Seroprevalence of Canine Herpesvirus-1 in Breeding Dogs with or Without Vaccination in Northwest Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs and Samples
2.2. Ethics Approval and Consent to Participate
2.3. Serum Neutralization Test
2.4. Statistical Analysis
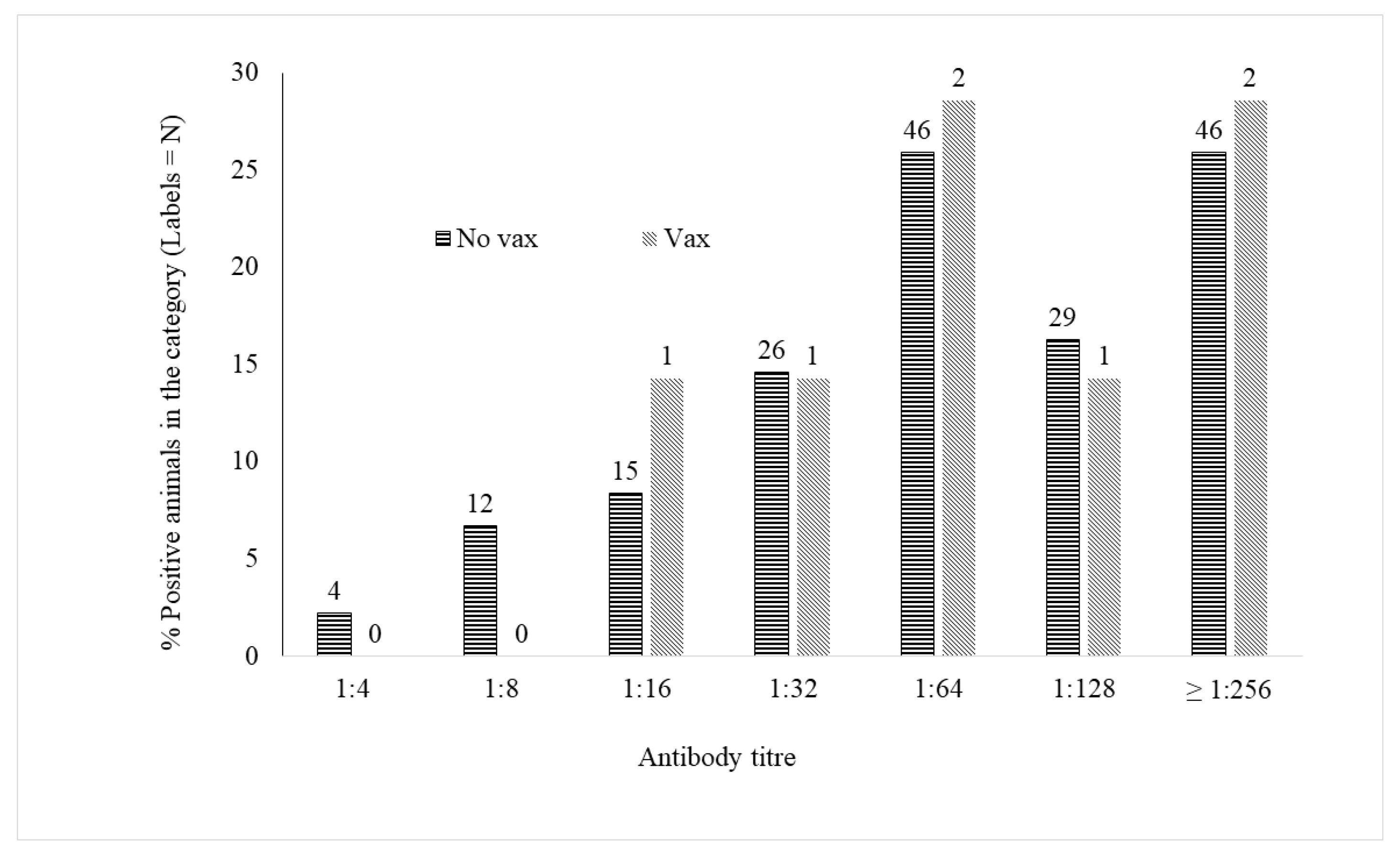
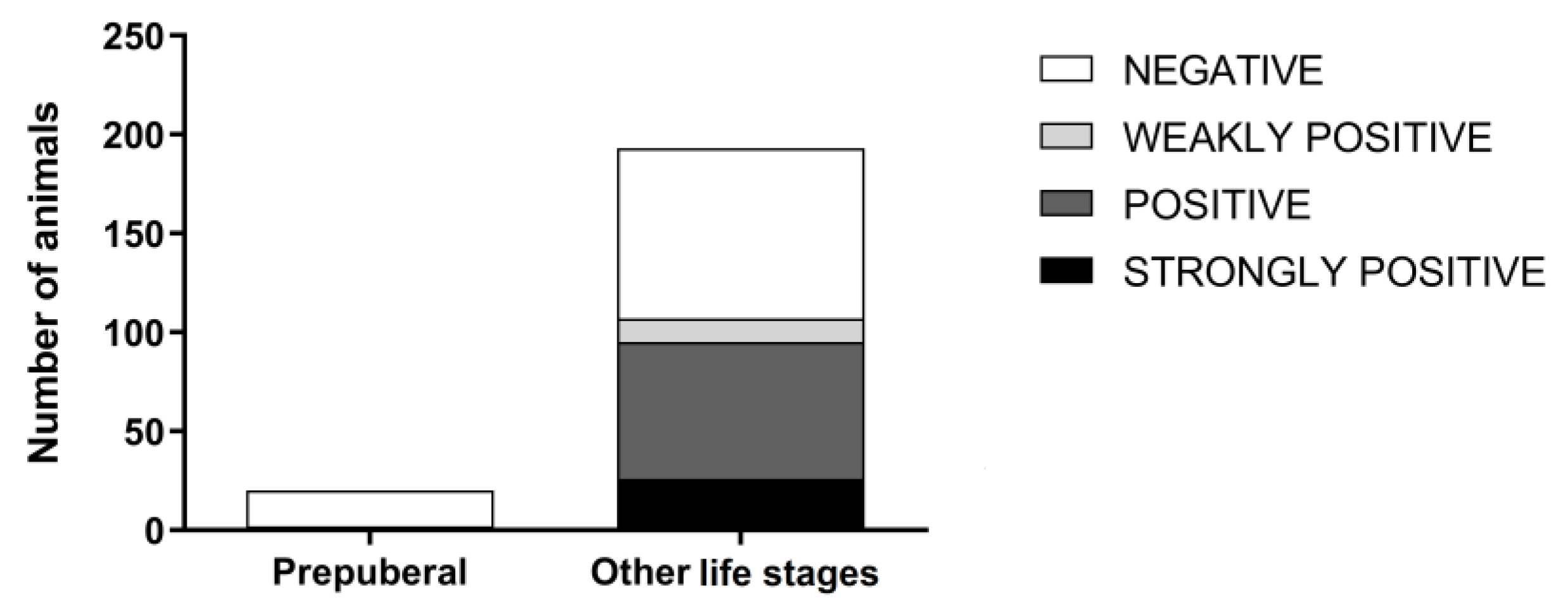
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carmichael, L.E.; Squire, R.A.; Krook, L. Clinical and pathologic features of a fatal viral disease of newborn puppies. Am. J. Vet. Res. 1965, 26, 803–814. [Google Scholar]
- Decaro, N.; Martella, V.; Buonavoglia, C. Canine adenoviruses and herpesvirus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 799–814. [Google Scholar] [CrossRef]
- Ronsse, V.; Verstegen, J.; Onclin, K.; Farnir, F.; Poulet, H. Risk factors and reproductive disorders associated with canine herpesvirus-1 (CHV-1). Theriogenology 2004, 61, 619–636. [Google Scholar] [CrossRef]
- Ronsse, V.; Verstegen, J.; Thiry, E.; Onclin, K.; Aeberlé, C.; Brunet, S.; Poulet, H. Canine herpesvirus-1 (CHV-1): Clinical, serological and virological patterns in breeding colonies. Theriogenology 2005, 64, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Dahlbom, M.; Johnsson, M.; Myllys, V.; Taponen, J.; Andersson, M. Seroprevalence of canine herpesvirus-1 and Brucella canis in Finnish breeding kennels with and without reproductive problems. Reprod. Domest. Anim. 2009, 44, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, L.E.; Greene, C.E. Canine Herpesvirus Infection. InInfectious Diseases of the Dog and Cat, 2nd ed.; Greene, C.E., Ed.; WB Saunders: Philadelphia, PA, USA, 1998; Volume 310, pp. 28–32. [Google Scholar]
- Miyoshi, M.; Ishii, Y.; Takiguchi, M.; Takada, A.; Yasuda, J.; Hashimoto, A.; Okazaki, K.; Kida, H. Detection of canine herpesvirus DNA in the ganglionic neurons and the lymphnode lymphocytes of latently infected dogs. J. Vet. Med. Sci. 1999, 61, 375–379. [Google Scholar] [CrossRef]
- Poulet, H.; Guigal, P.M.; Soulier, M.; Leroy, V.; Fayet, G.; Minke, J.; Merial, G.C. Protection of puppies against canine herpesvirus by vaccination of the dams. Vet. Rec. 2001, 148, 691–695. [Google Scholar] [CrossRef]
- Reagan, K.L.; Sykes, J.E. Canine Infectious Respiratory Disease. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 405–418. [Google Scholar] [CrossRef]
- Evermann, J.F.; Ledbetter, E.C.; Maes, R.K. Canine reproductive, respiratory and ocular diseases due to canine herpesvirus. Vet. Clin. Small Anim. 2011, 41, 1097–1120. [Google Scholar] [CrossRef]
- Ström Holst, B.; Hagberg Gustavsson, M.; Grapperon-Mathis, M.; Lilliehöök, I.; Johannisson, A.; Isaksson, M.; Lindhe, A.; Axnér, E. Canine Herpesvirus during pregnancy and non-pregnant luteal phase. Reprod. Domest. Anim. 2012, 47, 362–365. [Google Scholar] [CrossRef]
- Krogenæs, A.; Rootwelt, V.; Larsen, S.; Renström, L.; Farstad, W.; Lund, A. A serological study of canine herpesvirus-1 infection in a population of breeding bitches in Norway. Acta Vet. Scand 2014, 56, 19. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Dubovi, E.J.; Kim, S.G.; Maggs, D.J.; Bicalho, R.C. Experimental primary ocular canine herpesvirus-1 infection in adult dogs. Am. J. Vet. Res. 2009, 70, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kim, S.G.; Dubovi, E.J.; Bicalho, R.C. Experimental reactivation of latent canine herpesvirus-1 and induction of recurrent ocular disease in adult dogs. Vet. Microbiol. 2009, 138, 98–105. [Google Scholar] [CrossRef] [PubMed]
- König, M.; Neiseke, J.; Thiel, H.J. Prevalence of canine herpesvirus 1 (CHV-1) in German kennels. Tierärztl. Umsch. 2004, 59, 559–565. [Google Scholar]
- Nöthling, J.O.; Hüssy, D.; Steckler, D.; Ackermann, M. Seroprevalence of canine herpesvirus in breeding kennels in the Gauteng Province of South Africa. Theriogenology 2008, 69, 273–282. [Google Scholar] [CrossRef]
- Yeşilbağ, K.; Yalcin, E.; Tuncer, P.; Yilmaz, Z. Seroprevalence of Canine herpesvirus-1 in Turkish dog population. Res. Vet. Sci. 2012, 92, 36–39. [Google Scholar] [CrossRef]
- Krogenæs, A.; Rootwelt, V.; Larsen, S.; SjØberg, E.K.; Akselsen, B.; Skår, T.M.; Myhre, S.S.; Renström, L.H.M.; Klingeborn, B.; Lund, A. A serological study of canine herpes virus-1 infection in the adult dog population. Theriogenology 2012, 78, 153–158. [Google Scholar] [CrossRef]
- Engels, M.; Mayr-Bibrack, B.; Ruckstuhl, B.; Metzler, A.; Wyler, R. Seroepizootiology of canine herpes virus infection in Switzerland and preliminary studies with a vaccine. ZentralblVeterinarmed 1980, 27, 257–267. [Google Scholar]
- Van Gucht, S.; Nauwynck, H.; Pensaert, M. Prevalence of canine herpesvirus in kennels and the possible association with fertility problems and neonatal death. VlaamsDiergen Tijds 2001, 70, 204–211. [Google Scholar]
- Ronsse, V.; Verstegen, J.; Onclin, K.; Guiot, A.L.; Aeberle, C.; Nauwynck, H.J.; Poulet, H. Seroprevalence of Canine Herpesvirus-1 in the Belgian Dog Population in 2000. Reprod. Domestic. Anim. 2002, 37, 299–304. [Google Scholar] [CrossRef]
- Pratelli, A.; Colao, V.; Losurdo, M. Serological and virological detection of canine herpesvirus-1 in adult dogs with and without reproductive disorders. Vet. J. 2014, 200, 257–260. [Google Scholar] [CrossRef] [PubMed]


| Negative | Positive | Cytotoxic | Total | ||
| Female dog | no vax | 109 (48.02%) | 118 (51.98%) | 0 | 227 |
| vax | 21 (70%) | 7 (23.3%) | 2 (6.7%) | 30 | |
| Male dog | no vax | 52 (46.4%) | 60 (53.6%) | 0 | 112 |
| vax | 1 | 0 | 0 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rota, A.; Dogliero, A.; Biosa, T.; Messina, M.; Pregel, P.; Masoero, L. Seroprevalence of Canine Herpesvirus-1 in Breeding Dogs with or Without Vaccination in Northwest Italy. Animals 2020, 10, 1116. https://doi.org/10.3390/ani10071116
Rota A, Dogliero A, Biosa T, Messina M, Pregel P, Masoero L. Seroprevalence of Canine Herpesvirus-1 in Breeding Dogs with or Without Vaccination in Northwest Italy. Animals. 2020; 10(7):1116. https://doi.org/10.3390/ani10071116
Chicago/Turabian StyleRota, Ada, Andrea Dogliero, Teresa Biosa, Margherita Messina, Paola Pregel, and Loretta Masoero. 2020. "Seroprevalence of Canine Herpesvirus-1 in Breeding Dogs with or Without Vaccination in Northwest Italy" Animals 10, no. 7: 1116. https://doi.org/10.3390/ani10071116
APA StyleRota, A., Dogliero, A., Biosa, T., Messina, M., Pregel, P., & Masoero, L. (2020). Seroprevalence of Canine Herpesvirus-1 in Breeding Dogs with or Without Vaccination in Northwest Italy. Animals, 10(7), 1116. https://doi.org/10.3390/ani10071116







