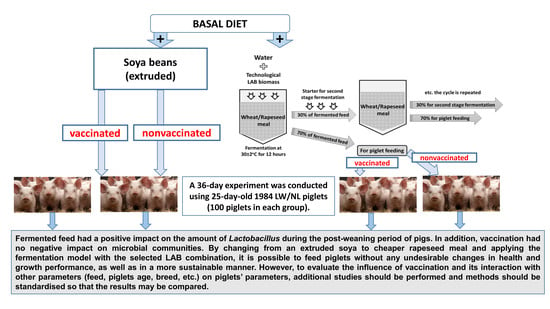
Influence of the Fermented Feed and Vaccination and Their Interaction on Parameters of Large White/Norwegian Landrace Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
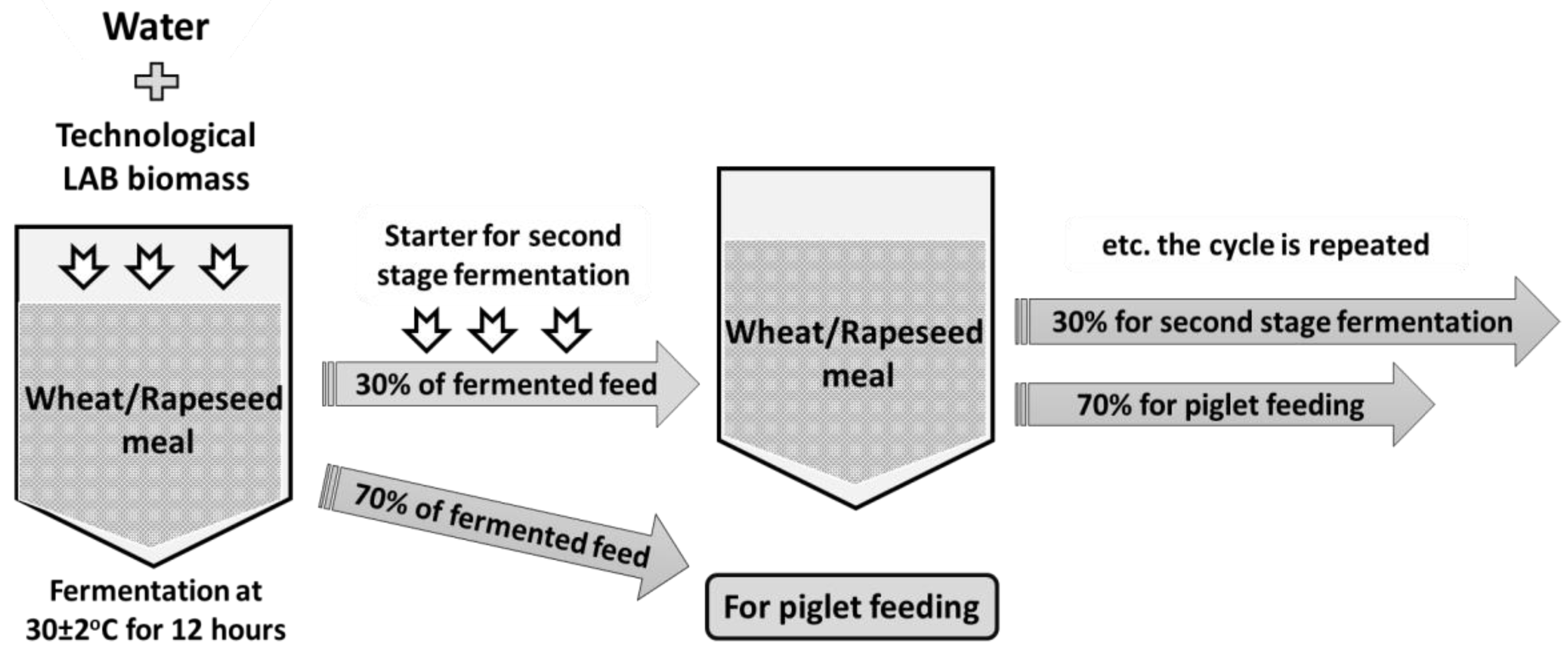
2.1. LAB Strains Used for Feed Fermentation, Feed Fermentation and Fermented Feed Parameters
2.2. Animals and Housing
2.3. Experimental Design and Diets
2.4. Metagenomics and Microbial Profiling Analysis
2.5. Microbiological Analysis of Faecal Samples
2.6. Blood Analysis
2.7. Evaluation of Piglets’ Growth Performance and Cases of Mortality and Diarrhoea
2.8. Analysis of Ammonia Emission
2.9. Statistical Analysis
3. Results and Discussion
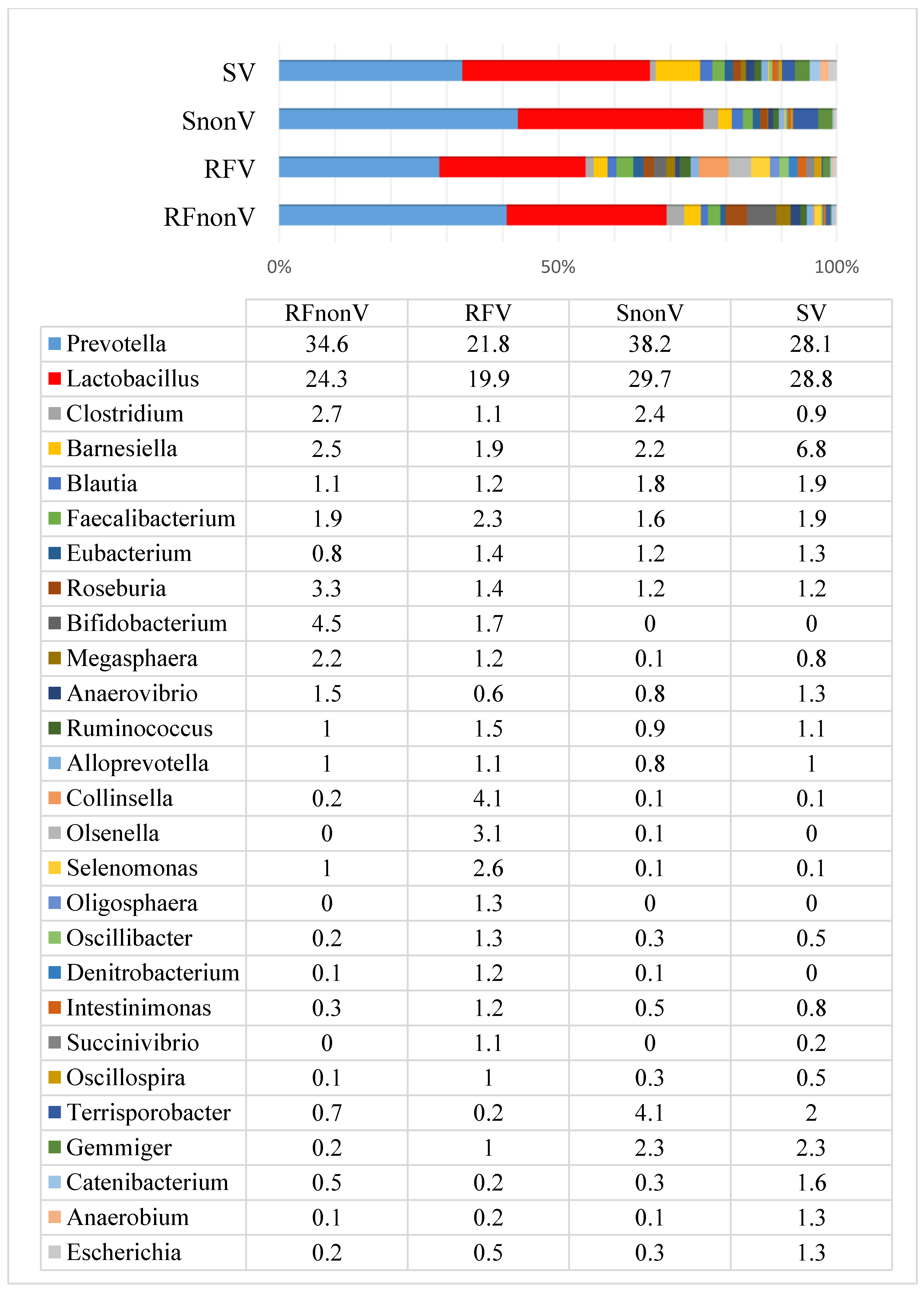
3.1. Microbial Profiles of Pig Faeces
3.2. LAB, TVC, TEC and M/Y Counts in Piglets’ Faeces
3.3. Piglet Blood Parameters
3.4. Piglets’ Growth Performance
3.5. Influence of Analysed Factors on Ammonia Emission
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tischer, I.; Mields, W.; Wolff, D.; Vagt, M.; Griem, W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 1986, 91, 271–276. [Google Scholar] [CrossRef]
- Ellis, J.; Hassard, L.; Clark, E.; Harding, J.; Allan, G.; Willson, P.; Strokappe, J.; Martin, K.; McNeilly, F.; Meehan, B.; et al. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 1998, 39, 44–51. [Google Scholar]
- Argilés, J.M.; Busquets, S.; Toledo, M.; López-Soriano, F.J. The role of cytokines in cancer cachexia. Curr. Opin. Support Palliat. Care 2009, 3, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Ottenhoff, T.H.M. The knowns and unknowns of the immunopathogenesis of tuberculosis. Int. J. Tuberc. Lung Dis. 2012, 16, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Csak, T. Inflammasomes in liver diseases. J. Hepatol. 2012, 57, 642–654. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Meng, X.-J. Porcine circoviruses: A minuscule yet mammoth paradox. Anim. Health Res. Rev. 2009, 10, 1–20. [Google Scholar] [CrossRef]
- Gillespie, J.; Opriessnig, T.; Meng, X.J.; Pelzer, K.; Buechner-Maxwell, V. Porcine circovirus type 2 and porcine circovirus-associated disease. J. Vet. Intern. Med. 2009, 23, 1151–1163. [Google Scholar] [CrossRef]
- Cságola, A.; Zádori, Z.; Mészáros, I.; Tuboly, T. Detection of porcine parvovirus 2 (Ungulate Tetraparvovirus 3) Specific antibodies and examination of the serological profile of an infected swine herd. PLoS ONE 2016, 11, e0151036. [Google Scholar] [CrossRef]
- Novosel, D.; Cadar, D.; Tuboly, T.; Jungic, A.; Stadejek, T.; Ait-Ali, T.; Cságola, A. Investigating porcine parvoviruses genogroup 2 infection using in situ polymerase chain reaction. BMC Vet. Res. 2018, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.; Smith, B.; Alan, G.; McNeilly, F.; McVicar, C. PDNS, PMWS and porcine circovirus type 2 in Scotland. Vet. Rec. 2000, 146, 651–652. [Google Scholar]
- Dán, Á.; Molnár, T.; Biksi, I.; Glávits, R.; Shaheim, M.; Harrach, B. Characterisation of Hungarian porcine circovirus 2 genomes associated with PMWS and PDNS cases. Acta Vet. Hung. 2003, 51, 551–562. [Google Scholar] [CrossRef]
- An, D.; Roh, I.; Song, D.; Park, C.; Park, B. Phylogenetic characterization of porcine circovirus type 2 in PMWS and PDNS Korean pigs between 1999 and 2006. Virus Res. 2007, 129, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Lu, Q.; Wang, F.; Xing, G.; Feng, H.; Jin, Q.; Guo, Z.; Teng, M.; Hao, H.; Li, D.; et al. Phylogenetic analysis of porcine circovirus type 2 (PCV2) between 2015 and 2018 in Henan Province, China. BMC Vet. Res. 2020, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef]
- Liu, J.; Wei, C.; Dai, A.; Lin, Z.; Fan, K.; Fan, J.; Liu, J.; Luo, M.; Yang, X. Detection of PCV2e strains in Southeast China. PeerJ 2018, 6, e4476. [Google Scholar] [CrossRef]
- Opriessnig, T.; Xiao, C.-T.; Gerber, P.F.; Halbur, P.G. Identification of recently described porcine parvoviruses in archived North American samples from 1996 and association with porcine circovirus associated disease. Vet. Microbiol. 2014, 173, 9–16. [Google Scholar] [CrossRef]
- Chen, N.; Huang, Y.; Ye, M.; Li, S.; Xiao, Y.; Cui, B.; Zhu, J. Co-infection status of classical swine fever virus (CSFV), porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circoviruses (PCV2 and PCV3) in eight regions of China from 2016 to 2018. Infect. Genet. Evol. 2019, 68, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Bartkevics, V.; Krungleviciute, V.; Pugajeva, I.; Zadeike, D.; Juodeikiene, G. Lactic acid bacteria combinations for wheat sourdough preparation and their influence on wheat bread quality and acrylamide formation. J. Food Sci. 2017, 82, 2371–2378. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Mickiene, R.; Zadeike, D.; Juodeikiene, G. A concept of mould spoilage prevention and acrylamide reduction in wheat bread: Application of lactobacilli in combination with a cranberry coating. Food Control 2018, 91, 284–293. [Google Scholar] [CrossRef]
- Bartkiene, E.; Sakiene, V.; Bartkevics, V.; Juodeikiene, G.; Lele, V.; Wiacek, C.; Braun, P.G. Modulation of the nutritional value of lupine wholemeal and protein isolates using submerged and solid-state fermentation with Pediococcus pentosaceus strains. Int. J. Food Sci. Technol. 2018, 53, 1896–1905. [Google Scholar] [CrossRef]
- Bartkiene, E.; Sakiene, V.; Lele, V.; Bartkevics, V.; Rusko, J.; Wiacek, C.; Ruzauskas, M.; Braun, P.G.; Matusevicius, P.; Zdunczyk, Z.; et al. Perspectives of lupine wholemeal protein and protein isolates biodegradation. Int. J. Food Sci. Technol. 2019, 54, 1989–2001. [Google Scholar] [CrossRef]
- Bartkiene, E.; Mozuriene, E.; Lele, V.; Zokaityte, E.; Gruzauskas, R.; Jakobsone, I.; Juodeikiene, G.; Ruibys, R.; Bartkevics, V. Changes of bioactive compounds in barley industry by-products during submerged and solid state fermentation with antimicrobial Pediococcus acidilactici strain LUHS29. Food Sci. Nutr. 2020, 8, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Badaras, S.; Klupsaite, D.; Mozuriene, E.; et al. Pigs’ feed fermentation model with antimicrobial lactic acid bacteria strains combination by changing extruded soya to biomodified local feed stock. Animals 2020, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- European Union Law. European Parliament Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes Text with EEA Relevance. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 13 July 2020).
- Lithuanian Director of the State Food and Veterinary Service. B1-866 Dėl Mokslo ir mokymo tikslais naudojamų gyvūnų laikymo, priežiūros ir naudojimo reikalavimų patvirtinimo. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.437081?positionInSearchResults=0&searchModelUUID=64a1f51f-6356-4b60-a21e-3a67ac3b1107 (accessed on 10 March 2020).
- National Research Council. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- AOAC. Official Methods of Analysis, 21st ed.; AOAC International: Rockland, MD, USA, 2019. [Google Scholar]
- Merkeviciene, L.; Ruzauskaite, N.; Klimiene, I.; Siugzdiniene, R.; Dailidaviciene, J.; Virgailis, M.; Mockeliunas, R.; Ruzauskas, M. Microbiome and antimicrobial resistance genes in microbiota of cloacal samples from European herring gulls (Larus Argentatus). J. Vet. Res. 2017, 4, 27–35. [Google Scholar] [CrossRef]
- Zavistanaviciute, P.; Poskiene, I.; Lele, V.; Antanaitis, R.; Kantautaite, J.; Bartkiene, E. The influence of the newly isolated Lactobacillus plantarum LUHS135 and Lactobacillus paracasei LUHS244 strains on blood and faeces parameters in endurance horses. Pol. J. Vet. Sci. 2019, 22, 513–521. [Google Scholar] [CrossRef]
- The Lithuanian Minister of Environment. D1-862. Dėl Lietuvos Respublikos Aplinkos Apsaugos Normatyvinio Dokumento LAND 88-2009 “Amoniako Koncentracijos Nustatymas Aplinkos Ore Spektrometriniu Metodu” Patvirtinimo. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.363222 (accessed on 13 July 2020).
- Pajarillo, E.A.B.; Chae, J.P.; Kim, H.B.; Kim, I.H.; Kang, D.-K. Barcoded pyrosequencing-based metagenomic analysis of the faecal microbiome of three purebred pig lines after cohabitation. Appl. Microbiol. Biotechnol. 2015, 99, 5647–5656. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Benson, A.K.; Kelly, S.A.; Legge, R.; Ma, F.; Low, S.J.; Kim, J.; Zhang, M.; Oh, P.L.; Nehrenberg, D.; Hua, K.; et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18933–18938. [Google Scholar] [CrossRef]
- Leamy, L.J.; Kelly, S.A.; Nietfeldt, J.; Legge, R.M.; Ma, F.; Hua, K.; Sinha, R.; Peterson, D.A.; Walter, J.; Benson, A.K.; et al. Host genetics and diet, but not immunoglobulin A expression, converge to shape compositional features of the gut microbiome in an advanced intercross population of mice. Genome Biol. 2014, 15, 552. [Google Scholar] [CrossRef]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Knudsen, C.; Gidenne, T.; Montagne, L.; Merlot, E. Impact of feed restriction on health, digestion and faecal microbiota of growing pigs housed in good or poor hygiene conditions. Animal 2014, 8, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huang, X.; Wang, P.; Yan, Z.; Sun, W.; Zhao, S. Longitudinal development of the gut microbiota in healthy and diarrheic piglets induced by age-related dietary changes. Microbiologyopen 2019, 8, e923. [Google Scholar] [CrossRef] [PubMed]
- Lamendella, R.; Domingo, J.W.; Ghosh, S.; Martinson, J.; Oerther, D.B. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 2011, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.A.B.; Chae, J.-P.; Balolong, M.P.; Kim, H.B.; Kang, D.-K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J. Gen. Appl. Microbiol. 2014, 60, 140–146. [Google Scholar] [CrossRef]
- Ivarsson, E.; Roos, S.; Liu, H.Y.; Lindberg, J.E. Fermentable non-starch polysaccharides increase the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.; Wright, D.P.; Christie, D.L.; Clinch, K.; Furneaux, R.H.; Roberton, A.M. A novel mechanism for desulfation of mucin: Identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J. Bacteriol. 2005, 187, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Delia, E.; Tafaj, M.; Männer, K. Efficiency of Probiotics in Farm Animals. Available online: https://www.intechopen.com/books/probiotic-in-animals/efficiency-of-probiotics-in-farm-animals (accessed on 17 May 2020).
- Valeriano, V.D.V.; Balolong, M.P.; Kang, D.-K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Awati, A.A.; Williams, B.A.; Miller, B.G.; Jones, P.; Stokes, C.R.; Akkermans, A.D.L.; Smidt, H.; de Vos, W.M. Post-natal development of the porcine microbiota composition and activities. Environ. Microbiol. 2006, 8, 1191–1199. [Google Scholar] [CrossRef]
- Dou, S.; Gadonna-Widehem, P.; Rome, V.; Hamoudi, D.; Rhazi, L.; Lakhal, L.; Larcher, T.; Bahi-Jaber, N.; Pinon-Quintana, A.; Guyonvarch, A.; et al. Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea. PLoS ONE 2017, 12, e0169851. [Google Scholar] [CrossRef]
- Canibe, N.; Jensen, B.B. Fermented liquid feed—Microbial and nutritional aspects and impact on enteric diseases in pigs. Anim. Feed Sci. Technol. 2012, 173, 17–40. [Google Scholar] [CrossRef]
- Grela, E.R.; Czech, A.; Kiesz, M.; Wlazło, Ł.; Nowakowicz-Dębek, B. A fermented rapeseed meal additive: Effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim. Nutr. 2019, 5, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Berri, M.; Estellé, J.; Levenez, F.; Lemonnier, G.; Denis, C.; Leplat, J.-J.; Chevaleyre, C.; Billon, Y.; Doré, J.; et al. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ. Microbiol. Rep. 2015, 7, 554–569. [Google Scholar] [CrossRef]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.-Y.; Choi, Y.-J.; Lee, H.K.; Hur, J.; Park, B.-Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Qin, S. Gut microbiota modulation on intestinal mucosal adaptive immunity. J. Immunol. Res. 2019, 2019, 4735040. [Google Scholar] [CrossRef] [PubMed]
- Slifierz, M.J.; Friendship, R.; Weese, J.S. Zinc oxide therapy increases prevalence and persistence of methicillin-resistant Staphylococcus aureus in pigs: A randomized controlled trial. Zoonoses Public Health 2015, 62, 301–308. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef]
- Bian, G.; Ma, S.; Zhu, Z.; Su, Y.; Zoetendal, E.G.; Mackie, R.; Liu, J.; Mu, C.; Huang, R.; Smidt, H.; et al. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ. Microbiol. 2016, 18, 1566–1577. [Google Scholar] [CrossRef]
- Levesque, C.L.; Hooda, S.; Swanson, K.S.; de Lange, K. Alterations in ileal mucosa bacteria related to diet complexity and growth performance in young pigs. PLoS ONE 2014, 9, e108472. [Google Scholar] [CrossRef]
- Zhu, L.; Liao, R.; Tu, W.; Lu, Y.; Cai, X. Pyrodextrin enhances intestinal function through changing the intestinal microbiota composition and metabolism in early weaned piglets. Appl. Microbiol. Biotechnol. 2020, 104, 4141–4154. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The influence of probiotics on vaccine responses—A systematic review. Vaccine 2018, 36, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P.; Singh, B.R. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE 2018, 13, e0192978. [Google Scholar] [CrossRef] [PubMed]
- Arfken, A.M.; Frey, J.F.; Ramsay, T.G.; Summers, K.L. Yeasts of burden: Exploring the mycobiome–bacteriome of the piglet GI tract. Front. Microbiol. 2019, 10, 2286. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, L.-M.; Li, B.; Guo, W.-J.; Ding, X.-L.; Xu, F.-Z. Effect of fermented biogas residue on growth performance, serum biochemical parameters, and meat quality in pigs. Asian Austral. J. Anim. Sci. 2017, 30, 1464–1470. [Google Scholar] [CrossRef]
- Czech, A.; Grela, E.; Kiesz, M.; Kłys, S. Biochemical and haematological blood parameters of sows and piglets fed a diet with a dried fermented rapeseed meal. Ann. Anim. Sci. 2020, 20, 535–550. [Google Scholar] [CrossRef]
- Shi, C.; He, J.; Wang, J.; Yu, J.; Yu, B.; Mao, X.; Zheng, P.; Huang, Z.; Chen, D. Effects of Aspergillus niger fermented rapeseed meal on nutrient digestibility, growth performance and serum parameters in growing pigs. Anim. Sci. J. 2016, 87, 557–563. [Google Scholar] [CrossRef]
- Cho, J.; Min, B.; Chen, Y.; Yoo, J.S.; Wang, Q. Evaluation of FSP (fermented soy protein) to replace soybean meal in weaned pigs: Growth performance, blood urea nitrogen and total protein concentrations in serum and nutrient digestibility. Asian Austral. J. Anim. Sci. 2007, 20, 1874–1879. [Google Scholar] [CrossRef]
- Min, B. Effects of diet complexity and fermented soy protein on growth performance and apparent ileal amino acid digestibility in weanling pigs. Asian Austral. J. Anim. Sci. 2010, 23, 1496–1502. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, M.; Zhang, R.; Sun, Z.; Wang, C.; Yang, F.; Huang, T.; Qu, S.; Zhao, L.; Li, Y.; et al. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb. Cell Fact. 2017, 16, 191. [Google Scholar] [CrossRef]
- Liu, X.; Feng, J.; Xu, Z. The effects of fermented soybean meal on growth performance and immune characteristics in weaned piglets. Turk. J. Vet. Anim. Sci. 2007, 31, 341–345. [Google Scholar]
- Jeong, Y.D.; Ko, H.S.; Hosseindoust, A.; Choi, Y.H.; Chae, B.J.; Yu, D.J.; Cho, E.S.; Cho, K.H.; Shim, S.M.; Ra, C.S.; et al. Lactobacillus-based fermentation product and lactose level in the feed for weanling pigs: Effects on intestinal morphology, microbiota, gas emission, and targeted intestinal coliforms. Livest. Sci. 2019, 227, 90–96. [Google Scholar] [CrossRef]
- Satessa, G.D.; Tamez-Hidalgo, P.; Hui, Y.; Cieplak, T.; Krych, L.; Kjærulff, S.; Brunsgaard, G.; Nielsen, D.S.; Nielsen, M.O. Impact of dietary supplementation of lactic acid bacteria fermented rapeseed with or without macroalgae on performance and health of piglets following omission of medicinal zinc from weaner diets. Animals 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet. Res. 2016, 47, 121. [Google Scholar] [CrossRef]
- Duivon, D.; Corrégé, I.; Hémonic, A.; Rigaut, M.; Roudaut, D.; Jolie, R. Field evaluation of piglet vaccination with a Mycoplasma hyopneumoniae bacterin as compared to a ready-to-use product including porcine circovirus 2 and M. hyopneumoniae in a conventional French farrow-to-finish farm. Porc. Health Manag. 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Park, C.; Choi, K.; Chae, C. Comparison of three commercial one-dose porcine circovirus type 2 (PCV2) vaccines in a herd with concurrent circulation of PCV2b and mutant PCV2b. Vet. Microbiol. 2015, 177, 43–52. [Google Scholar] [CrossRef] [PubMed]
- da Silva, N.; Carriquiry, A.; O’Neill, K.; Opriessnig, T.; O’Connor, A.M. Mixed treatment comparison meta-analysis of porcine circovirus type 2 (PCV2) vaccines used in piglets. Prev. Vet. Med. 2014, 117, 413–424. [Google Scholar] [CrossRef]
- Woźniak, A.; Miłek, D.; Matyba, P.; Stadejek, T. Real-time PCR detection patterns of porcine circovirus type 2 (PCV2) in Polish farms with different statuses of vaccination against PCV2. Viruses 2019, 11, 1135. [Google Scholar] [CrossRef]
- Opriessnig, T.; Xiao, C.-T.; Halbur, P.G.; Gerber, P.F.; Matzinger, S.R.; Meng, X.-J. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naïve pigs under experimental conditions. Vaccine 2017, 35, 248–254. [Google Scholar] [CrossRef]
- Afghah, Z.; Webb, B.; Meng, X.-J.; Ramamoorthy, S. Ten years of PCV2 vaccines and vaccination: Is eradication a possibility? Vet. Microbiol. 2017, 206, 21–28. [Google Scholar] [CrossRef]
- Chae, C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet. J. 2016, 212, 1–6. [Google Scholar] [CrossRef]
- Patterson, R.; Nevel, A.; Diaz, A.V.; Martineau, H.M.; Demmers, T.; Browne, C.; Mavrommatis, B.; Werling, D. Exposure to environmental stressors result in increased viral load and further reduction of production parameters in pigs experimentally infected with PCV2b. Vet. Microbiol. 2015, 177, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Yang, Y.; Jung, H.I.; Nguyen, D.H.; Kim, I.H. Effect of a protected blend of organic acids and medium-chain fatty acids on growth performance, nutrient digestibility, blood profiles, meat quality, faecal microflora, and faecal gas emission in finishing pigs. Can. J. Anim. Sci. 2019, 99, 448–455. [Google Scholar]
- Nguyen, D.H.; Nyachoti, C.; Kim, I.H. Evaluation of effect of probiotics mixture supplementation on growth performance, nutrient digestibility, faecal bacterial enumeration, and noxious gas emission in weaning pigs. Ital. J. Anim. Sci. 2019, 18, 466–473. [Google Scholar] [CrossRef]
- Bindas, L.; Bujnak, L.; Maskaľová, I.; Mihok, T.; Lacková, P.; Nad, P. Effects of feeding low protein diets on serum and faeces parameters in weaned piglets. Folia Vet. 2019, 63, 37–44. [Google Scholar] [CrossRef]

| Ingredients (%) | Control Group | Treated Group |
|---|---|---|
| Barley | 38.45 | 33.25 |
| Rapeseed meal | - | 25.00 |
| Wheat | 32.12 | 25.00 |
| Soya beans (extruded) | 9.30 | - |
| Potato protein | 5.00 | 2.00 |
| Soybean protein concentrate | 2.00 | - |
| Whey powder | 5.80 | 5.80 |
| Sunflower oil | 2.72 | 4.51 |
| Limestone | 1.48 | 1.1 |
| NaCl | 0.38 | 0.35 |
| Monocalcium phosphate | 0.33 | 0.41 |
| L-Lysine sulphate | 0.87 | 1.1 |
| DL-Methionine | 0.25 | 0.16 |
| Acidal NC (formic and acetic acids) | 0.30 | 0.30 |
| 1 Vitamins and trace elements (premix) | 1.00 | 1.00 |
| Nutritional value | ||
| ME swine (MJ/kg) | 13.86 | 13.95 |
| Crude protein (%) | 19.00 | 19.00 |
| Crude fat (%) | 6.51 | 6.51 |
| Crude fibre (%) | 3.15 | 5.14 |
| Lysine (%) | 1.45 | 1.45 |
| Methionine (%) | 0.55 | 0.55 |
| Threonine (%) | 0.93 | 0.94 |
| Tryptophan (%) | 0.26 | 0.25 |
| Methionine + Cystine (%) | 0.87 | 0.88 |
| Ca (%) | 0.90 | 0.90 |
| Total P (%) | 0.59 | 0.62 |
| Available P (%) | 0.37 | 0.38 |
| Na (%) | 0.20 | 0.21 |
| Microbiological Parameters (log10 CFU/g) | Day | Treatments | |||||
|---|---|---|---|---|---|---|---|
| SnonV | SV | RFnonV | RFV | ||||
| LAB | Baseline | 7.78 ± 0.03 A;a | 8.22 ± 0.04 A;b | 8.33 ± 0.05 A;a | 8.35 ± 0.06 A;a | ||
| 61 | 6.22 ± 0.04B;a | 8.47 ± 0.02 B;b | 5.17 ± 0.06 B;a | 6.24 ± 0.07 B;b | |||
| TVC | Baseline | 7.08 ± 0.02 A;a | 8.25 ± 0.05 A;b | 8.38 ± 0.07 A;b | 7.68 ± 0.07 A;a | ||
| 61 | 6.41 ± 0.04. B;a | 8.24 ± 0.03. A;b | 6.40 ± 0.03. B;a | 7.42 ± 0.08 B;b | |||
| TEC | Baseline | 7.22 ± 0.06 A;a | 7.79 ± 0.06 A;b | 7.40 ± 0.08 A;a | 7.58 ± 0.04 A;b | ||
| 61 | 6.36 ± 0.02 B;b | 6.18 ± 0.05 B;a | 6.87 ± 0.07 B;a | 7.12 ± 0.03 B;b | |||
| Y/M | Baseline | 6.73 ± 0.04 A;a | 7.71 ± 0.08 A;b | 6.21 ± 0.04 A;a | 6.63 ± 0.04 A;b | ||
| 61 | 6.36 ± 0.08 B;b | 4.31 ± 0.06 B;a | 5.72±0.05 B;a | 6.26±0.04 B;b | |||
| Tests of between-subject effects: influence of the analysed factors and their interactions on microbiological parameters in piglets’ faeces | |||||||
| Dependent Variable | Treatment Duration | Fermented Feed | Vaccination | Treatment Duration× Fermented Feed | Treatment Duration× Vaccination | Fermented Feed × Vaccination | Treatment Duration× Fermented Feed × Vaccination |
| Significance of the differences between groups (p) | |||||||
| LAB | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| TBC | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.246 | 0.0001 |
| TVC | 0.0001 | 0.0001 | 0.001 | 0.0001 | 0.021 | 0.0001 | 0.0001 |
| Y/M | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Blood Parameters | Day | Treatments | |||
|---|---|---|---|---|---|
| SnonV | SV | RFnonV | RFV | ||
| Aspartate aminotransferase (AST), U/L | Baseline | 29.4 A;a | 42.67 A;a | 51.4 A;a | 61.8 A;a |
| 61 | 34.0 B;a | 41.0. A;a | 44.0. A;a | 42.4. A;a | |
| Alanine aminotransferase (ALT), U/L | Baseline | 48.4 A;a | 43.67 A;a | 53.2 A;b | 60.8 A;a |
| 61 | 76.2. B;a | 69.6 B;b | 87.0 B;a | 80.8 A;b | |
| Cholesterol (Chol), mmol/L | Baseline | 1.64 A;a | 1.64 A;a | 1.88 A;a | 2.04 A;a |
| 61 | 2.06. B;a | 2.26. B;b | 2.34. A;a | 2.25. B;a | |
| High-density lipoprotein cholesterol (HDL-C), mmol/L | Baseline | 0.714 A;a | 0.744 A;b | 0.898 A;a | 0.846 A;a |
| 61 | 0.840 B;a | 0.944 B;b | 1.028 B;a | 0.872 A;b | |
| Low-density lipoprotein cholesterol (LDL-C), mmol/L | Baseline | 0.758 A;a | 0.726 A;a | 0.814 A;a | 0.976 A; a |
| 61 | 0.980 B;a | 1.102 B;a | 1.032 A;a | 0.860 A; b | |
| Triglycerides (TG), mmol/L | Baseline | 0.360 A;a | 0.372 A;a | 0.366 A;a | 0.478 A;a |
| 61 | 0.466 B;a | 0.466 A;a | 0.620 B;a | 0.650 B;b | |
| Total protein (TP), g/L | Baseline | 46.2 A;a | 44.9 A;a | 44.2 A;a | 45.6 A;a |
| 61 | 51.8 B;a | 56.3 B;b | 52.8 B;a | 49.5 B;b | |
| Albumin (ALB), g/L | Baseline | 30.0 A;a | 29.0 A;a | 32.6 A;a | 32.8 A;a |
| 61 | 35.8 A;a | 37.4 B;a | 36.2 A;a | 33.4 B;b | |
| Immunoglobulin IgG, g/L | Baseline | 2.65 A;a | 2.85 A;a | 2.35 A;a | 2.96 A;a |
| 61 | 3.74 B;a | 3.44 A;a | 3.05 B;a | 5.45 A;b | |
| Triiodothyronine (T3), nmol/L | Baseline | 1.21 A;a | 1.14 A;b | 1.29 A;a | 1.22 A;a |
| 61 | 2.14B;a | 2.02 B;b | 1.59 A;a | 1.49 A;a | |
| Thyroxine (T4), µ d/L | Baseline | 4.50 A;a | 4.82 A;a | 3.50 A;a | 5.22 A;a |
| 61 | 4.84 A;a | 3.84 A;b | 2.92 B;a | 2.90 A;a | |
| Glucose (GLU), nmol/L | Baseline | 5.84 A;a | 6.10 A;a | 6.12 A;a | 5.66 A;a |
| 61 | 5.74 A;a | 6.22 A;b | 6.08 A;a | 6.18 B;a | |
| Phosphorus (IP), mmol/L | Baseline | 2.94 A;a | 2.87 A;a | 2.61 A;a | 2.76 A;a |
| 61 | 3.50 B;a | 3.39 A;b | 3.28 B;a | 3.39 B;a | |
| Magnesium (Mg), mmol/L | Baseline | 1.02 A;a | 0.928 A;b | 0.996 A;a | 0.932 A;b |
| 61 | 1.07 A;a | 0.936 A;b | 0.960 A;a | 0.966 A;b | |
| Potassium (K) | Baseline | 4.96 A;a | 5.66 A;b | 4.65 A;a | 5.69 A;b |
| 61 | 5.81 B;a | 5.34 B;b | 4.96 A;a | 4.74 B;a | |
| Sodium (Na) | Baseline | 143.4 A;a | 141.0 A;a | 144.0 A;a | 142.0 A;b |
| 61 | 147.2 A;a | 147.2 B; a | 146.6 B;a | 144.4 A;a | |
| Iron (Fe), µmol/L | Baseline | 23.6 A;a | 23.8 A;a | 31.5 A;a | 23.5 A;b |
| 61 | 28.1 A;a | 26.9 B;a | 47.1 A;a | 51.4 B;a | |
| Calcium (Ca), nmol/L | Baseline | 2.60 A;a | 2.57 A;a | 2.71 A;a | 2.55 A;a |
| 61 | 2.87 A;a | 2.78 B;b | 2.79 A;a | 2.78 B;a | |
| Vitamin B12, pmol/L | Baseline | 142.2 A;a | 93.8 A;b | 78.2 A;a | 131.0 A;b |
| 61 | 214.6 A;a | 122.2 B;b | 94.2 A; a | 98.2 B;a | |
| Creatinine (CREA), µmol/L | Baseline | 64.2 A;a | 57.4 A;a | 78.8 A;a | 63.4 A;a |
| 61 | 57.4 A;a | 54.8 A;a | 48.2 B;a | 49.4 A;a | |
| Alkaline phosphatase (AP), U/L | Baseline | 336.2 A;a | 285.0 A;a | 408.6 A;a | 318.0 A;a |
| 61 | 263.6 A;a | 220.4 A;a | 242.6 B;a | 245.4 A;a | |
| Urea, mmol/L | Baseline | 2.36 A;a | 3.26 A;a | 2.64 A;a | 2.58 A;a |
| 61 | 2.02 A;a | 2.38 A;a | 3.19 B;a | 2.63 B;a | |
| Thyroid-stimulating hormone (TSH) | Baseline | 0.0200 A;a | 0.0190 A;a | 0.0208 A;a | 0.0236 A;a |
| 61 | 0.0208 A;a | 0.0228 A;a | 0.0230 A;a | 0.0260 B;a | |
| Total bilirubin (pmol/L) | Baseline | 1.88 A;a | 1.99 A;a | 1.99 A;a | 1.99 A;a |
| 61 | 1.98 A;a | 1.99 A;a | 1.99 A;a | 2.65 A;b | |
| Dependent Variable | TD | FF | V | TD × FF | TD × V | FF × V | TD × FF × V |
|---|---|---|---|---|---|---|---|
| Significance (p) | |||||||
| AST, U/L | 0.279 | 0.025 | 0.191 | 0.182 | 0.404 | 0.598 | 0.791 |
| ALT, U/L | 0.0001 | 0.017 | 0.557 | 0.997 | 0.358 | 0.453 | 0.481 |
| Chol, mmol/L | 0.004 | 0.088 | 0.608 | 0.467 | 0.907 | 0.809 | 0.396 |
| HDL-C, mmol/L | 0.069 | 0.123 | 0.768 | 0.501 | 0.905 | 0.185 | 0.482 |
| LDL-C, mmol/L | 0.045 | 0.723 | 0.807 | 0.143 | 0.584 | 0.760 | 0.149 |
| TG, mmol/L | 0.002 | 0.018 | 0.382 | 0.206 | 0.591 | 0.459 | 0.688 |
| TP, g/L | 0.0001 | 0.155 | 0.773 | 0.345 | 0.811 | 0.310 | 0.041 |
| ALB, g/L | 0.002 | 0.581 | 0.693 | 0.061 | 0.937 | 0.529 | 0.276 |
| IgG, g/L | 0.112 | 0.699 | 0.329 | 0.612 | 0.660 | 0.299 | 0.442 |
| T3, nmol/L | 0.001 | 0.120 | 0.522 | 0.038 | 0.909 | 0.994 | 0.949 |
| T4, µ d/L | 0.003 | 0.004 | 0.331 | 0.041 | 0.008 | 0.033 | 0.685 |
| GLU, nmol/L | 0.633 | 0.716 | 0.660 | 0.458 | 0.300 | 0.633 | 0.745 |
| IP, mmol/L | 0.001 | 0.179 | 0.878 | 0.658 | 0.851 | 0.349 | 0.993 |
| Mg, mmol/L | 0.675 | 0.487 | 0.060 | 0.655 | 0.867 | 0.250 | 0.422 |
| K | 0.885 | 0.041 | 0.203 | 0.153 | 0.007 | 0.462 | 0.902 |
| Na | 0.0001 | 0.548 | 0.039 | 0.108 | 0.464 | 0.548 | 0.388 |
| Fe, µmol/L | 0.001 | 0.001 | 0.694 | 0.008 | 0.370 | 0.830 | 0.267 |
| Ca, nmol/L | 0.001 | 0.985 | 0.176 | 0.379 | 0.657 | 0.816 | 0.313 |
| B12, pmol/L | 0.158 | 0.008 | 0.158 | 0.055 | 0.121 | 0.003 | 0.934 |
| CREA, µmol/L | 0.069 | 0.831 | 0.406 | 0.222 | 0.463 | 0.864 | 0.660 |
| AP, U/L | 0.027 | 0.490 | 0.256 | 0.521 | 0.521 | 0.966 | 0.588 |
| Urea, mmol/L | 0.164 | 0.797 | 0.899 | 0.286 | 0.075 | 0.014 | 0.456 |
| TSH | 0.604 | 0.523 | 0.736 | 0.854 | 0.951 | 0.854 | 0.691 |
| Total bilirubin (pmol/L) | 0.338 | 0.280 | 0.280 | 0.290 | 0.290 | 0.349 | 0.338 |
| Piglets Growth Performance | Average from day 25 to 61 | Treatments | |||
| SnonV | SV | RFnonV | RFV | ||
| FCR | 1.56 | 1.38 | 1.58 | 1.76 | |
| ADG | 0.395 | 0.392 | 0.397 | 0.399 | |
| Tests of between-subjects effects: influence of analysed factors and their interactions on FCR and ADG | |||||
| Dependent Variable | Fermented Feed | Vaccination | Fermented Feed × Vaccination | ||
| Significance (p) | |||||
| FCR | 0.154 | 1.000 | 0.194 | ||
| ADG | 0.962 | 0.996 | 0.979 | ||
| Differences among treatments (p < 0.05) | |||||
| SVvs.SnonV | RFVvs.RFnonV | SVvs.RFV | SnonVvs.RFnonV | SnonVvs.RFV | RFnonVvs.SV |
| ADG | |||||
| 0.901 | 0.783 | 0.55 | 0.943 | 0.91 | 0.286 |
| FCR | |||||
| 0.001 | 0.002 | 0.0001 | 0.773 | 0.91 | 0.074 |
| Ammonia Emission | Day | Treatments | ||||
| SnonV | SV | RFnonV | RFV | |||
| Baseline | 0.837 A;a | 1.172 A;b | 1.584 A;a | 1.252 A;b | ||
| 61 | 1.938 B;a | 1.063 A;b | 1.538 A;a | 0.810 B;b | ||
| Tests of between-subject effects: influence of the analysed factors and their interactions on ammonia emission | ||||||
| Treatment Duration | Fermented Feed | Vaccination | Treatment Duration× Fermented Feed | Treatment Duration× Vaccination | Fermented Feed × Vaccination | Treatment Duration× Fermented Feed × Vaccination |
| Significance (p) | ||||||
| 0.0001 | 0.003 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadopalas, L.; Badaras, S.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Klupsaite, D.; Mozuriene, E.; et al. Influence of the Fermented Feed and Vaccination and Their Interaction on Parameters of Large White/Norwegian Landrace Piglets. Animals 2020, 10, 1201. https://doi.org/10.3390/ani10071201
Vadopalas L, Badaras S, Ruzauskas M, Lele V, Starkute V, Zavistanaviciute P, Zokaityte E, Bartkevics V, Klupsaite D, Mozuriene E, et al. Influence of the Fermented Feed and Vaccination and Their Interaction on Parameters of Large White/Norwegian Landrace Piglets. Animals. 2020; 10(7):1201. https://doi.org/10.3390/ani10071201
Chicago/Turabian StyleVadopalas, Laurynas, Sarunas Badaras, Modestas Ruzauskas, Vita Lele, Vytaute Starkute, Paulina Zavistanaviciute, Egle Zokaityte, Vadims Bartkevics, Dovile Klupsaite, Erika Mozuriene, and et al. 2020. "Influence of the Fermented Feed and Vaccination and Their Interaction on Parameters of Large White/Norwegian Landrace Piglets" Animals 10, no. 7: 1201. https://doi.org/10.3390/ani10071201
APA StyleVadopalas, L., Badaras, S., Ruzauskas, M., Lele, V., Starkute, V., Zavistanaviciute, P., Zokaityte, E., Bartkevics, V., Klupsaite, D., Mozuriene, E., Dauksiene, A., Sidlauskiene, S., Gruzauskas, R., & Bartkiene, E. (2020). Influence of the Fermented Feed and Vaccination and Their Interaction on Parameters of Large White/Norwegian Landrace Piglets. Animals, 10(7), 1201. https://doi.org/10.3390/ani10071201