Meta-Transcriptomic Analysis of RNAseq Data Reveals Pacu and Loach Fish with Unusually High Levels of Myoglobin Expression in Skeletal Muscles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA Extraction
2.2. RNA Isolation, Library Construction, and Illumina Sequencing
2.3. De Novo Assembly of RNAseq
2.4. Identification of Oxygen-Binding Proteins in Muscle Transcriptome
2.5. PCA, Heatmap, and Clustering Analysis
3. Results and Discussion
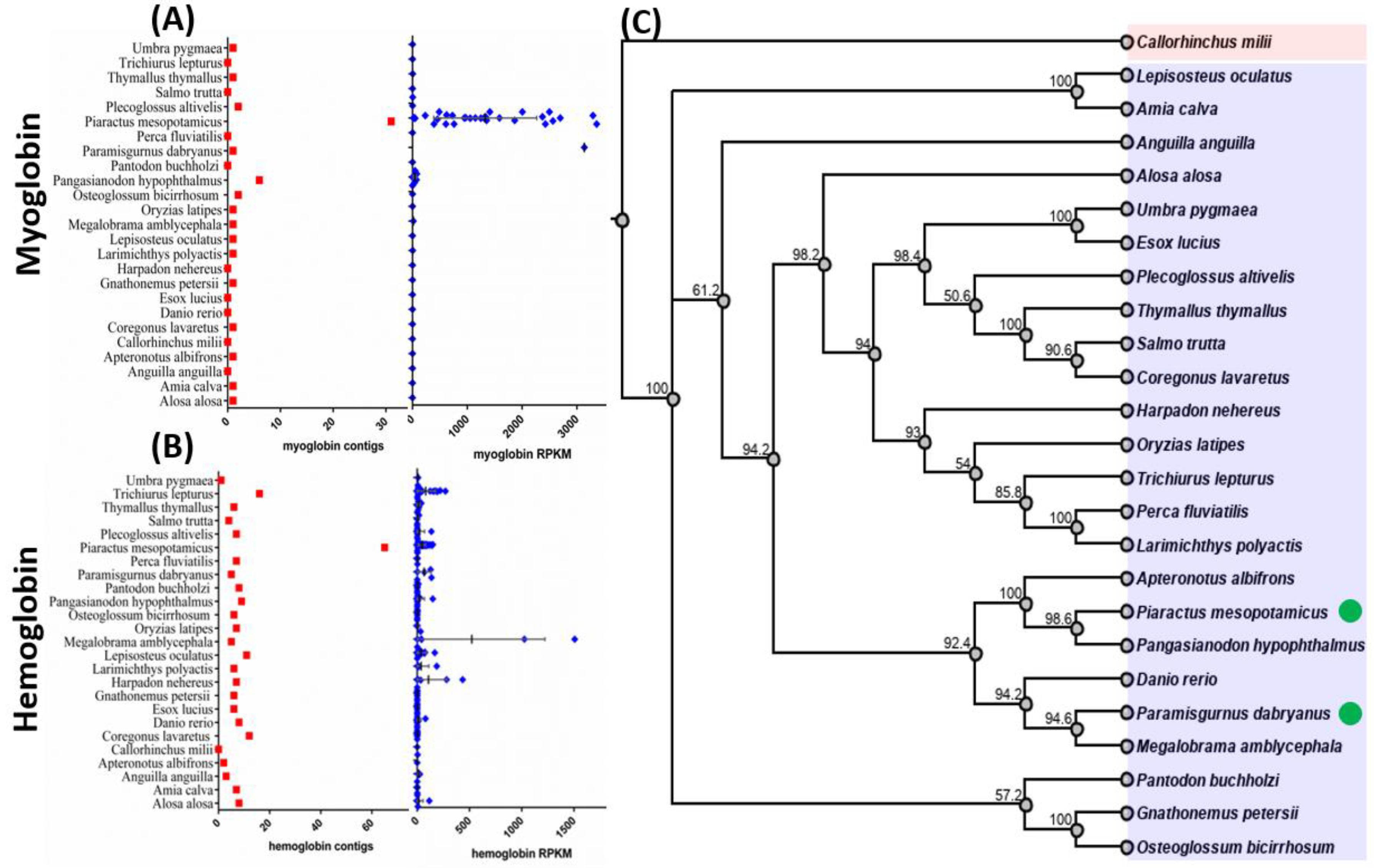
3.1. Meta-Transcriptomic Analysis of Oxygen-Binding Protein Expression
3.2. Comparison of the Expression Levels of Oxygen-Binding Protein Genes among Fish Species
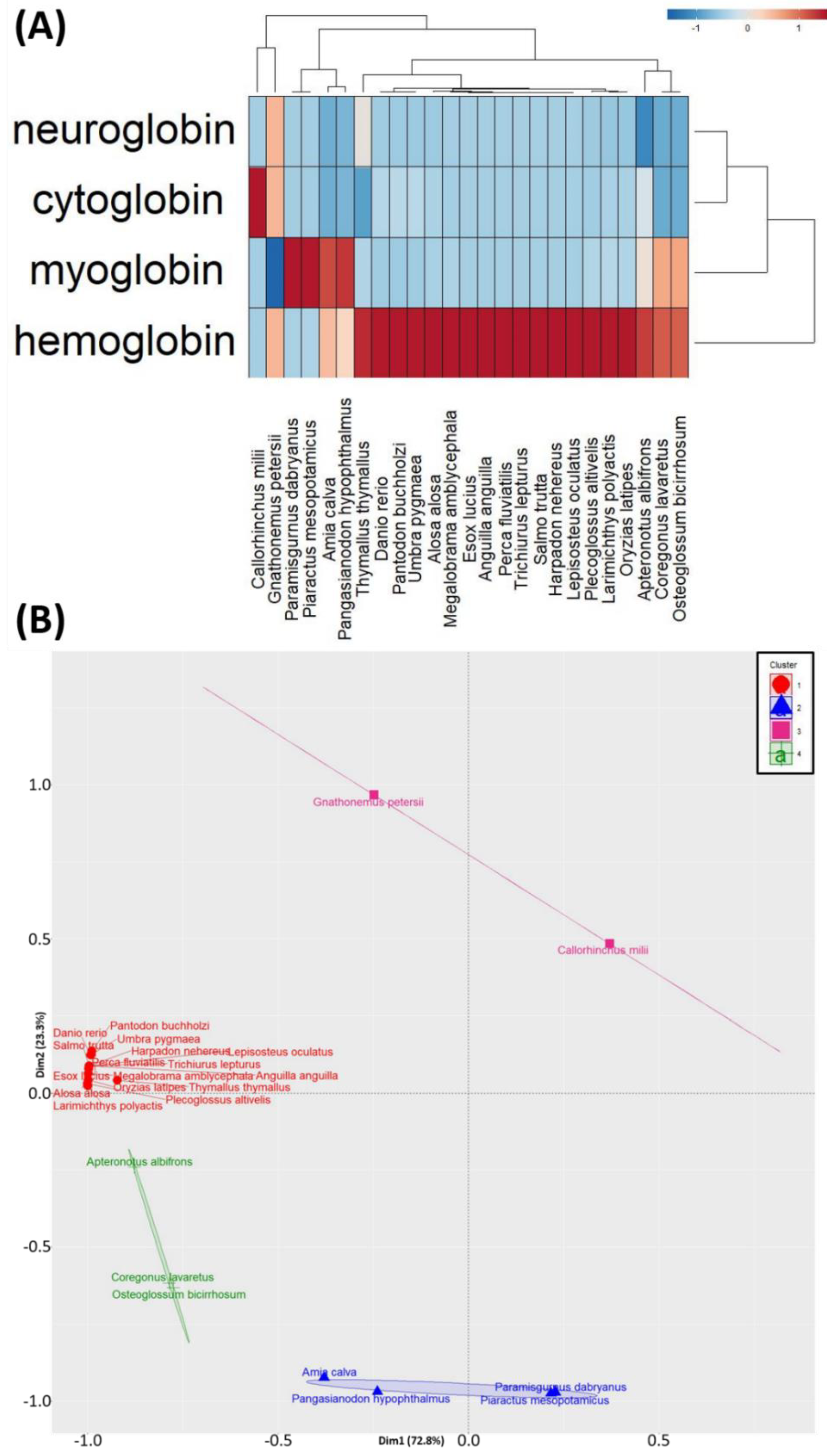
3.3. PCA and Hierarchical Clustering Analyses of the Oxygen-Binding Protein Genes Expression among Fish Species
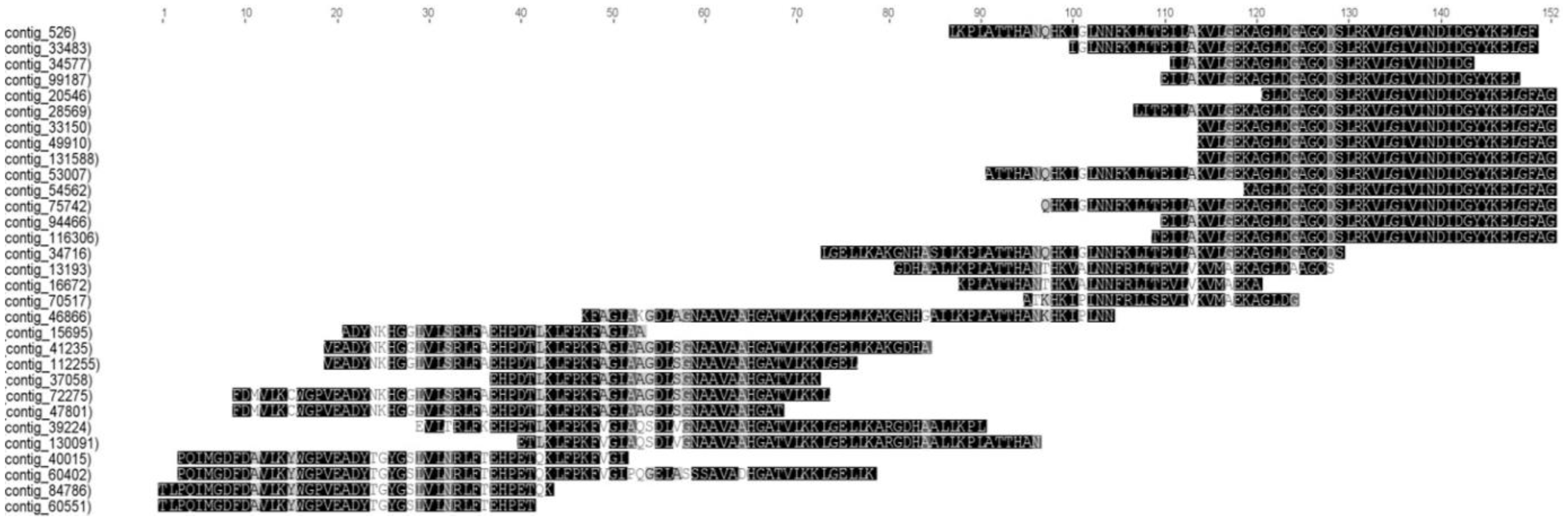
3.4. The Skeletal Muscle of Pacu Fish Has Multiple Myoglobin Transcripts with High Expression Level
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species Name | Hemoglobin | Myoglobin | Cytoglobin | Neuroglobin |
|---|---|---|---|---|
| Alosa alosa | 8 | 1 | 2 | 0 |
| Amia calva | 7 | 1 | 0 | 0 |
| Anguilla anguilla | 3 | 0 | 0 | 0 |
| Apteronotus albifrons | 2 | 1 | 1 | 0 |
| Callorhinchus milii | 0 | 0 | 1 | 0 |
| Coregonus lavaretus | 12 | 1 | 0 | 0 |
| Danio rerio | 8 | 0 | 2 | 0 |
| Esox lucius | 6 | 0 | 0 | 0 |
| Gnathonemus petersii | 6 | 1 | 1 | 1 |
| Harpadon nehereus | 7 | 0 | 0 | 0 |
| Larimichthys polyactis | 6 | 1 | 1 | 0 |
| Lepisosteus oculatus | 11 | 1 | 0 | 0 |
| Megalobrama amblycephala | 5 | 1 | 0 | 0 |
| Oryzias latipes | 7 | 1 | 2 | 0 |
| Osteoglossum bicirrhosum | 6 | 2 | 0 | 0 |
| Pangasianodon hypophthalmus | 9 | 6 | 0 | 0 |
| Pantodon buchholzi | 8 | 0 | 2 | 0 |
| Paramisgurnus dabryanus | 5 | 1 | 0 | 0 |
| Perca fluviatilis | 7 | 0 | 0 | 0 |
| Piaractus mesopotamicus | 65 | 31 | 0 | 0 |
| Plecoglossus altivelis | 7 | 2 | 1 | 0 |
| Salmo trutta | 4 | 0 | 0 | 0 |
| Thymallus thymallus | 6 | 1 | 2 | 1 |
| Trichiurus lepturus | 16 | 0 | 0 | 0 |
| Umbra pygmaea | 1 | 1 | 1 | 0 |
| Species Name | Hemoglobin | Myoglobin | Cytoglobin | Neuroglobin |
|---|---|---|---|---|
| Alosa alosa | 16.1 | 0.6 | 0.8 | 0 |
| Amia calva | 0.5 | 0.8 | 0 | 0 |
| Anguilla anguilla | 14.7 | 0 | 0 | 0 |
| Apteronotus albifrons | 2.2 | 1.1 | 0.9 | 0 |
| Callorhinchus milii | 0 | 0 | 2.4 | 0 |
| Coregonus lavaretus | 0.4 | 0.3 | 0 | 0 |
| Danio rerio | 10.4 | 0 | 0.7 | 0 |
| Esox lucius | 0.4 | 0 | 0 | 0 |
| Gnathonemus petersii | 0.3 | 0.2 | 0.3 | 0.3 |
| Harpadon nehereus | 108.5 | 0 | 0 | 0 |
| Larimichthys polyactis | 38.6 | 3.9 | 2.5 | 0 |
| Lepisosteus oculatus | 37.2 | 0 | 0 | 0 |
| Megalobrama amblycephala | 523.3 | 14.0 | 0 | 0 |
| Oryzias latipes | 5 | 0.4 | 0.3 | 0 |
| Osteoglossum bicirrhosum | 1.3 | 1 | 0 | 0 |
| Pangasianodon hypophthalmus | 20.8 | 43.5 | 0 | 0 |
| Pantodon buchholzi | 2.2 | 0 | 0.2 | 0 |
| Paramisgurnus dabryanus | 67.6 | 3136.4 | 0 | 0 |
| Perca fluviatilis | 1.4 | 0 | 0 | 0 |
| Piaractus mesopotamicus | 46 | 1327.8 | 0 | 0 |
| Plecoglossus altivelis | 20.8 | 1.4 | 0.3 | 0 |
| Salmo trutta | 4.5 | 0 | 0 | 0 |
| Thymallus thymallus | 13.6 | 3.2 | 0.3 | 5.6 |
| Trichiurus lepturus | 81.9 | 0 | 0 | 0 |
| Umbra pygmaea | 8.4 | 0.12 | 0.8 | 0 |
References
- Wilson, M.T.; Reeder, B.J. Oxygen-binding haem proteins. Exp. Physiol. 2008, 93, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, M.; Rees, B.B. Oxygen-dependent gene expression in fishes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1079–R1090. [Google Scholar] [CrossRef]
- Terwilliger, N.B. Functional adaptations of oxygen-transport proteins. J. Exp. Biol. 1998, 201, 1085–1098. [Google Scholar]
- Lekang, O.I. Aquaculture Engineering; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wittenberg, B.A.; Wittenberg, J.B. Myoglobin-mediated oxygen delivery to mitochondria of isolated cardiac myocytes. Proc. Natl. Acad. Sci. USA 1987, 84, 7503–7507. [Google Scholar] [CrossRef] [PubMed]
- Ordway, G.A.; Garry, D.J. Myoglobin: An essential hemoprotein in striated muscle. J. Exp. Biol. 2004, 207, 3441–3446. [Google Scholar] [CrossRef]
- Singh, S.; Canseco, D.C.; Manda, S.M.; Shelton, J.M.; Chirumamilla, R.R.; Goetsch, S.C.; Ye, Q.; Gerard, R.D.; Schneider, J.W.; Richardson, J.A. Cytoglobin modulates myogenic progenitor cell viability and muscle regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, E129–E138. [Google Scholar] [CrossRef] [PubMed]
- Burmester, T.; Ebner, B.; Weich, B.; Hankeln, T. Cytoglobin: A novel globin type ubiquitously expressed invertebrate tissues. Mol. Biol. Evol. 2002, 19, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Mao, X.O.; Xie, L.; Khan, A.A.; Greenberg, D.A. Neuroglobin protects against nitric oxide toxicity. Neurosci. Lett. 2008, 430, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Burmester, T.; Weich, B.; Reinhardt, S.; Hankeln, T. A vertebrate globin expressed in the brain. Nature 2000, 407, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.E.; Renshaw, G.M. Hypoxic survival strategies in two fishes: Extreme anoxia tolerance in the North European crucian carp and natural hypoxic preconditioning in a coral-reef shark. J. Exp. Biol. 2004, 207, 3131–3139. [Google Scholar] [CrossRef] [PubMed]
- Fagernes, C.E.; Stensløkken, K.-O.; Røhr, Å.K.; Berenbrink, M.; Ellefsen, S.; Nilsson, G.E. Extreme anoxia tolerance in crucian carp and goldfish through neofunctionalization of duplicated genes creating a new ethanol-producing pyruvate decarboxylase pathway. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Saint-Paul, U.; Bernardino, G. Behavioural and ecomorphological responses of the neotropical pacu Piaractus mesopotamicus (Teleostei, Serrasalmidae) to oxygen-deficient waters. Exp. Biol. 1988, 48, 19–26. [Google Scholar]
- Romagosa, E.; De Paiva, P.; Godinho, H.M. Pattern of oocyte diameter frequency distribution in females of the pacu, Piaractus mesopotamicus (Holmberg 1887)(= Colossoma mitrei Berg 1895), induced to spawn. Aquaculture 1990, 86, 105–110. [Google Scholar] [CrossRef]
- Souza, V.L.; Oliveira, E.; Urbinati, E. Effects of food restriction and refeeding on energy stores and growth of pacu, Piaractus mesopotamicus (Characidae). J. Aquac. Trop. 2000, 15, 371–379. [Google Scholar]
- Bechara, J.A.; Roux, J.P.; Ruiz Diaz, F.J.; Flores Quintana, C.I.; Longoni de Meabe, C.A. The effect of dietary protein level on pond water quality and feed utilization efficiency of pacuPiaractus mesopotamicus (Holmberg, 1887). Aquac. Res. 2005, 36, 546–553. [Google Scholar] [CrossRef]
- Takahashi, L.S.; de Abreu, J.S.; Biller, J.D.; Urbinati, E.C. Efeito do ambiente pós-transporte na recuperação dos indicadores de estresse de pacus juvenis, Piaractus mesopotamicus-doi:10.4025/actascianimsci. v28i4. 610. Acta Sci. Anim. Sci. 2008, 28, 469–475. [Google Scholar]
- Rantin, F.T.; Guerra, C.D.R.; Kalinin, A.L.; Glass, M.L. The influence of aquatic surface respiration (ASR) on cardio-respiratory function of the serrasalmid fish Piaractus mesopotamicus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 991–997. [Google Scholar] [CrossRef]
- Jasiński, A. Air-blood barrier in the respiratory intestine of the pond-loach, Misgurnus fossilis L. Cells Tissues Organs 1973, 86, 376–393. [Google Scholar] [CrossRef]
- Mendez-Sanchez, J.F.; Burggren, W.W. Hypoxia-induced developmental plasticity of larval growth, gill and labyrinth organ morphometrics in two anabantoid fish: The facultative air-breather Siamese fighting fish (Betta splendens) and the obligate air-breather the blue gourami (Trichopodus trichopterus). J. Morphol. 2019, 280, 193–204. [Google Scholar]
- Herbert, N.A.; Skjæraasen, J.E.; Nilsen, T.; Salvanes, A.G.; Steffensen, J.F. The hypoxia avoidance behaviour of juvenile Atlantic cod (Gadus morhua L.) depends on the provision and pressure level of an O2 refuge. Mar. Biol. 2011, 158, 737–746. [Google Scholar] [CrossRef]
- Salem, M.; Kenney, P.B.; Rexroad, C.E., 3rd; Yao, J. Microarray gene expression analysis in atrophying rainbow trout muscle: A unique nonmammalian muscle degradation model. Physiol. Genom. 2006, 28, 33–45. [Google Scholar] [CrossRef]
- Denslow, N.D.; Garcia-Reyero, N.; Barber, D.S. Fish ‘n’chips: The use of microarrays for aquatic toxicology. Mol. Biosyst. 2007, 3, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulou, E.; Kotoulas, G.; Power, D.M.; Geisler, R. Gene expression profiling of gilthead sea bream during early development and detection of stress-related genes by the application of cDNA microarray technology. Physiol. Genom. 2005, 23, 182–191. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Kassambara, A. Practical Guide To Principal Component Methods in R: PCA, M(CA), FAMD, MFA, HCPC, Factoextra; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2017. [Google Scholar]
- Zhang, H.; Audira, G.; Li, Y.; Xian, W.; Varikkodan, M.M.; Hsiao, C.-D. Comparative study the expression of calcium cycling genes in Bombay duck (Harpadon nehereus) and beltfish (Trichiurus lepturus) with different swimming activities. Genom. Data 2017, 12, 58–61. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, C.-M.; Shen, K.-N.; Xian, W.; Hsiao, C.-D. Identification of myogenic regulatory genes in the muscle transcriptome of beltfish (Trichiurus lepturus): A major commercial marine fish species with robust swimming ability. Genom. Data 2016, 8, 81–84. [Google Scholar] [CrossRef]
- Sidell, B.D.; O’Brien, K.M. When bad things happen to good fish: The loss of hemoglobin and myoglobin expression in Antarctic icefishes. J. Exp. Biol. 2006, 209, 1791–1802. [Google Scholar] [CrossRef]
- Grove, T.J.; Hendrickson, J.W.; Sidell, B.D. Two species of Antarctic icefishes (genus Champsocephalus) share a common genetic lesion leading to the loss of myoglobin expression. Polar Biol. 2004, 27, 579–585. [Google Scholar] [CrossRef]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef]
- Zhao, Z.-X.; Xu, P.; Cao, D.-C.; Kuang, Y.-Y.; Deng, H.-X.; Zhang, Y.; Xu, L.-M.; Li, J.-T.; Xu, J.; Sun, X.-W. Duplication and differentiation of common carp (Cyprinus carpio) myoglobin genes revealed by BAC analysis. Gene 2014, 548, 210–216. [Google Scholar] [CrossRef]
- Koch, J.; Lüdemann, J.; Spies, R.; Last, M.; Amemiya, C.T.; Burmester, T. Unusual diversity of myoglobin genes in the lungfish. Mol. Biol. 2016, 33, 3033–3041. [Google Scholar] [CrossRef] [PubMed]
- Bicker, A.; Dietrich, D.; Gleixner, E.; Kristiansen, G.; Gorr, T.A.; Hankeln, T. Extensive transcriptional complexity during hypoxia-regulated expression of the myoglobin gene in cancer. Hum. Mol. Genet. 2014, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]

| SRR ID | Species Name | Data Size (Gb) | Sequencing Platform | Sequencing Method | Assembled Contigs | N50 |
|---|---|---|---|---|---|---|
| SRR1613325 | Megalobrama amblycephala | 2.3 | Illumina HiSeq 2000 | 2X101 bp | 35052 | 557 |
| SRR1652342 | Paramisgurnus dabryanus | 3.6 | Illumina HiSeq 2000 | 2X102 bp | 49125 | 972 |
| SRR1533666 | Thymallus thymallus | 3 | Illumina HiSeq 2000 | 2X101 bp | 102377 | 489 |
| SRR1533644 | Pangasianodon hypophthalmus | 3.6 | Illumina HiSeq 2000 | 2X101 bp | 86400 | 454 |
| SRR1056356 | Piaractus mesopotamicus | 8.1 | Illumina HiSeq 2000 | 2X101 bp | 131747 | 483 |
| SRR514104 | Callorhinchus milii | 6.4 | Illumina Genome Analyzer II | 2X77 bp | 44417 | 338 |
| SRR1524253 | Lepisosteus oculatus | 3.7 | Illumina HiSeq 2000 | 2X101 bp | 82408 | 517 |
| SRR1524264 | Amia calva | 4.1 | Illumina HiSeq 2000 | 2X101 bp | 94702 | 554 |
| SRR1524274 | Oryzias latipes | 4.6 | Illumina HiSeq 2000 | 2X101 bp | 100420 | 560 |
| SRR1532750 | Apteronotus albifrons | 3.6 | Illumina HiSeq 2000 | 2X101 bp | 124794 | 551 |
| SRR1532760 | Anguilla anguilla | 2.7 | Illumina HiSeq 2000 | 2X101 bp | 86397 | 429 |
| SRR1532781 | Salmo trutta | 5.5 | Illumina HiSeq 2000 | 2X101 bp | 121092 | 486 |
| SRR1532792 | Osteoglossum bicirrhosum | 3.2 | Illumina HiSeq 2000 | 2X101 bp | 107973 | 469 |
| SRR1532803 | Alosa alosa | 4.8 | Illumina HiSeq 2000 | 2X101 bp | 115049 | 502 |
| SRR1532771 | Pantodon buchholzi | 2.6 | Illumina HiSeq 2000 | 2X101 bp | 93563 | 567 |
| SRR1533633 | Umbra pygmaea | 3.2 | Illumina HiSeq 2000 | 2X101 bp | 107513 | 583 |
| SRR1533655 | Esox lucius | 4 | Illumina HiSeq 2000 | 2X101 bp | 73132 | 550 |
| SRR1533677 | Coregonus lavaretus | 3.9 | Illumina HiSeq 2000 | 2X101 bp | 93899 | 481 |
| SRR1533689 | Perca fluviatilis | 5.1 | Illumina HiSeq 2000 | 2X101 bp | 75135 | 563 |
| SRR1533701 | Gnathonemus petersii | 4.3 | Illumina HiSeq 2000 | 2X101 bp | 136066 | 569 |
| SRR1533711 | Plecoglossus altivelis | 3.1 | Illumina HiSeq 2000 | 2X101 bp | 115114 | 537 |
| SRR3029231 | Larimichthys polyactis | 1.9 | Illumina HiSeq 2000 | 2X101 bp | 60237 | 715 |
| SRR1524241 | Danio rerio | 4.3 | Illumina HiSeq 2000 | 2X101 bp | 118459 | 509 |
| SRR332188 1 | Trichiurus lepturus | 5.7 | Illumina NextSeq 500 | 2X151 bp | 36214 | 860 |
| SRR338795 1 | Harpadon nehereus | 2.3 | Illumina NextSeq 500 | 2X151 bp | 21599 | 615 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.-Y.; Ngoc Hieu, B.T.; Audira, G.; Lou, B.; Lin, M.-D.; Hsiao, C.-D. Meta-Transcriptomic Analysis of RNAseq Data Reveals Pacu and Loach Fish with Unusually High Levels of Myoglobin Expression in Skeletal Muscles. Animals 2020, 10, 1130. https://doi.org/10.3390/ani10071130
Chen R-Y, Ngoc Hieu BT, Audira G, Lou B, Lin M-D, Hsiao C-D. Meta-Transcriptomic Analysis of RNAseq Data Reveals Pacu and Loach Fish with Unusually High Levels of Myoglobin Expression in Skeletal Muscles. Animals. 2020; 10(7):1130. https://doi.org/10.3390/ani10071130
Chicago/Turabian StyleChen, Rui-Yi, Bui Thi Ngoc Hieu, Gilbert Audira, Bao Lou, Ming-Der Lin, and Chung-Der Hsiao. 2020. "Meta-Transcriptomic Analysis of RNAseq Data Reveals Pacu and Loach Fish with Unusually High Levels of Myoglobin Expression in Skeletal Muscles" Animals 10, no. 7: 1130. https://doi.org/10.3390/ani10071130
APA StyleChen, R.-Y., Ngoc Hieu, B. T., Audira, G., Lou, B., Lin, M.-D., & Hsiao, C.-D. (2020). Meta-Transcriptomic Analysis of RNAseq Data Reveals Pacu and Loach Fish with Unusually High Levels of Myoglobin Expression in Skeletal Muscles. Animals, 10(7), 1130. https://doi.org/10.3390/ani10071130






