYY mosaicism as a cause of subfertility in boars: A single case study. Anim. Genet. 2003, 34, 51–54. [Google Scholar] [CrossRef]
- Słota, E.; Kozubska-Sobocińska, A.; Kościelny, M.; Danielak-Czech, B.; Rejduch, B. Detection of the XXY trisomy in a bull by using sex chromosome painting probes. J. Appl. Genet. 2003, 44, 379–382. [Google Scholar]
- Barasc, H.; Ferchaud, S.; Mary, N.; Cucchi, M.A.; Lucena, A.N.; Letron, I.R.; Calgaro, A.; Bonnet, N.; Dudez, A.M.; Yerle, M.; et al. Cytogenetic analysis of somatic and germinal cells from 38,XX/38,XY phenotypically normal boars. Theriogenology 2014, 81, 368–372. [Google Scholar] [CrossRef]
- Kozubska-Sobocińska, A.; Danielak-Czech, B.; Rejduch, B. Cytogenetic and molecular diagnostics of XX/XY chimerism in cattle, sheep, and goats—A review. Ann. Anim. Sci. 2016, 16, 989–1005. [Google Scholar] [CrossRef][Green Version]
- Kozubska-Sobocińska, A.; Rejduch, B. Identification of heterosomes in spermatozoa of rams with 54,XX/54,XY chimerism. Vet. Med. 2008, 53, 250–254. [Google Scholar] [CrossRef]
- Sanchez Sanchez, R.; De la Cruz Vigo, P.; Gomez Fidalgo, E.; Perez Garnelo, S.; Gonzales-Bulnes, A.; Martin-Lluch, M. A case of mosaicism (38XY/38XX) in a boar from an insemination center of Iberian pig population. Chromosome Res. 2016, 24 (Suppl. 1), S11. [Google Scholar]
- Szczerbal, I.; Dzimira, S.; Nowacka-Woszuk, J.; Świtoński, M. Leucocyte chimerism (XX/XY) in pigs with severe disorders of sex development. Chromosome Res. 2016, 24 (Suppl. 1), S10. [Google Scholar]
- Bickmore, W.A. Karyotype analysis and chromosome banding. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2001; pp. 1–7. [Google Scholar]
- Vorsanowa, S.G.; Burov, Y.B.; Iourov, I.Y. Human interphase chromosomes: A review of available molecular cytogenetic technologies. Mol. Cytogenet. 2010, 3, 2–15. [Google Scholar]
- Gustavsson, I. Standard karyotype of domestic pig. Hereditas 1988, 109, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Galman, O.; Yerle, M.; Echard, E. The high resolution G-banded karyotype of Sus scrofa domestica L. Genet. Sel. Evol. 1991, 23, 113–116. [Google Scholar] [CrossRef]
- Yerle, M.; Galman, O.; Echard, G. The high resolution GTG-banding pattern of pig chromosomes. Cytogenet Cell Genet. 1991, 56, 45–47. [Google Scholar] [CrossRef]
- Gustavsson, I. Chromosomes of the pig. Adv. Vet. Sci. Comp. Med. 1990, 34, 73–107. [Google Scholar]
- Rejduch, B.; Kozubska-Sobocińska, A.; Słota, E.; Sysa, P.; Wrzeska, M. Evaluation of chromosomal changes in gonads and their effect on boar fertility. Med. Weter. 2006, 62, 931–932. (In Polish) [Google Scholar]
- Pinkel, D.; Straume, T.; Gray, J.W. Cytogenetic analysis using quantitative, high sensitive, fluorescence hybridization. Proc. Nat. Acad. Sci. USA 1986, 83, 2934–2938. [Google Scholar] [CrossRef] [PubMed]
- Danielak-Czech, B.; Rejduch, B.; Kozubska-Sobocińska, A. Identification of telomeric sequences in pigs with rearranged karyotype using PRINS technique. Ann. Anim. Sci. 2013, 13, 495–502. [Google Scholar] [CrossRef]
- Chowdhary, B.P.; Raudsepp, T. Chromosome painting in farm, pet and wild animal species. Methods Cell Sci. 2001, 23, 37–55. [Google Scholar] [CrossRef]
- Fronicke, L.; Wienberg, J. Comparative chromosome painting defines the high rate of karyotype changes between pigs and bovids. Mamm. Genome 2001, 12, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Kozubska-Sobocińska, A.; Słota, E.; Pieńkowska, A. The application of FISH technique for diagnosing leukocyte chimerism in sheep. Med. Weter. 2003, 59, 987–989. (In Polish) [Google Scholar]
- Goureau, A.; Yerle, M.; Schmitz, A.; Riquet, D.; Milan, D.; Pinton, P.; Frelat, G.; Gellin, J. Human and porcine correspondence of chromosome segments using bidirectional chromosome painting. Genomics 1996, 36, 252–262. [Google Scholar] [CrossRef]
- Kozubska-Sobocińska, A.; Rejduch, B.; Danielak-Czech, B.; Babicz, M.; Bąk, A. Comparative sex chromosomes hybridizations in Ruminantia. Ann. Anim. Sci. 2012, 12, 495–500. [Google Scholar] [CrossRef][Green Version]
- Danielak-Czech, B.; Kozubska-Sobocińska, A.; Kruczek, K.; Babicz, M.; Rejduch, B. Physical mapping of the HSPB genes in the domestic and wild pigs. Chromosome Res. 2014, 22, 413. [Google Scholar]
- Fahrenkrug, S.C.; Rohrer, G.A.; Freking, B.A.; Smith, T.P.L.; Osoegawa, K.; Shu, C.L.; Catanese, J.J.; de Jong, P.J. A porcine BAC library with tenfold genome coverage: A resource for physical and genetic map integration. Mamm. Genome 2001, 12, 472–474. [Google Scholar] [CrossRef]
- Shizuya, H.; Kouros-Mehr, H. The development of applications of the bacterial artificial chromosome cloning system. Keio J. Med. 2001, 50, 21–30. [Google Scholar] [CrossRef]
- Telenius, H.; Pelmear, A.H.; Tunnacliffe, A.; Carter, N.P.; Behmel, A.; Ferguson-Smith, M.A.; Nordenskjold, M.; Pfragner, R.; Ponder, B.A. Cytogenetic analysis by chromosome painting using DOP-PCR amplified flow-sorted chromosomes. Genes Chromosome Cancer 1992, 4, 257–263. [Google Scholar] [CrossRef]
- Langford, C.F.; Telenius, H.; Miller, N.G.; Thomsen, P.D.; Tucker, E.M. Preparation of chromosome-specific paints and complete assignment of chromosome in the pig flow-sorted karyotype. Anim. Genet. 1993, 24, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Yerle, M.; Schmitz, A.; Milan, D.; Chaput, B.; Monteagudo, L.; Vaiman, M.; Frelat, G.; Gellin, J. Accurate characterization of porcine bivariate flow karyotype by PCR and fluorescence in situ hybridization. Genomics 1993, 16, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Pinton, A.; Ducos, A.; Yerle, M. Chromosomal rearrangements in cattle and pigs revealed by chromosome microdissection and chromosome painting. Genet. Sel. Evol. 2003, 35, 685–696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubickova, S.; Cernohorska, H.; Musilova, P.; Rubes, J. The use of laser microdissection for the preparation of chromosome-specific painting probes in farm animals. Chromosome Res. 2002, 10, 571–577. [Google Scholar] [CrossRef]
- Chaudhary, R.; Kijas, J.; Raudsepp, T.; Guan, X.Y.; Zhang, H.; Chowdhary, B.P. Microdissection of pig chromosomes: Dissection of whole chromosomes, arms and bands for construction of paints and libraries. Hereditas 1998, 128, 265–271. [Google Scholar] [CrossRef]
- Dolezel, J.; Vrana, J.; Safar, J.; Bartos, J.; Kubalakova, M.; Simkova, H. Chromosomes in the flow to simplify genome analysis. Funct. Integr. Genom. 2012, 12, 397–416. [Google Scholar] [CrossRef]
- Langford, C.F.; Telenius, H.; Carter, N.P.; Miller, N.G.; Tucker, E.M. Chromosome painting using chromosome-specific probes from flow-sorted pig chromosomes. Cytogenet. Cell Genet. 1992, 61, 221–223. [Google Scholar] [CrossRef]
- Schmitz, A.; Chardon, P.; Gainche, I.; Chaput, B.; Guilly, M.N.; Frelat, G.; Vaiman, M. Pig standard bivariate flow karyotype and peak assignment for chromosomes X, Y, 3, and 7. Genomics 1992, 14, 357–362. [Google Scholar] [CrossRef]
- Dixon, S.C.; Miller, N.G.; Carter, N.P.; Tucker, E.M. Bivariate flow cytometry of farm animal chromosomes: A potential tool for gene mapping. Anim. Genet. 1992, 23, 203–210. [Google Scholar] [CrossRef]
- Pellestor, F.; Girardet, A.; Lefort, G.; Andreo, B.; Charlieu, J.P. PRINS as a method for rapid chromosomal labeling on human spermatozoa. Mol. Reprod. Dev. 1995, 40, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Handkjaer, I.; Gustavsson, I.; Bolund, L. A signal of telomeric sequences on porcine chromosome 6q21-q22 detected by primed in situ labeling. Chromosome Res. 1996, 4, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Hindnkjaer, J.; Thomsen, P.D. A chromosomal basis for the differential organization of a porcine centromere-specific repeat. Cytogenet. Cell Genet. 1993, 62, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Rogel-Gaillard, C.; Hayes, H.; Coullin, P.; Chardon, P.; Vaiman, M. Swine centromeric DNA repeats revealed by primed in situ (PRINS) labeling. Cytogenet. Cell Genet. 1997, 79, 79–84. [Google Scholar] [CrossRef]
- Apiou, F.; Vincent-Naulleau, S.; Spatz, A.; Vielh, P.; Geffrotin, C.; Frelat, G.; Dutrillaux, B.; Le Chalony, C. Comparative genomic hybridization analysis of hereditary swine cutaneous melanoma revealed loss of the swine 13q36-49 chromosomal region in the nodular melanoma subtype. Int. J. Cancer 2004, 110, 232–238. [Google Scholar] [CrossRef]
- Hornak, M.; Hulinska, P.; Musilova, P.; Kubickova, S.; Rubes, J. Investigation of chromosome aneuploidies in early porcine embryos using comparative genomic hybridization. Cytogenet. Genome Res. 2009, 126, 210–216. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Ji, X.; Hu, H.; Zhang, J.; Meng, L.; Lin, Y.; Ma, D.; Jiang, T.; Jiang, H.; et al. Clinical application of whole-genome low-coverage next-generation sequencing to detect and characterize balanced chromosomal translocations. Clin Genet. 2017, 91, 605–610. [Google Scholar] [CrossRef]
- Grahofer, A.; Letko, A.; Hafliger, I.M.; Jagannathan, V.; Ducos, A.; Richard, O.; Peter, V.; Nathues, H.; Drogemuller, C. Chromosomal imbalance in pigs showing a syndromic form of cleft palate. BMC Genomics 2019, 20, 349. [Google Scholar] [CrossRef]
- O’Connor, R.E.; Fonseka, G.; Frodsham, R.; Archibald, A.L.; Lawrie, M.; Walling, G.A.; Griffin, D.K. Development of a porcine chromosomal translocation screening device reveals errors in the pig genome assembly. Chromosome Res. 2016, 24 (Suppl. 1), S33. [Google Scholar]
- Long, S. Reciprocal translocations in the pig (Sus scrofa): A review. Vet. Rec. 1991, 128, 275–278. [Google Scholar] [CrossRef]
- Tribout, T.; Ducos, A.; Maygnel, L.; Bidanel, J.P. Utilisation du système d’information BLUP pour la détection des verrats porteurs d’anomalies chromosomiques. Techniporc 2000, 23, 19–24. [Google Scholar]
- Bonneau, M.; Boscher, J.; Popescu, C.P. Consequences zootechniques des translocations reciproques dans un troupeau experimental porcin: Incidence economique. Journées Rech. Porcine 1991, 23, 395–400. [Google Scholar]
- Martin-Lluch, M.; De la Cruz-Vigo, P.; Ortuno, V.; Gomez-Fidalgo, E.; Carrascosa, C.; Sánchez-Sánchez, R. Cytogenetic study of a reciprocal translocation (1;6)(q17;p11) in a subfertile boar. Chromosome Res. 2014, 22, 393–437. [Google Scholar]
- Sanchez-Sánchez, R.; Gomez-Fidalgo, E.; Perez-Garnelo, S.; Martin-Lluch, M.; Cruz-Vigo, P.D.L. Prevalence of chromosomal aberrations in breeding pigs in Spain. Reprod Dom Anim. 2019, 54 (Suppl. 4), 98–101. [Google Scholar] [CrossRef] [PubMed]
- King, W.A.; Donaldson, B.; Rezaei, S.; Schmidt, C.; Revay, T.; Villagomez, D.A.; Kuschke, K. Chromosomal abnormalities in swine and their impact on production and profitability. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon Press: Oxford, UK, 2019; pp. 508–518. [Google Scholar]
- Calgaro, A.; Mouney-Bonnet, N.; Loustau, A.M.; Revel, C.; Barasc, H.; Mary, N.; Ducos, A.; Pinton, A. Chromosomal control of pig populations in France. Chromosome Res. 2016, 24 (Suppl. 1), S16. [Google Scholar]
- Available online: http://www.envt.fr/menu-og-34/plateforme-de-cytogénetique-animale (accessed on 15 July 2020).
- Roca, J.; Broekhuijse, M.L.W.J.; Parrilla, I.; Rodriguez-Martinez, H.; Martinez, E.A.; Bolarin, A. Boar differences in artificial insemination outcomes: Can they be minimized? Reprod. Dom. Anim. 2015, 50 (Suppl. 2), 48–55. [Google Scholar] [CrossRef]
- Villagomez, D.A.; Revay, T.; Donaldson, B.; Rezaei, S.; Pinton, A.; Palomino, M.; King, W.A. Azoospermia and testicular hypoplasia in a boar carrier of a novel Y-autosome translocation. Sex. Dev. 2017, 11, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Danielak-Czech, B.; Słota, E. Mutagen-induced chromosome instability in farm animals. J. Anim. Feed Sci. 2004, 13, 257–267. [Google Scholar] [CrossRef]
- Genualdo, V.; Rossetti, C.; Pauciullo, A.; Musilova, P.; Incaenato, D.; Perucatti, A. A de novo reciprocal chromosomal translocation t(3;6)(p14;q26) in the black Lucano pig. Reprod. Domest. Anim. 2020. [Google Scholar] [CrossRef]
- Raudsepp, T.; Chowdhary, B.P. Chromosome aberrations and fertility disorders in domestic animals. Annu. Rev. Anim. Biosci. 2016, 4, 15–43. [Google Scholar] [CrossRef]
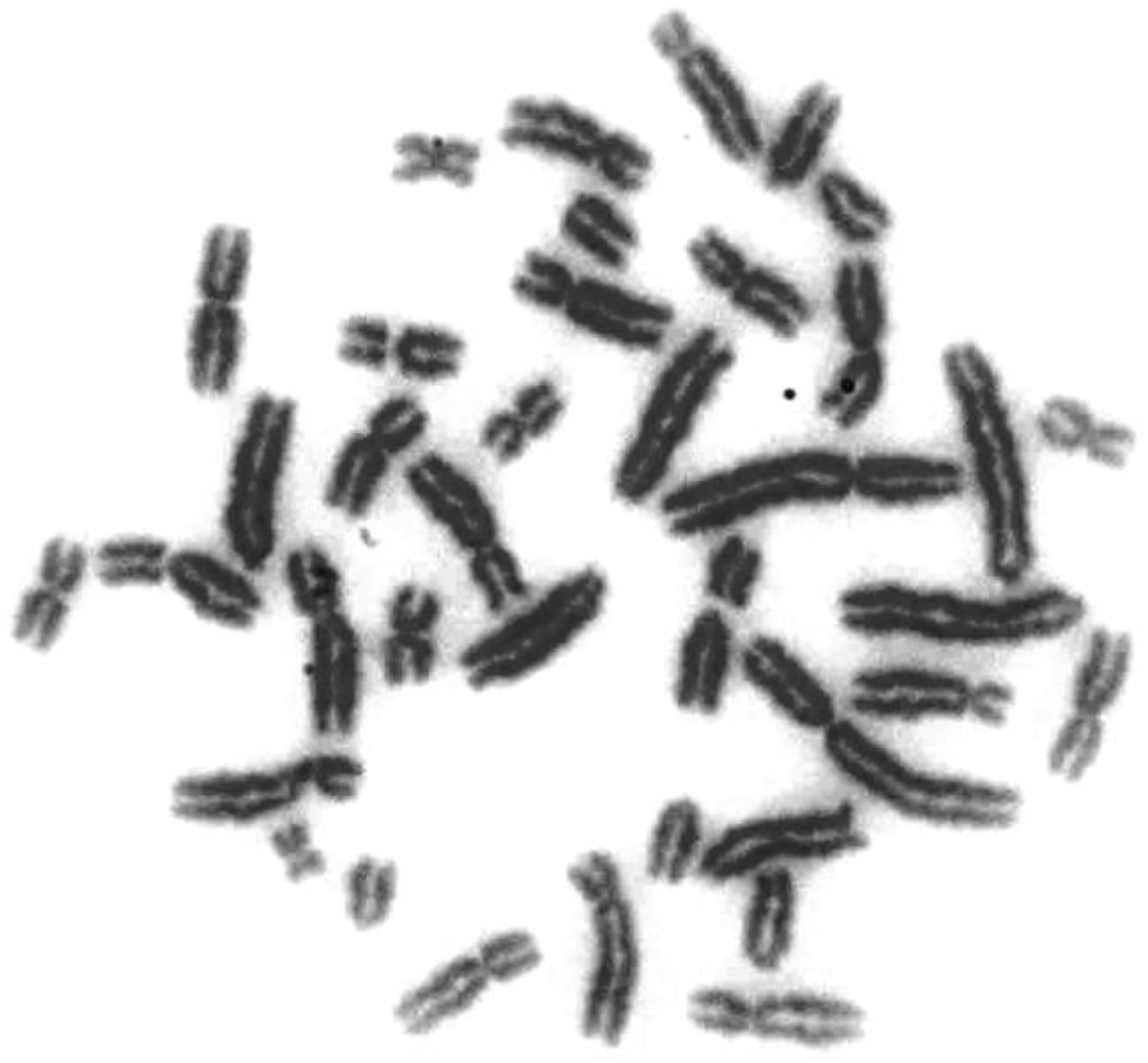
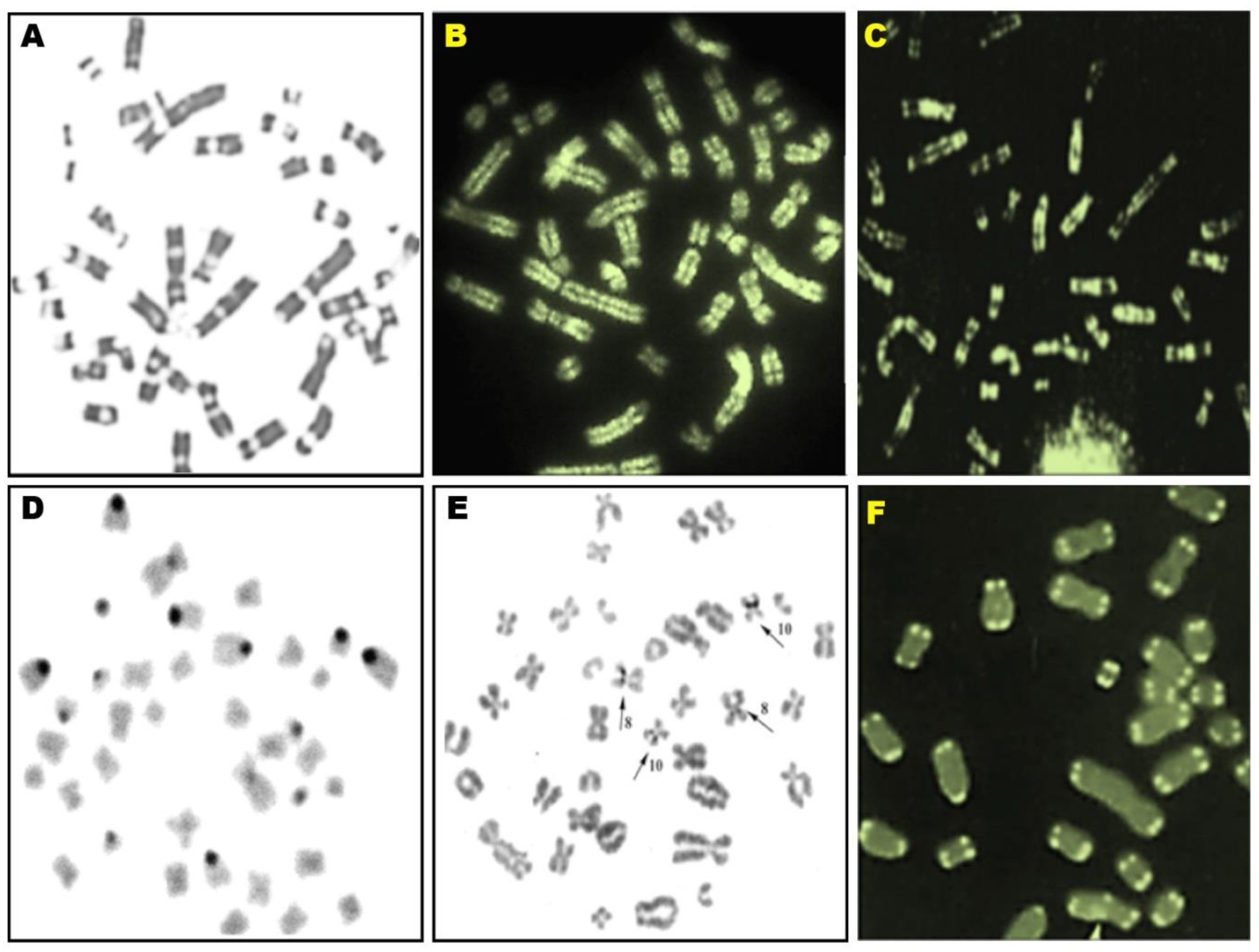
| Pig Karyotype Abnormalities | Effect on Carrier Fertility | Cytomolecular Diagnostics | Comments |
|---|---|---|---|
| Reciprocal translocations | Reduced fertility of sires and herds (5–100%) | Giemsa staining, differential banding techniques (GTG, RBA, QFQ), FISH | Techniques used for routine analysis: Giemsa staining, GTG technique, and FISH; in special cases, a multiprobe system for diagnostics of cryptic micro-rearrangement |
| Robertsonian translocations and tandem fusions | Reduced fertility (5–22%) | Giemsa staining, differential banding techniques (GTG, RBA, QFQ), FISH | Techniques used for routine analysis: Giemsa staining, GTG technique, and FISH in difficult cases |
| Peri- and paracentric inversions | Reduced fertility (less than 10%) | Giemsa staining, differential banding techniques (GTG, RBA, QFQ), FISH | Techniques used for routine analysis: Giemsa staining, GTG technique, and FISH in difficult cases |
| Sex chromosome aneuploidies and leukocyte chimerism | Reduced fertility or infertility | Giemsa staining, differential banding techniques (GTG, RBA, QFQ), FISH | Techniques used for routine analysis: Giemsa staining, GTG technique, and FISH with heterosome probes or the array–CGH method in difficult cases |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danielak-Czech, B.; Kozubska-Sobocińska, A.; Smołucha, G.; Babicz, M. Breeding and Economic Aspects of Cytogenetic Screening Studies of Pigs Qualified for Reproduction. Animals 2020, 10, 1200. https://doi.org/10.3390/ani10071200
Danielak-Czech B, Kozubska-Sobocińska A, Smołucha G, Babicz M. Breeding and Economic Aspects of Cytogenetic Screening Studies of Pigs Qualified for Reproduction. Animals. 2020; 10(7):1200. https://doi.org/10.3390/ani10071200
Chicago/Turabian StyleDanielak-Czech, Barbara, Anna Kozubska-Sobocińska, Grzegorz Smołucha, and Marek Babicz. 2020. "Breeding and Economic Aspects of Cytogenetic Screening Studies of Pigs Qualified for Reproduction" Animals 10, no. 7: 1200. https://doi.org/10.3390/ani10071200
APA StyleDanielak-Czech, B., Kozubska-Sobocińska, A., Smołucha, G., & Babicz, M. (2020). Breeding and Economic Aspects of Cytogenetic Screening Studies of Pigs Qualified for Reproduction. Animals, 10(7), 1200. https://doi.org/10.3390/ani10071200





