A Dietary Sugarcane-Derived Polyphenol Mix Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status, and Meat Quality in Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Experimental Design and Diets
2.3. Slaughter and Objective Meat Quality Measures
2.4. Biochemical and Hormone Analyses
2.5. Statistical Analyses
3. Results
3.1. Body Weight and Feed Consumption
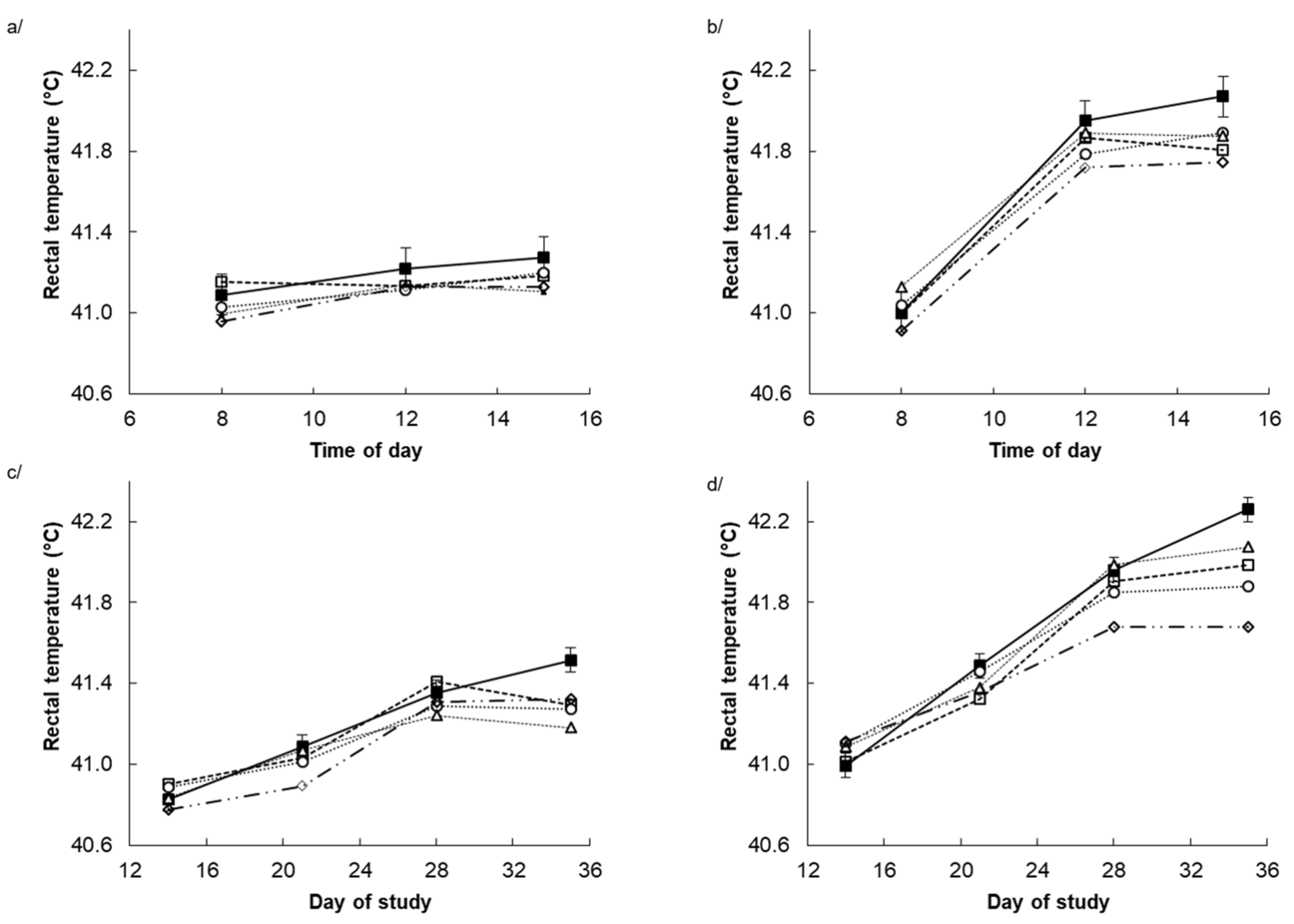
3.2. Rectal Temperature and Respiration Rate
3.3. Blood and Plasma Parameters and Muscle Betaine
3.4. Objective Meat Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Ringuet, M.; Furness, J.; Dunshea, F.R. Betaine and Antioxidants Improve Growth Performance, Breast Muscle Development and Ameliorate Thermoregulatory Responses to Cyclic Heat Exposure in Broiler Chickens. Animals 2018, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Al-Fataftah, A.A.; Abu-Dieyeh, Z. Effect of chronic heat stress on broiler performance in Jordan. Int. J. Poult 2007, 6, 64–70. [Google Scholar]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef]
- Davies, S.F.; Iber, C.; Keene, S.A.; McArthur, C.D.; Path, M.J. Effect of respiratory alkalosis during exercise on blood lactate. J. Appl. Physiol. 1986, 61, 948–952. [Google Scholar] [CrossRef]
- Akşit, M.; Yalcin, S.; Özkan, S.; Metin, K.; Özdemir, D. Effects of temperature during rearing and crating on stress parameters and meat quality of broilers. Poult. Sci. 2006, 85, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Love, J.D.; Pearson, A.M. Lipid oxidation in meat and meat products—A review. J. Am. Oil Chem. Soc. 1971, 48, 547–549. [Google Scholar] [CrossRef]
- Rajagopal, K.; Oommen, G.T. Myofibril fragmentation index as an immediate postmortem predictor of Buffalo meat tenderness. J. Food Process. Preserv. 2015, 39, 1166–1171. [Google Scholar] [CrossRef]
- Huang, C.; Jiao, H.; Song, Z.; Zhao, J.; Wang, X.; Lin, H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens. J. Anim. Sci. 2015, 93, 2144–2153. [Google Scholar] [CrossRef]
- Cottrell, J.; Liu, F.; Hung, A.; DiGiacomo, K.; Chauhan, S.; Leury, B.; Furness, J.; Celi, P.; Dunshea, F. Nutritional strategies to alleviate heat stress in pigs. Animal Prod. Sci. 2015, 55, 1391–1402. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Budzyńska, A.; Różalska, B.; Sadowska, B. Immunomodulacyjna rola polifenoli roślinnych The immunomodulatory role of plant polyphenols. Postepy Hig Med Dosw (Online) 2012, 66, 637–646. [Google Scholar] [CrossRef]
- Landete, J. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Czeczot, H. Flawonoidy w profilaktyce i terapii. Farmakol. Polska 2009, 65, 369–377. [Google Scholar]
- D Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348. [Google Scholar]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant Activity and Polyphenol Composition of Sugarcane Molasses Extract. Food Chem. 2020. [Google Scholar] [CrossRef]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. Dietary Betaine Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status and Meat Quality in Broiler Chickens. Agriculture 2020, 10, 176. [Google Scholar] [CrossRef]
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. Growth Performance and Characterization of Meat Quality of Broiler Chickens Supplemented with Betaine and Antioxidants under Cyclic Heat Stress. Antioxidants 2019, 8, 336. [Google Scholar] [CrossRef]
- Fagan, J.M.; Sleczka, B.G.; Sohar, I. Quantitation of oxidative damage to tissue proteins. Int. J. Biochem. Cell Biol. 1999, 31, 751–757. [Google Scholar] [CrossRef]
- He, S.; Zhao, S.; Dai, S.; Liu, D.; Bokhari, S.G. Effects of dietary betaine on growth performance, fat deposition and serum lipids in broilers subjected to chronic heat stress. Anim. Sci. J. 2015, 86, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Nofal, M.E.; Magda, A.G.; Mousa, S.M.M.; Doaa, M.M.Y.; Bealsh, A.M.A. Effect of dietary betaine supplementation on productive, physiological and immunological performance and carcass characteristic of growing developed chicks uinder the condition of heat stress. Egypt. Poult. Sci. 2015, 35, 237–259. [Google Scholar]
- Abu Hafsa, S.; Ibrahim, S. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J. Anim. Physiol. Anim. Nutr. (Berl.) 2018, 102, 268–275. [Google Scholar] [CrossRef]
- Starčević, K.; Krstulović, L.; Brozić, D.; Maurić, M.; Stojević, Z.; Mikulec, Ž.; Bajić, M.; Mašek, T. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J. Sci. Food Agric. 2015, 95, 1172–1178. [Google Scholar] [CrossRef]
- Hu, R.; He, Y.; Arowolo, M.A.; Wu, S.; He, J. Polyphenols as Potential Attenuators of Heat Stress in Poultry Production. Antioxidants 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; Washburn, K. The relationships of body temperature to weight gain, feed consumption, and feed utilization in broilers under heat stress. Poult. Sci. 1998, 77, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wen, J.; Zhang, H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult. Sci. 2007, 86, 1059–1064. [Google Scholar] [CrossRef]
- Yalcin, S.; Özkan, S.; Türkmut, L.; Siegel, P. Responses to heat stress in commercial and local broiler stocks. 1. Performance traits. Br. Poult. Sci. 2001, 42, 149–152. [Google Scholar] [CrossRef]
- Deeb, N.; Shlosberg, A.; Cahaner, A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 4. Association between responses to heat stress and to cold-induced ascites. Poult. Sci. 2002, 81, 1454–1462. [Google Scholar] [CrossRef]
- Dev, N.; Sankar, J.; Vinay, M. Functions of Thyroid Hormones. In Thyroid Disorders; Springer: Berlin/Heidelberg, Germany, 2016; pp. 11–25. [Google Scholar]
- Mansourian, A.R. A literature review on the adverse effects of hypothyroidism on kidney function. Pak. J. Biol. Sci. 2012, 15, 709–719. [Google Scholar] [CrossRef]
- Bowen, S.; Washburn, K. Thyroid and adrenal response to heat stress in chickens and quail differing in heat tolerance. Poult. Sci. 1985, 64, 149–154. [Google Scholar] [CrossRef]
- Chiang, W.; Booren, A.; Strasburg, G. The effect of heat stress on thyroid hormone response and meat quality in turkeys of two genetic lines. Meat Sci. 2008, 80, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Leeson, S.; Summers, J. Nutrition of the Chicken; Univ. Books: Guelph, CA, USA, 2001; pp. 366–377. [Google Scholar]
- NRC. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Ait-Boulahsen, A.; Garlich, J.D.; Edens, F.W. Potassium chloride improves the thermotolerance of chickens exposed to acute heat stress. Poult. Sci. 1995, 74, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Calpain activity, myofibrillar protein profile, and physicochemical properties of beef Semimembranosus and Biceps femoris from culled dairy cows during aging. J. Food Process. Preserv. 2018, 42, e13835. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Huang, Y.; Chen, L.; Bao, P.; Fang, H.; Zhou, C. L-arginine and L-lysine degrade troponin-T, and L-arginine dissociates actomyosin: Their roles in improving the tenderness of chicken breast. Food Chem. 2020. [Google Scholar] [CrossRef]
- Qiao, M.; Fletcher, D.; Smith, D.; Northcutt, J. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001, 80, 676–680. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- Sams, A. The effect of seasonal heat stress on rigor development and the incidence of pale, exudative turkey meat. Poult. Sci. 1997, 76, 1616–1620. [Google Scholar]
- Akter, Y.; Libinaki, R.; Hutchison, C.; Hopcroft, R.; Edwards, A.C.; Edwards, M.; O’Shea, C.J. Optimisation and investigations into the effect of a phosphorylated tocopherol mixture on growth performance, meat quality and plasma inflammatory biomarkers in broilers. Anim. Feed Sci. Technol. 2019, 253, 181–190. [Google Scholar] [CrossRef]
- Fouad, A.M.; Chen, W.; Ruan, D.; Wang, S.; Xia, W.G.; Zheng, C.T. Impact of heat stress on meat, egg quality, immunity and fertility in poultry and nutritional factors that overcome these effects: A review. Int. J. Poult. 2016, 15, 81. [Google Scholar]
- Chauhan, S.; Celi, P.; Leury, B.; Clarke, I.; Dunshea, F. Dietary antioxidants at supranutritional doses improve oxidative status and reduce the negative effects of heat stress in sheep. J. Anim. Sci. 2014, 92, 3364–3374. [Google Scholar] [CrossRef]
- Liu, F.; Cottrell, J.J.; Furness, J.B.; Rivera, L.R.; Kelly, F.W.; Wijesiriwardana, U.; Pustovit, R.V.; Fothergill, L.J.; Bravo, D.M.; Celi, P.; et al. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 2016, 101, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, X.; Peng, Z. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 2009, 88, 1078–1084. [Google Scholar] [CrossRef]
- Imik, H.; Atasever, M.A.; Urcar, S.; Ozlu, H.; Gumus, R.; Atasever, M. Meat quality of heat stress exposed broilers and effect of protein and vitamin E. Br. Poult. Sci. 2012, 53, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Gao, F.; Xu, X.; Zhang, W.; Song, S.; Zhou, G. Effects of dietary glutamine and gamma-aminobutyric acid on meat colour, pH, composition, and water-holding characteristic in broilers under cyclic heat stress. Br. Poult. Sci. 2012, 53, 471–481. [Google Scholar] [CrossRef] [PubMed]

| Radicals | ORAC (μmol TE/100 g) * |
|---|---|
| Peroxyl radicals | 33,300 |
| Hydroxyl radicals | 162,400 |
| Peroxynitrite | 7300 |
| Super oxide anion | 45,100 |
| Singlet oxygen | 27,700 |
| Minerals | Per 100 g |
| Sodium | 50–200 mg |
| Potassium | 2000–4000 mg |
| Selenium | <0.05 mg |
| Chromium | 0.20–0.50 mg |
| Calcium | 300–500 mg |
| Iron | 10–15 mg |
| Magnesium | 3000–5000 mg |
| Zinc | 0.5–1.5 mg |
| Nutritional Analysis | Per 100 g |
| Energy | 600 kJ |
| Protein | 6.9 g |
| Total fat | <0.1 g |
| Carbohydrate | 26.6 g |
| Dose of Polygain, g/kg | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 10 | SED 1 | Significance 2,3 | ||
| Daily body gain, g/d | ||||||||
| 0 to 21 days | TN | 33.3 | 30.6 | 33.9 | 29.8 | 37.0 | 2.49 | |
| HS | 31.8 | 33.8 | 31.4 | 31.4 | 32.8 | |||
| 21 to 35 days | TN | 84.0 | 88.4 | 88.8 | 94.0 | 96.4 | 5.04 | T *, L ** |
| HS | 80.1 | 82.8 | 86.3 | 89.0 | 87.6 | |||
| 0 to 35 days | TN | 53.9 | 53.7 | 55.9 | 55.5 | 60.4 | 2.46 | T +, L ** |
| HS | 51.1 | 53.5 | 54.7 | 54.2 | 55.4 | |||
| Daily feed intake, g/d | ||||||||
| 0 to 21 days | TN | 48.7 | 52.6 | 52.1 | 52.2 | 48.0 | 5.2 | |
| HS | 45.4 | 52.7 | 51.8 | 47.2 | 48.3 | |||
| 21 to 35 days | TN | 147 | 135 | 145 | 140 | 149 | 8.04 | T * |
| HS | 128 | 147 | 135 | 128 | 136 | |||
| 0 to 35 days | TN | 87.9 | 85.5 | 89.3 | 87.5 | 88.4 | 5.05 | T + |
| HS | 78.5 | 90.2 | 85.0 | 79.7 | 83.3 | |||
| Feed conversion ratio | ||||||||
| 0 to 21 days | TN | 1.46 | 1.72 | 1.54 | 1.77 | 1.30 | 0.186 | |
| HS | 1.45 | 1.56 | 1.68 | 1.51 | 1.50 | |||
| 21 to 35 days | TN | 1.76 | 1.53 | 1.64 | 1.50 | 1.55 | 0.098 | L * |
| HS | 1.60 | 1.78 | 1.57 | 1.44 | 1.55 | |||
| 0 to 35 days | TN | 1.65 | 1.59 | 1.60 | 1.58 | 1.46 | 0.101 | L * |
| HS | 1.54 | 1.69 | 1.61 | 1.46 | 1.53 | |||
| Slaughter weight, g | TN | 1925 | 1917 | 1994 | 1978 | 2154 | 86.4 | T +, L ** |
| HS | 1828 | 1912 | 1950 | 1936 | 1976 | |||
| Dose of Polygain, g/kg | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 10 | SED 1 | Significance 2,3 | ||
| Rectal temperature, °C | TN | 41.6 | 41.3 | 41.3 | 41.3 | 41.4 | 0.13 | T ***, L ***, Q *, T.L ** |
| HS | 42.7 | 42.3 | 42.2 | 42.4 | 42.0 | |||
| Respiration rate, bpm | TN | 66.0 | 64.0 | 62.0 | 63.0 | 64.0 | 8.20 | T ***, L *, T.L * |
| HS | 177 | 163 | 160 | 154 | 148 | |||
| pH | TN | 7.27 | 7.40 | 7.35 | 7.32 | 7.40 | 0.037 | T ***, L + |
| HS | 7.45 | 7.39 | 7.44 | 7.48 | 7.43 | |||
| pCO2, mm Hg | TN | 55.6 | 41.5 | 54.6 | 55.4 | 50.7 | 4.73 | T *** |
| HS | 40.1 | 45.3 | 42.5 | 35.0 | 42.4 | |||
| Total CO2, mM | TN | 27.4 | 26.9 | 31.3 | 30.1 | 31.8 | 1.56 | L +, T.L * |
| HS | 29.8 | 29.0 | 29.8 | 26.9 | 29.5 | |||
| pO2, mm Hg | TN | 30.6 | 45.6 | 36.7 | 42.0 | 29.3 | 4.11 | Q *** |
| HS | 32.8 | 36.9 | 36.4 | 40.2 | 31.9 | |||
| O2 saturation,% | TN | 48.6 | 81.2 | 65.6 | 70.7 | 54.1 | 7.59 | Q *** |
| HS | 64.8 | 68.9 | 71.1 | 78.7 | 62.8 | |||
| HCO3, mM | TN | 25.6 | 25.6 | 29.6 | 28.4 | 31.5 | 1.54 | L *, T.L ** |
| HS | 28.5 | 27.6 | 28.5 | 25.8 | 28.2 | |||
| Anion gap, mM | TN | 20.8 | 19.0 | 16.0 | 17.7 | 14.3 | 0.92 | T ***, L ***, T.L *** |
| HS | 15.0 | 15.3 | 14.7 | 16.7 | 16.0 | |||
| Base excess, mM | TN | -1.17 | 0.72 | 3.40 | 2.03 | 6.23 | 1.55 | L **, T.L ** |
| HS | 4.23 | 2.43 | 3.93 | 2.10 | 3.50 | |||
| Dose of Polygain, g/kg | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 10 | SED 1 | Significance 2,3 | ||
| Haematocrit,% | TN | 19.5 | 19.0 | 21.7 | 19.7 | 18.7 | 1.10 | T +, Q * |
| HS | 19.0 | 19.0 | 19.3 | 19.0 | 18.0 | |||
| Hgb 4, g/dL | TN | 6.65 | 6.30 | 7.37 | 6.60 | 6.30 | 0.383 | Q + |
| HS | 6.40 | 6.37 | 6.60 | 6.47 | 6.10 | |||
| Potassium, mM | TN | 6.78 | 6.49 | 6.43 | 6.33 | 5.97 | 0.46 | T ***, T.L * |
| HS | 5.20 | 5.33 | 5.50 | 5.60 | 5.87 | |||
| Sodium, mM | TN | 151 | 148 | 151 | 149 | 148 | 1.0 | T *** |
| HS | 148 | 147 | 148 | 148 | 148 | |||
| Chloride, mM | TN | 112 | 111 | 112 | 110 | 108 | 1.2 | L *, T.L + |
| HS | 110 | 109 | 110 | 111 | 109 | |||
| Calcium, mM | TN | 1.52 | 1.40 | 1.50 | 1.52 | 1.46 | 0.043 | Q +, T.Q * |
| HS | 1.48 | 1.44 | 1.47 | 1.34 | 1.51 | |||
| Lactate, mM | TN | 10.8 | 7.65 | 7.09 | 7.39 | 4.67 | 1.25 | T ***, L **, T.L ** |
| HS | 3.76 | 6.24 | 4.26 | 4.30 | 5.31 | |||
| Glucose, mM | TN | 14.2 | 14.0 | 14.7 | 14.3 | 15.0 | 0.59 | T + |
| HS | 15.1 | 15.1 | 15.1 | 14.3 | 15.2 | |||
| T3 4, pg/mL | TN | 4.11 | 6.68 | 4.31 | 4.88 | 6.16 | 0.754 | T.L + |
| HS | 5.61 | 6.07 | 4.76 | 4.27 | 5.44 | |||
| T4 4, pg/mL | TN | 6.95 | 6.56 | 6.21 | 7.63 | 5.85 | 1.261 | T *** |
| HS | 9.56 | 9.34 | 9.16 | 8.89 | 9.39 | |||
| Plasma betaine, µmol/L | TN | 133 | 149 | 142 | 136 | 133 | 7.76 | T ***, Q * |
| HS | 102 | 115 | 116 | 107 | 101 | |||
| Muscle betaine, µmol/g | TN | 246 | 152 | 144 | 117 | 115 | 18.3 | T **, L ***, Q **, T.L *** T.Q ** |
| HS | 131 | 148 | 122 | 118 | 113 | |||
| Dose of Polygain, g/kg | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 10 | SED 1 | Significance 2,3 | ||
| Cooking loss,% | TN | 22.8 | 21.6 | 23.0 | 20.9 | 22.4 | 1.28 | |
| HS | 21.5 | 22.2 | 21.2 | 22.5 | 21.4 | |||
| Drip loss,% | TN | 2.95 | 2.11 | 2.21 | 1.92 | 1.58 | 0.356 | L **, T.L * |
| HS | 2.13 | 1.71 | 2.10 | 2.49 | 1.76 | |||
| Water content,% | TN | 76.2 | 76.6 | 77.3 | 77.1 | 78.7 | 1.02 | T *, L ** |
| HS | 74.7 | 75.1 | 78.3 | 75.4 | 76.7 | |||
| Shear force, N | TN | 24.1 | 20.1 | 20.9 | 19.9 | 19.4 | 1.61 | T ***, L ** |
| HS | 24.8 | 26.3 | 23.3 | 23.1 | 23.1 | |||
| MFI 4 | TN | 82.0 | 69.8 | 106 | 97.8 | 113 | 19.13 | T **, L + |
| HS | 64.9 | 60.0 | 86.0 | 69.1 | 68.7 | |||
| Carbonyl | TN | 3.22 | 3.00 | 3.08 | 2.84 | 2.89 | 0.403 | Q + |
| nmol/mg protein | HS | 3.77 | 3.45 | 2.94 | 2.90 | 3.35 | ||
| Dose of Polygain, g/kg | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (TM) | 0 | 2 | 4 | 6 | 10 | SED 1 | Significance 2,3 | ||
| L* | 24 h | TN | 55.5 | 53.0 | 56.3 | 56.3 | 55.2 | 1.95 | TM ***, T * |
| HS | 55.7 | 55.3 | 60.3 | 56.2 | 56.3 | ||||
| 72 h | TN | 50.1 | 47.5 | 49.8 | 51.4 | 51.8 | |||
| HS | 50.6 | 50.9 | 53.1 | 50.8 | 51.7 | ||||
| a* | 24 h | TN | 1.99 | 1.99 | 1.82 | 2.06 | 2.38 | 0.420 | TM **, T *** |
| HS | 1.54 | 1.26 | 1.42 | 1.19 | 1.29 | ||||
| 72 h | TN | 1.55 | 2.21 | 1.49 | 1.26 | 1.79 | |||
| HS | 0.69 | 1.29 | 1.29 | 1.21 | 0.62 | ||||
| b* | 24 h | TN | 6.07 | 5.60 | 6.25 | 6.79 | 6.26 | 0.606 | TM ***, L *, Q *, T.L * |
| HS | 5.11 | 6.01 | 6.50 | 6.44 | 6.00 | ||||
| 72 h | TN | 4.46 | 3.97 | 4.43 | 5.21 | 5.53 | |||
| HS | 4.50 | 4.28 | 6.09 | 4.74 | 4.31 | ||||
| pH | 10 min | TN | 6.77 | 6.88 | 6.88 | 6.90 | 6.90 | 0.112 | T ***, TM ***, L ***, T.TM *** |
| HS | 6.83 | 6.78 | 6.86 | 6.84 | 6.99 | TM.L *, T.TM.L * | |||
| 1 h | TN | 6.44 | 6.72 | 6.64 | 6.75 | 6.60 | |||
| HS | 6.60 | 6.49 | 6.64 | 6.58 | 6.67 | ||||
| 24 h | TN | 5.72 | 5.91 | 5.98 | 6.15 | 6.33 | |||
| HS | 5.61 | 5.60 | 5.86 | 5.81 | 5.73 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Le, H.H.; Suleria, H.A.R.; Warner, R.D.; Dunshea, F.R. A Dietary Sugarcane-Derived Polyphenol Mix Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status, and Meat Quality in Broiler Chickens. Animals 2020, 10, 1158. https://doi.org/10.3390/ani10071158
Shakeri M, Cottrell JJ, Wilkinson S, Le HH, Suleria HAR, Warner RD, Dunshea FR. A Dietary Sugarcane-Derived Polyphenol Mix Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status, and Meat Quality in Broiler Chickens. Animals. 2020; 10(7):1158. https://doi.org/10.3390/ani10071158
Chicago/Turabian StyleShakeri, Majid, Jeremy J. Cottrell, Stuart Wilkinson, Hieu H. Le, Hafiz A. R. Suleria, Robyn D. Warner, and Frank R. Dunshea. 2020. "A Dietary Sugarcane-Derived Polyphenol Mix Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status, and Meat Quality in Broiler Chickens" Animals 10, no. 7: 1158. https://doi.org/10.3390/ani10071158
APA StyleShakeri, M., Cottrell, J. J., Wilkinson, S., Le, H. H., Suleria, H. A. R., Warner, R. D., & Dunshea, F. R. (2020). A Dietary Sugarcane-Derived Polyphenol Mix Reduces the Negative Effects of Cyclic Heat Exposure on Growth Performance, Blood Gas Status, and Meat Quality in Broiler Chickens. Animals, 10(7), 1158. https://doi.org/10.3390/ani10071158









