Methane Emissions and Milk Fatty Acid Profiles in Dairy Cows Fed Linseed, Measured at the Group Level in a Naturally Ventilated Housing and Individually in Respiration Chambers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
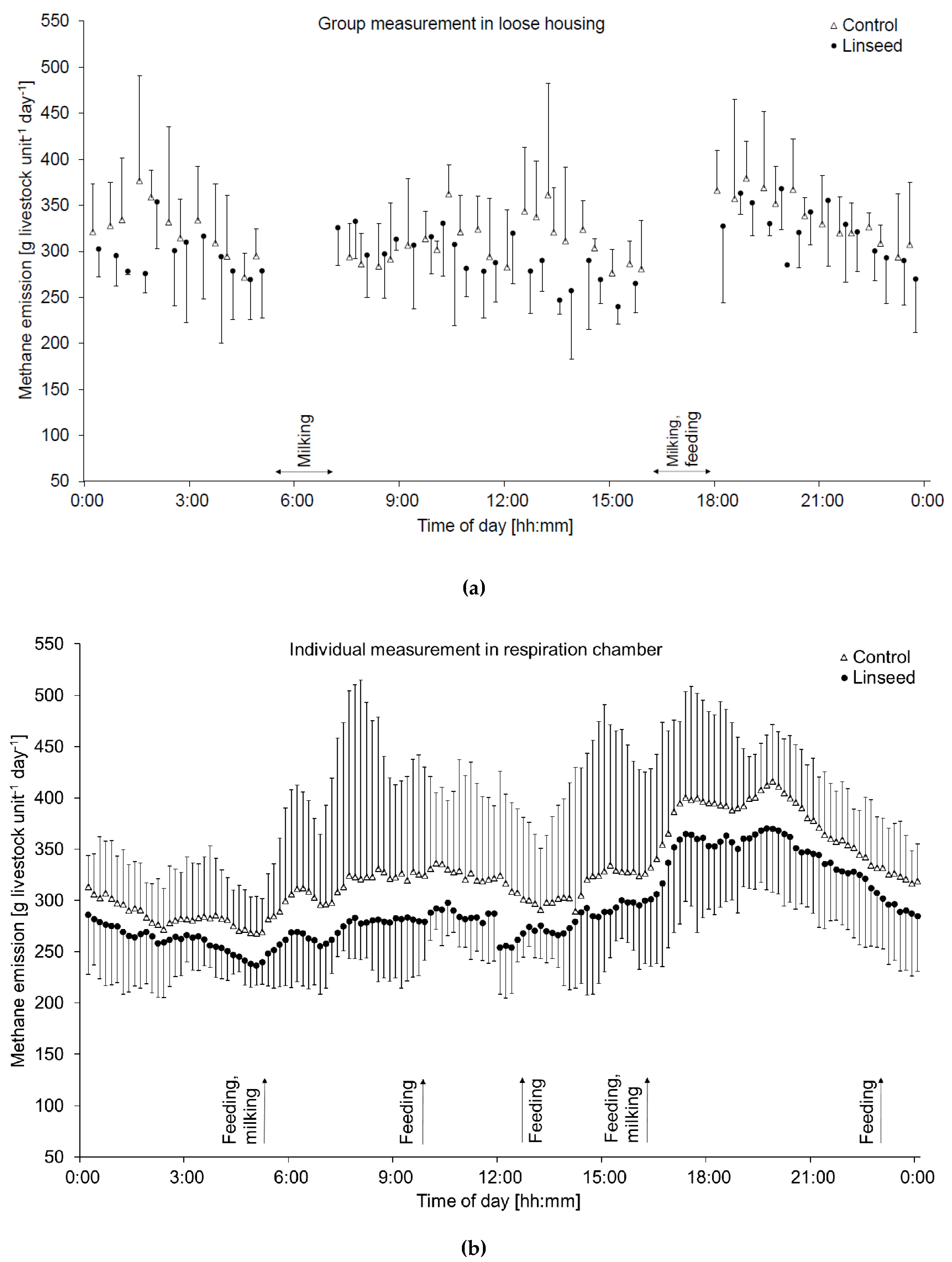
2.1.1. Measurements at the Group Level in the Loose-Housing System
2.1.2. Measurements at the Individual Level in the Tied-Housing System and in the Respiration Chambers
2.2. Feeding
2.3. Measurement of Methane and Carbon Dioxide Emissions
2.3.1. Emission Measurement in the Experimental Dairy Loose-Housing System (Group Level)
2.3.2. Emission Measurement in the Respiration Chambers (Individual Level)
2.4. Performance and Feeding Behavior (Individual Measurements in the Loose and Tied-Housing Systems)
2.5. Sampling
2.6. Laboratory Analyses
2.7. Calculations and Statistical Analysis
3. Results
3.1. Individual Performance, Digestibility, and Feeding Behavior (Loose- and Tied-Housing Systems)
3.2. Methane and Carbon Dioxide Emissions (Group and Individual Level)
3.3. Milk Composition (Group and Individual Level)
4. Discussion
4.1. Effects of Linseed on the Intake Pattern and Milk Yield in the Loose- and Tied-Housing Systems
4.2. Effects of Linseed on Digestibility as well as Nitrogen and Energy Utilization (Individual Level)
4.3. Effects of Linseed on Methane Emission at Group and Individual Level
4.4. Effects of Linseed Supplementation on Milk Composition at the Group and Individual Levels
4.5. Comparability of Methane Emission Measurements in an Experimental Housing System and Individually in Respiration Chambers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Item | Control | Linseed | p-Value |
|---|---|---|---|
| N balance (g·day–1) | |||
| Intake | 526 ± 49 | 552 ± 59 | 0.419 |
| Feces | 176 ± 26 | 167 ± 26 | 0.561 |
| Urine | 167 ± 67 | 214 ± 68 | 0.250 |
| Milk | 125 ± 33 | 156 ± 53 | 0.246 |
| Body | 58 ± 66 | 15 ± 59 | 0.255 |
| N balance (g·kg−1) | |||
| Feces | 334 ± 29 | 303 ± 45 | 0.187 |
| Urine | 312 ± 97 | 383 ± 89 | 0.211 |
| Milk | 235 ± 45 | 277 ± 65 | 0.222 |
| Body | 119 ± 122 | 36 ± 105 | 0.234 |
| N utilization | |||
| Apparent N digestibility (%) | 64.6 ± 11.2 | 68.7 ± 7.2 | 0.468 |
| N intake (g·kg−1 ECM1) | 20.5 ± 4.1 | 19.1 ± 4.0 | 0.551 |
| Feces N (g·kg−1 ECM) | 6.9 ± 1.9 | 5.8 ± 1.5 | 0.266 |
| Manure N traits | |||
| Manure N (g·kg−1 of N intake) | 250 ± 16 | 232 ± 26 | 0.174 |
| Urinary N (g·kg−1 of manure N) | 473 ± 73 | 554 ± 78 | 0.094 |
| Item | Control | Linseed | p-Value |
|---|---|---|---|
| Energy intake (MJ/day) | |||
| Gross energy (GE) | 357.8 ± 30.8 | 361.9 ± 37.6 | 0.840 |
| Digestible energy | 273.5 ± 14.3 | 276.5 ± 33.8 | 0.843 |
| Metabolizable energy (ME) | 236.5 ± 14.8 | 241.5 ± 34.3 | 0.750 |
| Energy loss (MJ/day) | |||
| Feces | 84.3 ± 17.1 | 85.3 ± 13.3 | 0.906 |
| Urine | 9.9 ± 0.9 | 10.4 ± 3.1 | 0.717 |
| Methane | 27.1 ± 2.2 | 24.7 ± 1.9 | 0.063 |
| Heat | 119.1 ± 10.7 | 118.2 ± 6.2 | 0.858 |
| Total energy loss | 240.4 ± 25.5 | 238.6 ± 16.7 | 0.887 |
| Energy retention (MJ/day) | |||
| Milk | 83.1 ± 17.7 | 95.7 ± 29.2 | 0.386 |
| Body | 34.3 ± 12.8 | 27.6 ± 17.1 | 0.458 |
| Energy turnover (kJ·MJ−1 of GE intake) | |||
| Feces | 234 ± 26 | 236 ± 34 | 0.885 |
| Urine | 28 ± 4 | 29 ± 10 | 0.750 |
| Methane | 76 ± 10 | 69 ± 6 | 0.131 |
| Heat | 333 ± 17 | 328 ± 24 | 0.700 |
| Total energy loss | 671 ± 17 | 663 ± 52 | 0.712 |
| Milk | 231 ± 40 | 260 ± 54 | 0.313 |
| Body | 98 ± 41 | 77 ± 47 | 0.440 |
| Heat (kJ·MJ−1 of ME intake) | 503 ± 24 | 495 ± 54 | 0.743 |
| Energy utilization (%) | |||
| Apparent digestibility | 76.6 ± 2.6 | 76.4 ± 3.4 | 0.885 |
| Metabolizability (q) | 66.2 ± 1.6 | 66.6 ± 3.7 | 0.822 |
| kL1 (estimated from q) | 62.2 ± 0.4 | 62.3 ± 0.9 | 0.882 |
| kL1 (direct calculation) | 57.0 ± 2.6 | 58.1 ± 2.4 | 0.493 |
| Supply over estimated requirements | |||
| Net energy for lactation (MJ) | 21.8 ± 12.6 | 12.5 ± 16.6 | 0.299 |
| % deviation from requirements | 18.8 ± 14.1 | 10.7 ± 13.6 | 0.335 |
| ME (MJ) | 32.5 ± 19.9 | 17.3 ± 26.0 | 0.282 |
| % deviation from requirements | 17.3 ± 13.5 | 9.3 ± 13.0 | 0.327 |
References
- Hristov, A.N.; Hanigan, M.; Cole, A.; Todd, R.; McAllister, T.A.; Ndegwa, P.M.; Rotz, A. Ammonia emissions from dairy farms and beef feedlots. Can. J. Anim. Sci. 2011, 91, 1–35. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [PubMed]
- Cottle, D.J.; Nolan, J.V.; Wiedemann, S.G. Ruminant enteric methane mitigation: A review. Anim. Prod. Sci. 2011, 51, 491–514. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agr. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Martin, C.; Rouel, J.; Jouany, J.P.; Doreau, M.; Chilliard, Y. Methane output and diet digestibility in response to feeding dairy cows crude linseed, extruded linseed, or linseed oil. J. Anim. Sci. 2008, 86, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Ferlay, A.; Mosoni, P.; Rochette, Y.; Chilliard, Y.; Doreau, M. Increasing linseed supply in dairy cow diets based on hay or corn silage: Effect on enteric methane emission, rumen microbial fermentation, and digestion. J. Dairy Sci. 2016, 99, 3445–3456. [Google Scholar] [CrossRef]
- Hammond, K.J.; Humphries, D.J.; Crompton, L.A.; Kirton, P.; Reynolds, C.K. Effects of forage source and extruded linseed supplementation on methane emissions from growing dairy cattle of differing body weights. J. Dairy Sci. 2015, 98, 8066–8077. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Humphries, D.J.; Kirton, P.; Kliem, K.E.; Givens, D.I.; Reynolds, C.K. Effects of forage type and extruded linseed supplementation on methane production and milk fatty acid composition of lactating dairy cows. J. Dairy Sci. 2015, 98, 4000–4011. [Google Scholar] [CrossRef]
- Judy, J.V.; Bachman, G.C.; Brown-Brandl, T.M.; Fernando, S.C.; Hales, K.E.; Harvatine, K.J.; Miller, P.S.; Kononoff, P.J. Increasing the concentration of linolenic acid in diets fed to Jersey cows in late lactation does not affect methane production. J. Dairy Sci. 2019, 102, 2085–2093. [Google Scholar] [CrossRef]
- Engelke, S.W.; Daş, G.; Derno, M.; Tuchscherer, A.; Wimmers, K.; Rychlik, M.; Kienberger, H.; Berg, W.; Kuhla, B.; Metges, C.C. Methane prediction based on individual or groups of milk fatty acids for dairy cows fed rations with or without linseed. J. Dairy. Sci. 2019, 102, 1788–1802. [Google Scholar] [CrossRef]
- Focant, M.; Froidmont, E.; Archambeau, Q.; Dang Van, Q.C.; Larondelle, Y. The effect of oak tannin (Quercus robur) and hops (Humulus lupulus) on dietary nitrogen efficiency, methane emission, and milk fatty acid composition of dairy cows fed a low-protein diet including linseed. J. Dairy Sci. 2019, 102, 1144–1159. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Romero-Pérez, G.A.; Chouinard, P.Y.; Hassanat, F.; Eugene, M.; Petit, H.V.; Côrtes, C. Supplementation of increasing amounts of linseed oil to dairy cows fed total mixed rations: Effects on digestion, ruminal fermentation characteristics, protozoal populations, and milk fatty acid composition. J. Dairy. Sci. 2012, 95, 4578–4590. [Google Scholar] [CrossRef] [PubMed]
- Troy, S.H.; Duthie, C.-A.; Ross, D.W.; Hyslop, J.J.; Roehe, R.; Waterhouse, A.; Rooke, J.A. A comparison of methane emissions from beef cattle measured using methane hoods with those measured using respiration chambers. Anim. Feed. Sci. Technol. 2016, 211, 227–240. [Google Scholar] [CrossRef]
- Storm, I.M.; Hellwing, A.L.F.; Nielsen, N.I.; Madsen, J. Methods for measuring and estimating methane emission from ruminants. Animals 2012, 2, 160–183. [Google Scholar] [CrossRef] [PubMed]
- Huhtanen, P.; Cabezas-Garcia, E.H.; Utsumi, S.; Zimmerman, S. Comparison of methods to determine methane emissions from dairy cows in farm conditions. J. Dairy Sci. 2015, 98, 3394–3409. [Google Scholar] [CrossRef]
- Schmithausen, A.J.; Schiefler, I.; Trimborn, M.; Gerlach, K.; Südekum, K.-H.; Pries, M.; Büscher, W. Quantification of methane and ammonia emissions in a naturally ventilated barn by using defined criteria to calculate emission rates. Animals 2018, 8, 75. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; McGinn, S.M.; Auldist, M.J.; Beauchemin, K.A.; Hannah, M.C.; Waghorn, G.C.; Clark, H.; Eckard, R.J. Methane emissions from dairy cows measured using the sulfur hexafluoride (SF6) tracer and chamber techniques. J. Dairy Sci. 2007, 90, 2755–2766. [Google Scholar] [CrossRef]
- Hammond, K.J.; Humphries, D.J.; Crompton, L.A.; Green, C.; Reynolds, C.K. Methane emissions from cattle: Estimates from short-term measurements using a GreenFeed system compared with measurements obtained using respiration chambers or sulphur hexafluoride tracer. Anim. Feed. Sci. Technol. 2015, 203, 41–52. [Google Scholar] [CrossRef]
- Agroscope. Feeding Recommendations and Nutrient Tables for Ruminants. 2020. Available online: https://www.db-agroscope.ch/member/fmdb/7Fuumltterungsempfehlungenfuum_113.pdf (accessed on 11 January 2020). (In German).
- Mohn, J.; Zeyer, K.; Keck, M.; Keller, M.; Zähner, M.; Poteko, J.; Emmenegger, L.; Schrade, S. A dual tracer ratio method for comparative emission measurements in an experimental dairy housing. Atmos. Environ. 2018, 179, 12–22. [Google Scholar] [CrossRef]
- Schrade, S.; Zeyer, K.; Gygax, L.; Emmenegger, L.; Hartung, E.; Keck, M. Ammonia emissions and emission factors of naturally ventilated dairy housing with solid floors and an outdoor exercise area in Switzerland. Atmos. Environ. 2012, 47, 183–194. [Google Scholar] [CrossRef]
- Buehler, K.; Wanner, M. Chapter 6: Metabolic Centre of the University of Zurich and ETH Zurich (under construction). In Technical Manual on Respiration Chamber Designs; Leahy, S., Parlane, K., Eds.; Ministry of Agriculture and Forestry: Wellington, New Zealand, 2018; pp. 89–106. Available online: https://globalresearchalliance.org/wp-content/uploads/2018/02/LRG-Manual-Facility-BestPract-Sept-2018.pdf (accessed on 23 April 2020).
- Werner, J.; Leso, L.; Umstatter, C.; Niederhauser, J.; Kennedy, E.; Geoghegan, A.; Shalloo, L.; Schick, M.; O’Brien, B. Evaluation of the RumiWatchSystem for measuring grazing behaviour of cows. J. Neurosci. Meth. 2018, 300, 138–146. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Grandl, F.; Zeitz, J.O.; Clauss, M.; Furger, M.; Kreuzer, M.; Schwarm, A. Evidence for increasing digestive and metabolic efficiency of energy utilization with age of dairy cattle as determined in two feeding regimes. Animal 2018, 12, 515–527. [Google Scholar] [CrossRef] [PubMed]
- ISO (International Organization for Standardization). ISO 14637: Milk—Determination of Urea Content–Enzymatic Method Using Difference in pH (Reference Method); ISO: Geneva, Switzerland, 2004. [Google Scholar]
- IUPAC (International Union of Pure and Applied Chemistry). Standard Methods for the Analysis of Oils, Fats and Derivates, 7th ed.; Blackwell Scientific Publications: Oxford, UK, 1987. [Google Scholar]
- Wettstein, H.-R.; Scheeder, M.R.L.; Sutter, F.; Kreuzer, M. Effect of lecithins partly replacing rumen-protected fat on fatty acid digestion and composition of cow milk. Eur. J. Lipid. Sci. Tech. 2001, 103, 12–22. [Google Scholar] [CrossRef]
- Suter, B.; Grob, K.; Pacciarelli, B. Determination of fat content and fatty acid composition through 1-min transesterification in the food sample; principles. Z. Lebensmitteluntersuchungs- Und -Forschung A 1997, 204, 252–258. [Google Scholar] [CrossRef]
- Collomb, M.; Bühler, T. Analysis of the fatty acid composition of the milk fat. I. Optimisation and validation of a general method adapted to the research. Mitt. Lebensm. Hyg. 2000, 91, 306–332. [Google Scholar]
- KTBL (Kuratorium für Technik und Bauwesen in der Landwirtschaft), 2019. Grossvieheinheitenrechner 2.1. Available online: http://daten.ktbl.de/gvrechner/gvHome.do;jsessionid=C389DE 28C48F29CD7A3EEA53225A478E#start (accessed on 23 April 2020).
- Kinsman, R.; Sauer, F.D.; Jackson, H.A.; Wolynetz, M.S. Methane and Carbon Dioxide Emissions from Dairy Cows in Full Lactation Monitored over a Six-Month Period. J. Dairy Sci. 1995, 78, 2760–2766. [Google Scholar] [CrossRef]
- Sauer, F.D.; Fellner, V.; Kinsman, R.; Kramer, J.K.G.; Jackson, H.A.; Lee, A.J.; Chen, S. Methane output and lactation response in Holstein cattle with monensin or unsaturated fat added to the diet. J. Anim. Sci. 1998, 76, 906–914. [Google Scholar] [CrossRef]
- Machmüller, A.; Soliva, C.R.; Kreuzer, M. Methane-suppressing effect of myristic acid in sheep as affected by dietary calcium and forage proportion. Br. J. Nutr. 2003, 90, 529–540. [Google Scholar] [CrossRef]
- Zhou, X.; Zeitz, J.O.; Meile, L.; Kreuzer, M.; Schwarm, A. Influence of pH and the degree of protonation on the inhibitory effect of fatty acids in the ruminal methanogen Methanobrevibacter ruminantium strain M1. J. Appl. Microbiol. 2015, 119, 1482–1493. [Google Scholar] [CrossRef]
- Zetouni, L.; Difford, G.F.; Lassen, J.; Byskov, M.V.; Norberg, E.; Løvendahl, P. Is rumination time an indicator of methane production in dairy cows? J. Dairy Sci. 2018, 101, 11074–11085. [Google Scholar] [CrossRef] [PubMed]
- Watt, L.J.; Clark, C.E.F.; Krebs, G.L.; Petzel, C.E.; Nielsen, S.; Utsumi, S.A. Differential rumination, intake, and enteric methane production of dairy cows in a pasture-based automatic milking system. J. Dairy Sci. 2015, 98, 7248–7263. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, K.A. Current perspectives on eating and rumination activity in dairy cows. J. Dairy Sci. 2018, 101, 4762–4784. [Google Scholar] [CrossRef]
- Van Zijderveld, S.M.; Dijkstra, J.; Perdok, H.B.; Newbold, J.R.; Gerrits, W.J.J. Dietary inclusion of diallyl disulfide, yucca powder, calcium fumarate, an extruded linseed product, or medium-chain fatty acids does not affect methane production in lactating dairy cows. J. Dairy Sci. 2011, 94, 3094–3104. [Google Scholar] [CrossRef] [PubMed]
- Doreau, M.M.; Ferlay, A. Linseed: A valuable feedstuff for ruminants. Oilseeds. Fats. Crops. Lipids. 2015, 22, 1–9. [Google Scholar] [CrossRef]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Eur. J. Lipid. Sci. Tech. 2007, 109, 828–855. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Methane emissions of manure from dairy cows fed red clover or corn silage-based diets supplemented with linseed oil. J. Dairy Sci. 2019, 102, 11766–11776. [Google Scholar] [CrossRef]
| Mixed Part of the Diet | Supplementary Concentrates | Linseed | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Component | Grass Silage | Corn Silage | Hay | Beet Pulp | Concentrate1 | Rich in Protein2 | Rich in Energy3 | Corn Flour | Product |
| Dry matter (g·kg−1) | 459 | 417 | 899 | 260 | 882 | 863 | 865 | 859 | 933 |
| Nutrients (g·kg−1 of dry matter) | |||||||||
| Organic matter | 917 | 970 | 914 | 900 | 917 | 919 | 923 | 989 | 957 |
| Crude protein | 94 | 62 | 109 | 83 | 563 | 413 | 182 | 79 | 180 |
| Neutral detergent fiber | 463 | 328 | 506 | 435 | 221 | 127 | 161 | 161 | 398 |
| Acid detergent fiber | 303 | 193 | 295 | 230 | 135 | 95 | 85 | 52 | 215 |
| Acid detergent lignin | 37.7 | 33.8 | 37.1 | 29.2 | 50.4 | 32.5 | 32.5 | 22.1 | 103 |
| Ether extract4 | 20.8 | 29.6 | 17.5 | 3.9 | 42.0 | 37.4 | 62.1 | 35.7 | 255 |
| Realized DM intake (kg·day–1 per cow) | |||||||||
| Loose housing (group observations)5 | |||||||||
| Control | 6.38 ± 0.13 | 3.30 ± 0.07 | 2.32 ± 0.05 | 0.94 ± 0.02 | 1.50 ± 0.03 | 1.55 ± 0.53 | 1.05 ± 1.72 | 2.16 ± 0.05 | - |
| Linseed | 6.40 ± 0.14 | 3.31 ± 0.07 | 2.32 ± 0.05 | 0.94 ± 0.02 | 1.50 ± 0.03 | 1.73 | 1.14 ± 1.39 | - | 2.19 ± 0.05 |
| Tied housing (individual observations)6 | |||||||||
| Control | 6.60 ± 0.80 | 3.41 ± 0.42 | 2.40 ± 0.29 | 0.97 ± 0.12 | 1.55 ± 0.19 | 1.73 | 1.14 ± 1.62 | 2.23 ± 0.27 | - |
| Linseed | 6.40 ± 0.82 | 3.31 ± 0.43 | 2.33 ± 0.30 | 0.94 ± 0.12 | 1.51 ± 0.19 | 1.73 | 1.15 ± 1.67 | - | 2.19 ± 0.28 |
| Housing | Loose (Group) | Tied (Individual) | ||||
|---|---|---|---|---|---|---|
| Item | Control | Linseed | p–Value | Control | Linseed | p–Value |
| Body weight (BW, kg)1 | 681 ± 93 | 682 ± 84 | 0.971 | 756 ± 71 | 761 ± 38 | 0.883 |
| Energy-corrected milk (ECM, kg·day−1)1 | 33.2 ± 7.9 | 36.3 ± 10.0 | 0.294 | 26.4 ± 5.7 | 30.5 ± 9.3 | 0.386 |
| Milk fat (kg·day−1)1 | 1.40 ± 0.34 | 1.46 ± 0.51 | 0.661 | 1.08 ± 0.21 | 1.16 ± 0.38 | 0.652 |
| Intake (kg·day−1)2 | ||||||
| Dry matter (DMI) | 19.3 ± 0.4 | 19.5 ± 0.4 | 0.417 | 20.1 ± 1.6 | 19.6 ± 2.0 | 0.665 |
| Organic matter | 17.9 ± 0.4 | 18.0 ± 0.4 | 0.754 | 18.7 ± 1.5 | 18.1 ± 1.9 | 0.564 |
| Neutral detergent fiber | 6.58 ± 0.14 | 7.14 ± 0.17 | 0.002 | 6.91 ± 0.29 | 7.24 ± 0.36 | 0.108 |
| Acid detergent fiber | 4.01 ± 0.09 | 4.40 ± 0.11 | 0.001 | 4.15 ± 0.16 | 4.39 ± 0.20 | 0.045 |
| Efficiency | ||||||
| ECM per BW (kg·100 kg−1)1 | 4.93 ± 0.12 | 5.37 ± 0.14 | 0.312 | 3.55 ± 0.97 | 4.02 ± 1.25 | 0.488 |
| ECM per BW (g·kg−0.75)1 | 251 ± 59 | 273 ± 71 | 0.292 | 185 ± 48 | 211 ± 65 | 0.458 |
| ECM1 per DMI2 (kg·kg−1) | 1.72 ± 0.41 | 1.86 ± 0.51 | 0.111 | 1.31 ± 0.23 | 1.53 ± 0.32 | 0.204 |
| Housing | Loose (Group) | Tied (Individual) | ||||
|---|---|---|---|---|---|---|
| Item | Control | Linseed | p–Value | Control | Linseed | p–Value |
| Eating1 | ||||||
| min | 406 ± 27 | 455 ± 15 | 0.019 | 422 ± 96 | 445 ± 36 | 0.354 |
| min·kg−1 DMI2 | 21.1 ± 1.64 | 23.3 ± 0.95 | 0.051 | 20.4 ± 4.9 | 22.1 ± 3.0 | 0.219 |
| Chews·kg−1 DMI | 1439 ± 142 | 1598 ± 58 | 0.072 | 1386 ± 386 | 1536 ± 273 | 0.182 |
| Rumination1 | ||||||
| min | 466 ± 38 | 497 ± 9 | 0.170 | 411 ± 56 | 445 ± 34 | 0.078 |
| min·kg−1 DMI | 24.2 ± 2.2 | 25.4 ± 0.9 | 0.275 | 19.8 ± 2.2 | 22.0 ± 1.7 | 0.008 |
| Chews·kg−1 DMI | 1546 ± 146 | 1664 ± 66 | 0.162 | 1197 ± 172 | 1386 ± 188 | 0.006 |
| Digestibility (%) | ||||||
| Organic matter (OM) | - | - | - | 76.1 ± 2.9 | 75.9 ± 3.1 | 0.935 |
| Neutral detergent fiber (NDF) | - | - | - | 61.5 ± 6.2 | 63.2 ± 4.1 | 0.580 |
| Acid detergent fiber | - | - | - | 63.2 ± 6.5 | 63.9 ± 4.2 | 0.829 |
| Carbon dioxide (kg·LU−1)3,4 | 9.18 ± 0.35 | 9.20 ± 0.55 | 0.952 | 9.07 ± 1.02 | 8.54 ± 0.60 | 0.295 |
| Methane yield4 | ||||||
| g·kg−1 DMI | 22.3 ± 1.6 | 21.4 ± 2.1 | 0.496 | 24.6 ± 3.1 | 22.9 ± 2.1 | 0.285 |
| g·kg−1 digestible OM intake | - | - | - | 34.7 ± 3.6 | 32.7 ± 3.6 | 0.361 |
| g·kg−1 digestible NDF intake | - | - | - | 116.3 ± 10.0 | 97.9 ± 8.4 | 0.006 |
| kJ·MJ−1 gross energy intake (Ym) | 69.4 ± 5.0 | 64.1 ± 6.2 | 0.232 | 76.4 ± 9.7 | 68.6 ± 6.4 | 0.131 |
| Methane emission intensity4 | ||||||
| g·kg−1 ECM5 | 13.6 ± 1.2 | 12.6 ± 1.2 | 0.266 | 19.2 ± 3.8 | 15.7 ± 4.4 | 0.165 |
| g·LU−1;4 | 321 ± 5.1 | 301 ± 6.7 | 0.292 | 327 ± 44 | 294 ± 30 | 0.155 |
| Housing | Loose (Group)1 | Tied (Individual)2 | ||||
|---|---|---|---|---|---|---|
| Item | Control | Linseed | p–Value | Control | Linseed | p–Value |
| Fat (g·kg−1 milk) | 42.9 ± 2.5 | 34.3 ± 2.6 | 0.003 | 45.6 ± 6.2 | 36.5 ± 3.1 | 0.009 |
| Protein (g·kg−1 milk) | 37.7 ± 0.4 | 33.7 ± 0.3 | <0.001 | 38.8 ± 3.5 | 33.1 ± 1.8 | 0.006 |
| Lactose (g·kg−1 milk) | 47.6 ± 0.4 | 49.1 ± 0.2 | <0.001 | 48.1 ± 0.9 | 48.7 ± 0.9 | 0.248 |
| MUN (mg·100−1 mL milk) | ||||||
| Enzymatic | 10.9 ± 0.5 | 13.5 ± 2.0 | 0.041 | 12.1 ± 2.0 | 15.6 ± 2.4 | 0.022 |
| FTIR3 | 10.8 ± 1.0 | 10.5 ± 1.7 | 0.777 | 12.0 ± 2.1 | 12.8 ± 2.0 | 0.521 |
| Fatty acids (FA; g 100 g−1 of total FA) | ||||||
| C18:0 | 7.24 ± 0.07 | 8.89 ± 0.19 | <0.001 | 8.99 ± 1.64 | 11.02 ± 1.19 | 0.034 |
| C18:1 trans-6, trans-8 | 0.25 ± 0.01 | 0.56 ± 0.05 | <0.001 | 0.34 ± 0.05 | 0.67 ± 0.11 | <0.001 |
| C18:1 trans-9 | 0.19 ± 0.01 | 0.44 ± 0.03 | <0.001 | 0.18 ± 0.02 | 0.44 ± 0.06 | <0.001 |
| C18:1 trans-10 | 0.34 ± 0.00 | 0.75 ± 0.02 | <0.001 | 0.42 ± 0.06 | 1.05 ± 0.55 | 0.018 |
| C18:1 trans-11 | 0.67 ± 0.01 | 4.39 ± 0.27 | <0.001 | 0.80 ± 0.29 | 4.37 ± 1.37 | <0.001 |
| C18:1 trans-12 | 0.045 ± 0.004 | 0.105 ± 0.004 | <0.001 | 0.043 ± 0.013 | 0.097 ± 0.044 | 0.016 |
| C18:1 trans-13, trans-14, cis-6, cis-8 | 0.31 ± 0.00 | 0.72 ± 0.04 | <0.001 | 0.36 ± 0.04 | 0.83 ± 0.06 | <0.001 |
| C18:1 cis-9 | 16.6 ± 0.6 | 19.4 ± 0.4 | <0.001 | 17.4 ± 2.1 | 24.0 ± 3.3 | 0.002 |
| C18:1 cis-10 | 0.105 ± 0.025 | 0.263 ± 0.146 | 0.076 | 0.078 ± 0.017 | 0.137 ± 0.037 | 0.005 |
| C18:1 cis-11 | 0.67 ± 0.03 | 1.07 ± 0.08 | <0.001 | 0.74 ± 0.13 | 1.28 ± 0.12 | <0.001 |
| C18:1 cis-12 | 0.208 ± 0.010 | 0.514 ± 0.028 | <0.001 | 0.259 ± 0.035 | 0.490 ± 0.072 | <0.001 |
| C18:1 cis-13 | 0.077 ± 0.002 | 0.185 ± 0.019 | <0.001 | 0.092 ± 0.025 | 0.212 ± 0.019 | <0.001 |
| C18:1 cis-14, trans-16 | 0.17 ± 0.01 | 0.33 ± 0.04 | <0.001 | 0.23 ± 0.03 | 0.54 ± 0.16 | <0.001 |
| C18:2 n-6 trans | 0.11 ± 0.01 | 0.54 ± 0.07 | <0.001 | 0.12 ± 0.03 | 0.51 ± 0.16 | <0.001 |
| C18:2 cis-9, trans-13, trans-8, cis-12 | 0.22 ± 0.02 | 0.81 ± 0.08 | <0.001 | 0.26 ± 0.04 | 1.21 ± 0.30 | <0.001 |
| C18:2 cis-9, trans-12 | 0.068 ± 0.013 | 0.178 ± 0.010 | <0.001 | 0.079 ± 0.010 | 0.257 ± 0.077 | <0.001 |
| C18:2 trans-11, cis-15, trans-9, cis-12 | 0.080 ± 0.013 | 1.282 ± 0.109 | <0.001 | 0.177 ± 0.075 | 1.482 ± 0.349 | <0.001 |
| C18:2 n-6 cis | 1.95 ± 0.05 | 1.93 ± 0.05 | 0.599 | 2.05 ± 0.23 | 1.92 ± 0.14 | 0.278 |
| C18:2 cis-9, cis-15 | 0.11 ± 0.01 | 0.18 ± 0.01 | <0.001 | 0.14 ± 0.02 | 0.20 ± 0.20 | <0.001 |
| C18:2 cis-9, trans-11 | 0.21 ± 0.02 | 0.32 ± 0.07 | 0.022 | 0.33 ± 0.07 | 0.36 ± 0.09 | 0.523 |
| C18:2 cis-9, cis-11 | 0.054 ± 0.003 | 0.241 ± 0.016 | <0.001 | 0.064 ± 0.014 | 0.250 ± 0.047 | <0.001 |
| C18:2 trans-9, trans-11 | 0.015 ± 0.001 | 0.148 ± 0.009 | <0.001 | 0.015 ± 0.007 | 0.158 ± 0.046 | <0.001 |
| C18:3 n-6 | 0.062 ± 0.004 | 0.087 ± 0.012 | 0.008 | 0.065 ± 0.017 | 0.048 ± 0.022 | 0.181 |
| C18:3 n-3 | 0.45 ± 0.01 | 1.10 ± 0.05 | <0.001 | 0.49 ± 0.09 | 1.23 ± 0.06 | <0.001 |
| Σ C18 FA | 30.2 ± 0.6 | 44.4 ± 0.8 | <0.001 | 33.7 ± 4.0 | 52.8 ± 4.9 | <0.001 |
| Σ SFA | 72.2 ± 0.7 | 60.4 ± 0.7 | <0.001 | 70.8 ± 3.3 | 54.5 ± 4.7 | <0.001 |
| Σ MUFA | 24.0 ± 0.6 | 32.4 ± 0.7 | <0.001 | 25.0 ± 2.7 | 37.6 ± 4.1 | <0.001 |
| Σ PUFA | 3.80 ± 0.11 | 7.24 ± 0.24 | <0.001 | 4.26 ± 0.56 | 7.97 ± 0.79 | <0.001 |
| Σ n-6 FA | 2.42 ± 0.06 | 2.79 ± 0.08 | <0.001 | 2.55 ± 0.30 | 2.68 ± 0.14 | 0.368 |
| Σ n-3 FA | 0.57 ± 0.01 | 1.24 ± 0.04 | <0.001 | 0.60 ± 0.11 | 1.35 ± 0.06 | <0.001 |
| n-6:n-3 FA | 4.28 ± 0.08 | 2.25 ± 0.13 | <0.001 | 4.29 ± 0.34 | 1.99 ± 0.07 | <0.001 |
| Trait Housing System | ECM1 (kg·day−1) | Methane2 (g·day−1) | Rumination1 (min·day−1) | Eating1 (min·day−1) |
|---|---|---|---|---|
| DMI (kg·day−1)2 | ||||
| Loose | 0.494 | –0.538 | –0.211 | –0.107 |
| Tied | 0.777** | 0.156 | 0.610* | –0.156 |
| ECM (kg·day−1)1 | ||||
| Loose | –0.387 | 0.143 | 0.397 | |
| Tied | 0.151 | 0.466 | 0.156 | |
| Methane (g·day−1)2 | ||||
| Loose | –0.322 | –0.184 | ||
| Tied | 0.198 | 0.254 | ||
| Rumination (min·day−1)1 | ||||
| Loose | 0.828* | |||
| Tied | –0.296 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poteko, J.; Schrade, S.; Zeyer, K.; Mohn, J.; Zaehner, M.; Zeitz, J.O.; Kreuzer, M.; Schwarm, A. Methane Emissions and Milk Fatty Acid Profiles in Dairy Cows Fed Linseed, Measured at the Group Level in a Naturally Ventilated Housing and Individually in Respiration Chambers. Animals 2020, 10, 1091. https://doi.org/10.3390/ani10061091
Poteko J, Schrade S, Zeyer K, Mohn J, Zaehner M, Zeitz JO, Kreuzer M, Schwarm A. Methane Emissions and Milk Fatty Acid Profiles in Dairy Cows Fed Linseed, Measured at the Group Level in a Naturally Ventilated Housing and Individually in Respiration Chambers. Animals. 2020; 10(6):1091. https://doi.org/10.3390/ani10061091
Chicago/Turabian StylePoteko, Jernej, Sabine Schrade, Kerstin Zeyer, Joachim Mohn, Michael Zaehner, Johanna O. Zeitz, Michael Kreuzer, and Angela Schwarm. 2020. "Methane Emissions and Milk Fatty Acid Profiles in Dairy Cows Fed Linseed, Measured at the Group Level in a Naturally Ventilated Housing and Individually in Respiration Chambers" Animals 10, no. 6: 1091. https://doi.org/10.3390/ani10061091
APA StylePoteko, J., Schrade, S., Zeyer, K., Mohn, J., Zaehner, M., Zeitz, J. O., Kreuzer, M., & Schwarm, A. (2020). Methane Emissions and Milk Fatty Acid Profiles in Dairy Cows Fed Linseed, Measured at the Group Level in a Naturally Ventilated Housing and Individually in Respiration Chambers. Animals, 10(6), 1091. https://doi.org/10.3390/ani10061091





