Consequences of Transition Treatments on Fertility and Associated Metabolic Status for Dairy Cows in Early Lactation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
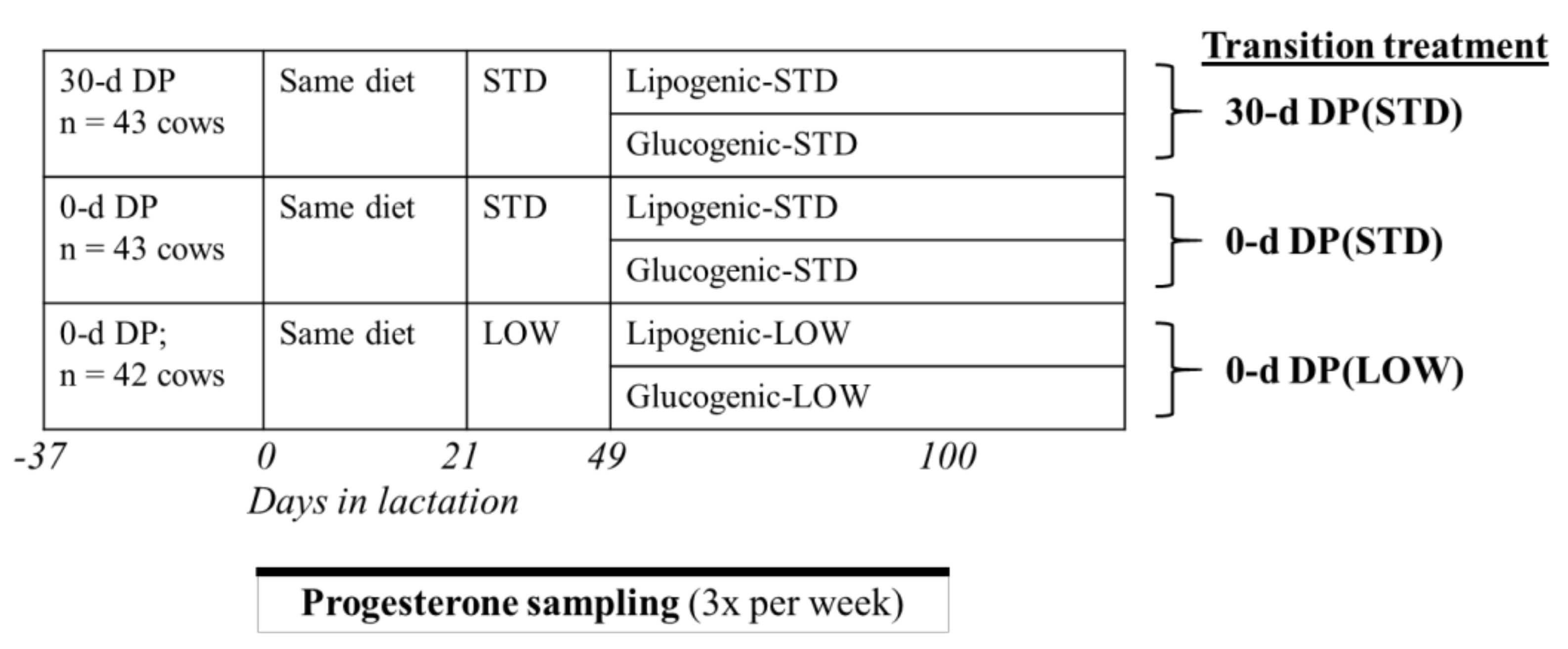
2.2. Experimental Design
2.3. Measurements
2.3.1. Feed Intake and Energy Balance
2.3.2. Milk Sampling and Progesterone Assay
2.3.3. Blood Sampling and Analysis
2.3.4. Definitions of Ovarian Cyclicity
- Normal ovarian cycle: cycles with 18–24 days in length.
- Prolonged ovarian cycle: cycles with more than 24 days in length.
- Short ovarian cycle: cycles with less than 18 days in length.
2.4. Reproduction Protocol
2.5. Statistical Analysis
3. Results
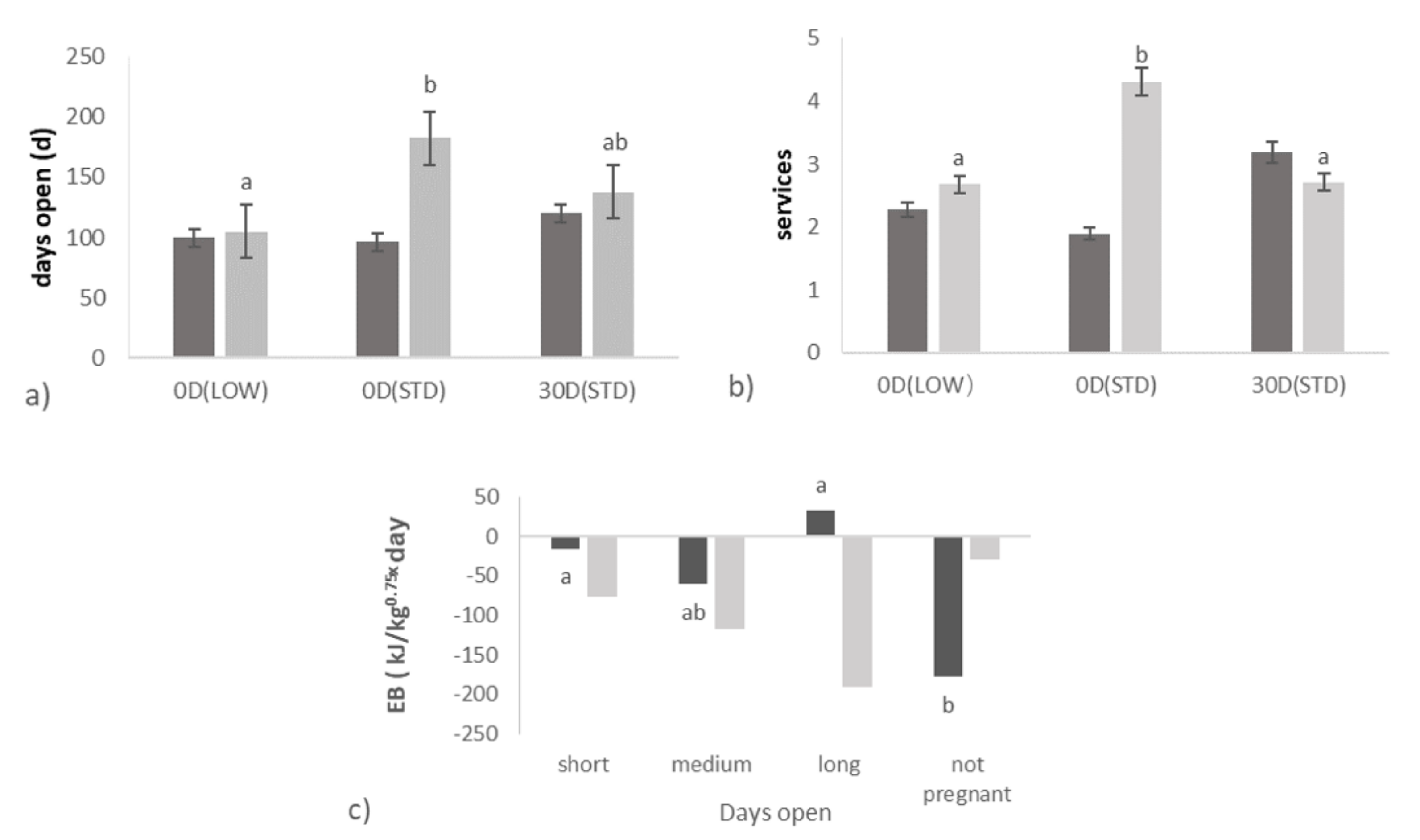
3.1. Effect of Transition Treatments on Days Open and Ovarian Activity
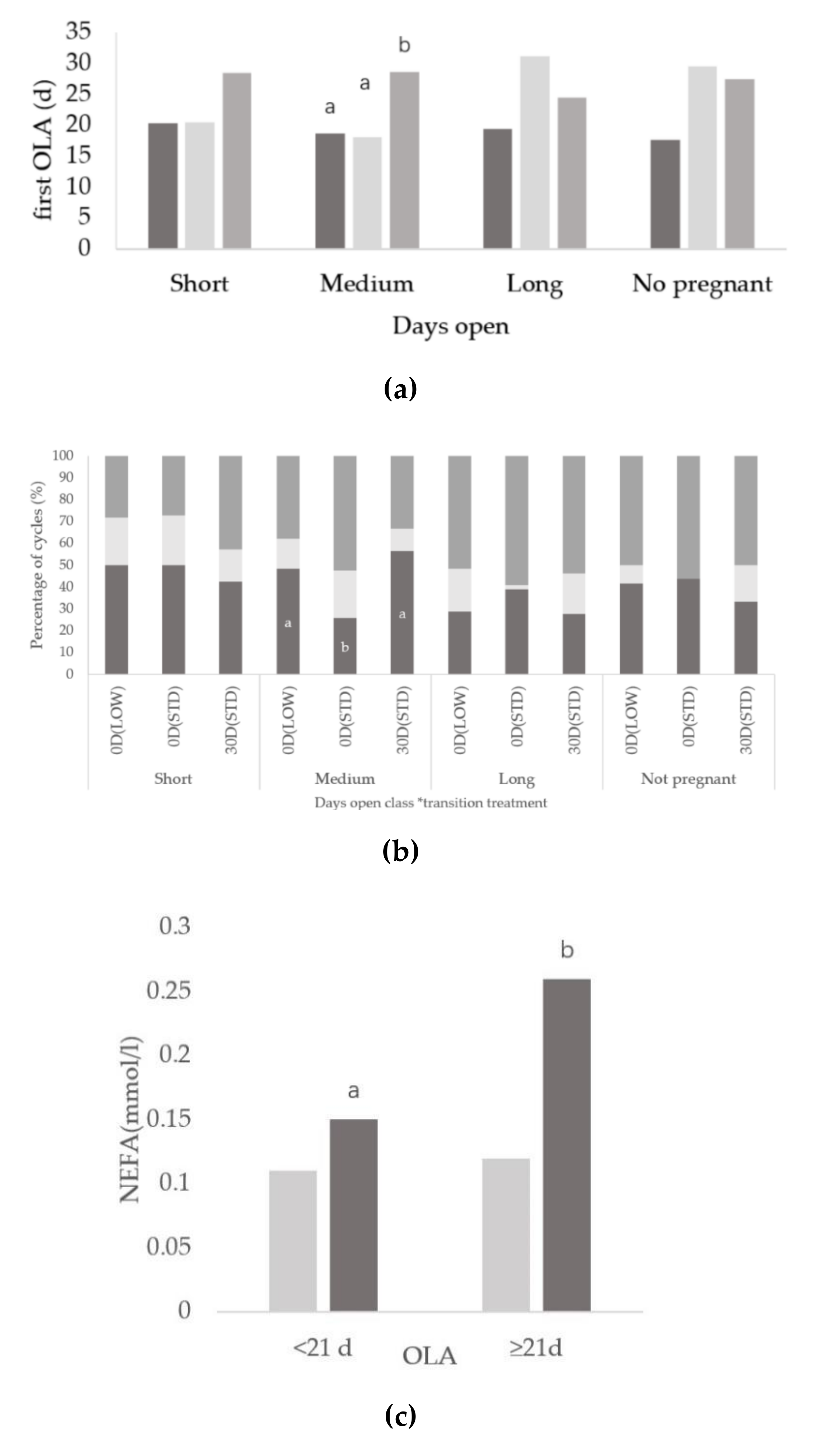
3.2. Relationships between Days Open and Ovarian Activity
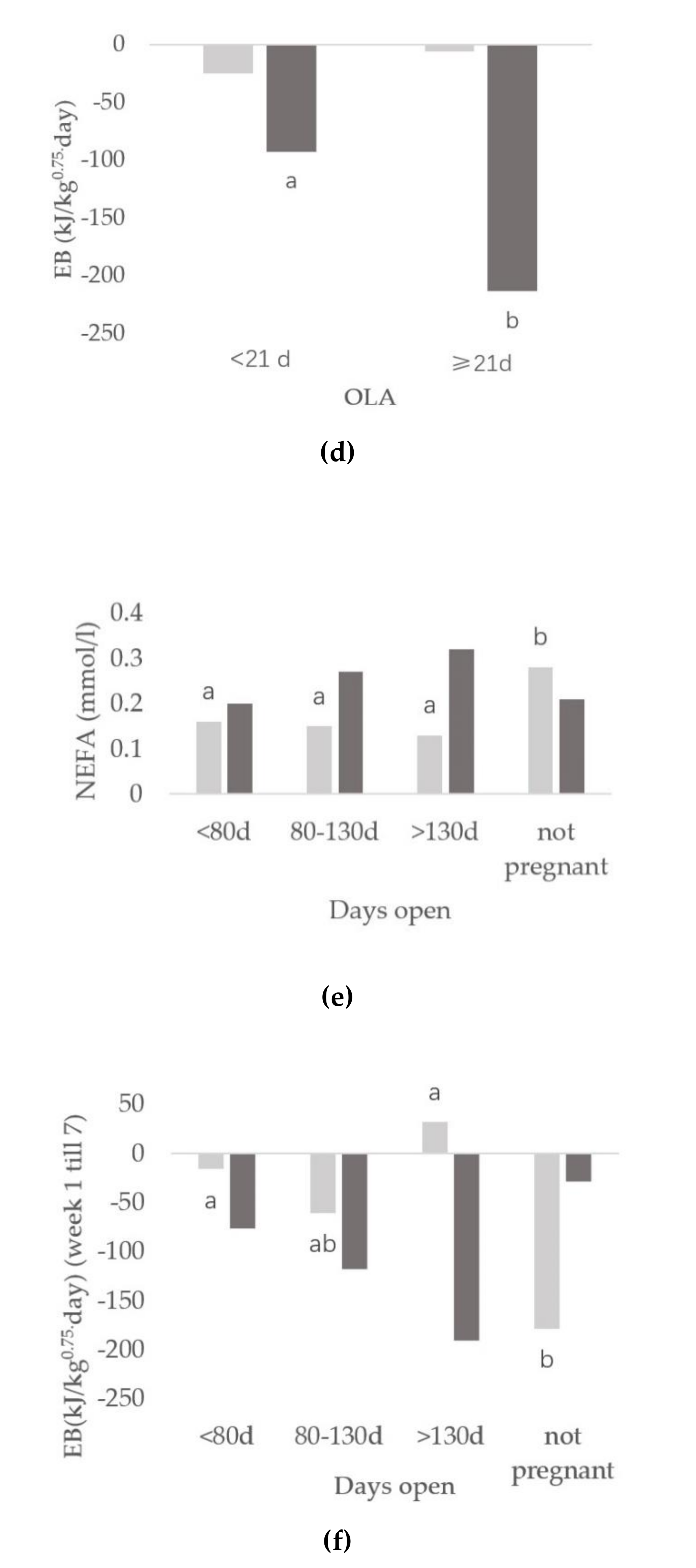
3.3. Relationships between Onset of Luteal Activity and Metabolic Status
3.4. Relationships between Days Open and Metabolic Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Watters, R.; Wiltbank, M.C.; Guenther, J.; Brickner, A.; Rastani, R.; Fricke, P.; Grummer, R. Effect of dry period length on reproduction during the subsequent lactation. J. Dairy Sci. 2009, 92, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Santschi, D.E.; Lefebvre, D.; Cue, R.; Girard, C.; Pellerin, D. Incidence of metabolic disorders and reproductive performance following a short (35-d) or conventional (60-d) dry period management in commercial Holstein herds. J. Dairy Sci. 2011, 94, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Soede, N.; Van Dorland, H.; Remmelink, G.; Bruckmaier, R.; Kemp, B.; Van Knegsel, A.T. Relationship between metabolism and ovarian activity in dairy cows with different dry period lengths. Theriogenology 2015, 84, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Gümen, A.; Rastani, R.; Grummer, R.; Wiltbank, M.C. Reduced Dry Periods and Varying Prepartum Diets Alter Postpartum Ovulation and Reproductive Measures. J. Dairy Sci. 2005, 88, 2401–2411. [Google Scholar] [CrossRef]
- Pezeshki, A.; Mehrzad, J.; Ghorbani, G.; Rahmani, H.; Collier, R.; Burvenich, C. Effects of Short Dry Periods on Performance and Metabolic Status in Holstein Dairy Cows. J. Dairy Sci. 2007, 90, 5531–5541. [Google Scholar] [CrossRef]
- Pezeshki, A.; Mehrzad, J.; Ghorbani, G.R.; De Spiegeleer, B.; Collier, R.; Burvenich, C. The effect of dry period length reduction to 28 days on the performance of multiparous dairy cows in the subsequent lactation. Can. J. Anim. Sci. 2008, 88, 449–456. [Google Scholar] [CrossRef]
- Chen, J.; Gross, J.J.; Van Dorland, H.; Remmelink, G.; Bruckmaier, R.; Kemp, B.; Van Knegsel, A.T. Effects of dry period length and dietary energy source on metabolic status and hepatic gene expression of dairy cows in early lactation. J. Dairy Sci. 2015, 98, 1033–1045. [Google Scholar] [CrossRef]
- Chen, J.; Remmelink, G.; Gross, J.J.; Bruckmaier, R.; Kemp, B.; Van Knegsel, A.T. Effects of dry period length and dietary energy source on milk yield, energy balance, and metabolic status of dairy cows over 2 consecutive years: Effects in the second year. J. Dairy Sci. 2016, 99, 4826–4838. [Google Scholar] [CrossRef]
- Van Hoeij, R.; Dijkstra, J.; Bruckmaier, R.; Gross, J.J.; Lam, T.; Remmelink, G.; Kemp, B.; Van Knegsel, A.; Van Knegsel, A.T. The effect of dry period length and postpartum level of concentrate on milk production, energy balance, and plasma metabolites of dairy cows across the dry period and in early lactation. J. Dairy Sci. 2017, 100, 5863–5879. [Google Scholar] [CrossRef]
- Britt, J.H.; Kittok, R.J.; Harrison, D.S. Ovulation, Estrus and Endocrine Response after GnRH in Early Postpartum Cows. J. Anim. Sci. 1974, 39, 915–919. [Google Scholar] [CrossRef]
- Pushpakumara, P.; Gardner, N.; Reynolds, C.; Beever, D.; Wathes, D. Relationships between transition period diet, metabolic parameters and fertility in lactating dairy cows. Theriogenology 2003, 60, 1165–1185. [Google Scholar] [CrossRef]
- Butler, W. Nutritional interactions with reproductive performance in dairy cattle. Anim. Reprod. Sci. 2000, 60, 449–457. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Gong, J.G.; Webb, R. Interactions between nutrition and ovarian activity in cattle: Physiological, cellular and molecular mechanisms. Reprod. (Camb. Eng.) Suppl. 2003, 61, 403–414. [Google Scholar] [CrossRef]
- Lucy, M. Mechanisms linking nutrition and reproduction in postpartum cows. Reprod. (Camb. Eng.) Suppl. 2003, 61, 415–427. [Google Scholar] [CrossRef]
- Fouladi-Nashta, A.A.; Gutierrez, C.G.; Gong, J.G.; Garnsworthy, P.; Webb, R. Impact of Dietary Fatty Acids on Oocyte Quality and Development in Lactating Dairy Cows1. Boil. Reprod. 2007, 77, 9–17. [Google Scholar] [CrossRef]
- Garnsworthy, P.; Fouladi-Nashta, A.A.; Mann, G.; Sinclair, K.D.; Webb, R. Effect of dietary-induced changes in plasma insulin concentrations during the early post partum period on pregnancy rate in dairy cows. Reproduction 2009, 137, 759–768. [Google Scholar] [CrossRef]
- Van Knegsel, A.; Remmelink, G.; Jorjong, S.; Fievez, V.; Kemp, B.; Van Knegsel, A.T. Effect of dry period length and dietary energy source on energy balance, milk yield, and milk composition of dairy cows. J. Dairy Sci. 2014, 97, 1499–1512. [Google Scholar] [CrossRef]
- Gutierrez, C.G.; Dobrinski, I.; Smith, T.T.; Suarez, S.S.; Ball, B.A. Development of a long-term bovine granulosa cell culture system: Induction and maintenance of estradiol production, response to follicle- stimulating hormone, and morphological characteristics. Boil. Reprod. 1997, 56, 608–616. [Google Scholar] [CrossRef]
- Glister, C.; Tannetta, D.; Groome, N.P.; Knight, P.G. Interactions between follicle-stimulating hormone and growth factors in modulating secretion of steroids and inhibin-related peptides by nonluteinized bovine granulosa cells. Boil. Reprod. 2001, 65, 1020–1028. [Google Scholar] [CrossRef]
- Spicer, L.J.; Alpizar, E.; Echternkamp, S.E. Effects of insulin, insulin-like growth factor I, and gonadotropins on bovine granulosa cell proliferation, progesterone production, estradiol production, and(or) insulin-like growth factor I production in vitro1. J. Anim. Sci. 1993, 71, 1232–1241. [Google Scholar] [CrossRef]
- Spicer, L.J.; Stewart, R.E. Interactions among basic fibroblast growth factor, epidermal growth factor, insulin, and insulin-like growth factor-I (IGF-I) on cell numbers and steroidogenesis of bovine thecal cells: Role of IGF-I receptors. Boil. Reprod. 1996, 54, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Graber, M.; Kohler, S.; Müller, A.; Burgermeister, K.; Kaufmann, T.; Bruckmaier, R.; Van Dorland, H. Identification of plasma and hepatic parameters related to metabolic robustness in dairy cows. J. Anim. Physiol. Anim. Nutr. 2011, 96, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Van Hoeij, R.; Dijkstra, J.; Bruckmaier, R.; Gross, J.J.; Lam, T.; Remmelink, G.; Kemp, B.; Van Knegsel, A. Consequences of dietary energy source and energy level on energy balance, lactogenic hormones, and lactation curve characteristics of cows after a short or omitted dry period. J. Dairy Sci. 2017, 100, 8544–8564. [Google Scholar] [CrossRef] [PubMed]
- De Feu, M.; Evans, A.; Lonergan, P.; Butler, S. The effect of dry period duration and dietary energy density on milk production, bioenergetic status, and postpartum ovarian function in Holstein-Friesian dairy cows. J. Dairy Sci. 2009, 92, 6011–6022. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.; Staples, C.R.; Thatcher, W.W.; Erickson, P.; Cleale, R.M.; Firkins, J.L.; Clark, J.H.; Murphy, M.R.; Brodie, B.O. Influence of diet composition, dry-matter intake, milk production and energy balance on time of post-partum ovulation and fertility in dairy cows. Anim. Sci. 1992, 54, 323–331. [Google Scholar] [CrossRef]
- Darwash, A.O.; Lamming, G.E.; Wooliams, J.A. The phenotypic association between the interval to post-partum ovulation and traditional measures of fertility in dairy cattle. Anim. Sci. 1997, 65, 9–16. [Google Scholar] [CrossRef]
- Galvão, K.; Frajblat, M.; Butler, W.; Brittin, S.; Guard, C.; Gilbert, R.; Gilbert, R. Effect of Early Postpartum Ovulation on Fertility in Dairy Cows. Reprod. Domest. Anim. 2009, 45, 207–211. [Google Scholar] [CrossRef]
- Butler, W.; Smith, R. Interrelationships Between Energy Balance and Postpartum Reproductive Function in Dairy Cattle. J. Dairy Sci. 1989, 72, 767–783. [Google Scholar] [CrossRef]
- Smith, M.C.A.; Wallace, J.M. Influence of early post partum ovulation on the re-establishment of pregnancy in multiparous and primiparous dairy cattle. Reprod. Fertil. Dev. 1998, 10, 207–216. [Google Scholar] [CrossRef]
- Shrestha, H.K.; Nakao, T.; Suzuki, T.; Higaki, T.; Akita, M. Effects of abnormal ovarian cycles during pre-service period postpartum on subsequent reproductive performance of high-producing Holstein cows. Theriogenology 2004, 61, 1559–1571. [Google Scholar] [CrossRef]
- Lamming, G.E.; Darwash, A.O. The use of milk progesterone profiles to characterise components of subfertility in milked dairy cows. Anim. Reprod. Sci. 1998, 52, 175–190. [Google Scholar] [CrossRef]
- Royal, M.; Mann, G.; Flint, A. Strategies for Reversing the Trend Towards Subfertility in Dairy Cattle. Veter. J. 2000, 160, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Kawakami, S. The roles of PGF2α and PGE2 in regression of the corpus luteum after intrauterine infusion of Arcanobacterium pyogenes in cows. Theriogenology 2009, 71, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Fischer, D.P.; Werling, D.; Williams, E.; Lilly, S.T.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 2005, 147, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Opsomer, G.; Gröhn, Y.T.; Hertl, J.; Coryn, M.; Deluyker, H.; De Kruif, A. Risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: A field study. Theriogenology 2000, 53, 841–857. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Nakao, T.; Yamada, K.; Koike, K.; Hayashi, A.; Dematawewa, C. Characteristics of prolonged luteal phase identified by milk progesterone concentrations and its effects on reproductive performance in Holstein cows. J. Dairy Sci. 2011, 94, 116–127. [Google Scholar] [CrossRef]
- Mateus, L.; Lopes-Da-Costa, L.; Diniz, P.; Ziecik, A.J. Relationship between endotoxin and prostaglandin (PGE2 and PGFM) concentrations and ovarian function in dairy cows with puerperal endometritis. Anim. Reprod. Sci. 2003, 76, 143–154. [Google Scholar] [CrossRef]
- Opsomer, G.; Coryn, M.; Deluyker, H.; Kruif, A. An Analysis of Ovarian Dysfunction in High Yielding Dairy Cows After Calving Based on Progesterone Profiles. Reprod. Domest. Anim. 1998, 33, 193–204. [Google Scholar] [CrossRef]
- Kafi, M.; Mirzaei, A.; Tamadon, A.; Saeb, M. Factors affecting the occurrence of postpartum prolonged luteal activity in clinically healthy high-producing dairy cows. Theriogenology 2012, 77, 421–429. [Google Scholar] [CrossRef]
- Harrison, D.S. Reproduction, Milk Production and Culling in Dairy Cows Inseminated at First Estrus after 40 or 60 Days Postpartum. Master’s Thesis, Michigan State University. Department of Dairy Science, East Lansing, MI, USA, 1975. [Google Scholar]
- González-Recio, O.; Alenda, R. Genetic Parameters for Female Fertility Traits and a Fertility Index in Spanish Dairy Cattle. J. Dairy Sci. 2005, 88, 3282–3289. [Google Scholar] [CrossRef]
- Gautam, G.; Nakao, T.; Yamada, K.; Yoshida, C. Defining delayed resumption of ovarian activity postpartum and its impact on subsequent reproductive performance in Holstein cows. Theriogenology 2010, 73, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M. Regulation of Ovarian Follicular Growth by Somatotropin and Insulin-Like Growth Factors in Cattle. J. Dairy Sci. 2000, 83, 1635–1647. [Google Scholar] [CrossRef]
- Beam, S.W.; Butler, W. Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J. Reprod. Fertil. Suppl. 1999, 54, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Gong, J.; Bramley, T.; Webb, R. Effects of genetic selection for milk yield on metabolic hormones and follicular development in postpartum dairy cattle. J. Reprod. Fertil. Abst. Ser. 1999, 24, 32–36. [Google Scholar]
- Garverick, H.A.; Harris, M.; Vogel-Bluel, R.; Sampson, J.; Bader, J.; Lamberson, W.; Spain, J.; Lucy, M.; Youngquist, R. Concentrations of nonesterified fatty acids and glucose in blood of periparturient dairy cows are indicative of pregnancy success at first insemination. J. Dairy Sci. 2013, 96, 181–188. [Google Scholar] [CrossRef]
- Huszenicza, G.; Jánosi, S.; Kulcsár, M.; Kóródi, P.; Reiczigel, J.; Kátai, L.; Peters, A.; De Rensis, F. Effects of Clinical Mastitis on Ovarian Function in Post-partum Dairy Cows. Reprod. Domest. Anim. 2005, 40, 199–204. [Google Scholar] [CrossRef]
- Petersson, K.-J.; Strandberg, E.; Gustafsson, H.; Berglund, B. Environmental effects on progesterone profile measures of dairy cow fertility. Anim. Reprod. Sci. 2006, 91, 201–214. [Google Scholar] [CrossRef]
- Jorritsma, R.; Wensing, T.; Kruip, T.A.; Vos, P.L.; Noordhuizen, J.P. Metabolic changes in early lactation and impaired reproductive performance in dairy cows. Veter. Res. 2003, 34, 11–26. [Google Scholar] [CrossRef]
- Rutter, S.; Jackson, D.; Johnson, C.; Forbes, J. Automatically recorded competitive feeding behaviour as a measure of social dominance in dairy cows. Appl. Anim. Behav. Sci. 1987, 17, 41–50. [Google Scholar] [CrossRef]
- Ospina, P.; Nydam, D.; Stokol, T.; Overton, T. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef]
- Ospina, P.; Nydam, D.; Stokol, T.; Overton, T. Association between the proportion of sampled transition cows with increased nonesterified fatty acids and β-hydroxybutyrate and disease incidence, pregnancy rate, and milk production at the herd level. J. Dairy Sci. 2010, 93, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Mihandoost, B.; Mogheiseh, A.; Nazifi, S.; Ahmadi, M.R.; Ansari-Lari, M. Metabolic status and ultrasound traits of reproductive tract of estrus dairy cows in early postpartum period. Comp. Haematol. Int. 2019, 28, 689–693. [Google Scholar] [CrossRef]
- Lucy, M.; Staples, C.; Michel, F.; Thatcher, W.; Bolt, D. Effect of Feeding Calcium Soaps to Early Postpartum Dairy Cows on Plasma Prostaglandin F2α, Luteinizing Hormone, and Follicular Growth. J. Dairy Sci. 1991, 74, 483–489. [Google Scholar] [CrossRef]
- Keskin, A.; Mecitoğlu, G.; Bilen, E.; Güner, B.; Orman, A.; Okut, H.; Gumen, A. The effect of ovulatory follicle size at the time of insemination onpregnancy rate in lactating dairy cows. Turk. J. Veter. Anim. Sci. 2016, 40, 68–74. [Google Scholar] [CrossRef]
- Adamiak, S.; Mackie, K.; Watt, R.; Webb, R.; Sinclair, K.D. Impact of Nutrition on Oocyte Quality: Cumulative Effects of Body Composition and Diet Leading to Hyperinsulinemia in Cattle1. Boil. Reprod. 2005, 73, 918–926. [Google Scholar] [CrossRef]
- Adamiak, S.J.; Powell, K.; Rooke, J.A.; Webb, R.; Sinclair, K.D. Body composition, dietary carbohydrates and fatty acids determine post-fertilisation development of bovine oocytes in vitro. Reproduction 2006, 131, 247–258. [Google Scholar] [CrossRef]
- Fouladi-Nashta, A.A.; Campbell, K.H.S. Dissociation of oocyte nuclear and cytoplasmic maturation by the addition of insulin in cultured bovine antral follicles. Reproduction 2006, 131, 449–460. [Google Scholar] [CrossRef]

| Cows, n | 0-d DP (LOW) | 0-d DP (STD) | 30-d DP (STD) | Total |
|---|---|---|---|---|
| Cows in experiment | 42 | 43 | 43 | 128 |
| Cows with OLA activity within 100 DIM 1 | 42 | 42 | 43 | 127 |
| Cows with complete 1st ovarian cycle | 41 | 39 | 39 | 119 |
| Cows with complete 2nd ovarian cycle | 32 | 26 | 29 | 87 |
| Transition Treatments | SEM | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0-d DP (LOW) | 0-d DP (STD) | 30-d DP (STD) | TT | P | TT × P | |||||
| Parity | 2 | ≥3 | 2 | ≥3 | 2 | ≥3 | ||||
| Cows (n) | 21 | 21 | 22 | 21 | 21 | 22 | ||||
| Days open (days) | 99.9 a | 110.7 a | 96.6 a | 159.3 b | 114.9 a,b | 127.5 a,b | 15.0 | <0.01 | <0.01 | <0.01 |
| Calving to first AI 3 (days) | 73.5 | 66.9 | 66.1 | 71.9 | 67.7 | 69.0 | 5.0 | 0.97 | 0.48 | 0.50 |
| Services per conception | 2.3 b | 2.7 b | 2.1 b | 4.3 a | 2.9 a,b | 2.7 b | 0.4 | 0.27 | 0.02 | <0.01 |
| Pregnant within 44 weeks (%) | 95.2 | 95.2 | 95.4 | 87.1 | 85.2 | 95.5 | 7.1 | 0.96 | 0.90 | 0.99 |
| Pregnant within 100 DIM (%) | 66.7 a | 57.1 a,b | 59.1 a,b | 23.8 b | 42.9 a,b | 22.7 b | 10.4 | 0.02 | 0.04 | 0.63 |
| Days to 1st OLA 4 | 17.0 a | 21.9 a,b | 20.2 a,b | 27.6 b | 26.3 a,b | 28.3 b | 2.6 | <0.01 | <0.01 | 0.31 |
| Cycle number per cow within 100 DIM | 2.1 a,b | 2.0 a,b | 2.2 a | 1.8 a,b | 1.9 a,b | 1.7 b | 0.2 | <0.01 | <0.01 | 0.07 |
| Normal cycles (per cow within 100 DIM) (%) | 48.6 | 39.6 | 41.7 | 39.7 | 42.0 | 42.1 | 8.4 | 0.48 | 0.44 | 0.29 |
| Short cycles (per cow within 100 DIM) (%) | 16.5 a,b | 18.1 a,b | 18.5 a,b | 5.9 a | 23.2 b | 7.0 a,b | 5.6 | 0.06 | <0.01 | <0.01 |
| Prolonged cycles (per cow within 100 DIM) (%) | 34.9 a | 42.4 0 a,b | 40.9 a,b | 54.4 b | 34.8 a,b | 50.9 a,b | 8.6 | 0.02 | 0.09 | 0.45 |
| 1st ovarian cycle postpartum (days) | ||||||||||
| Luteal phase length (days) | 18.0 a,b | 18.9 a,b | 21.0 a,b | 21.8 a | 13.9 b | 17.9 a,b | 2.7 | <0.01 | 0.10 | 0.15 |
| Follicular phase length (days) | 6.9 a,b | 10.4 a | 7.4 a,b | 6.5 b | 8.2 a,b | 9.4 a,b | 1.3 | <0.01 | <0.01 | <0.01 |
| Cycle length (days) | 24.9 | 28.3 | 28.4 | 28.3 | 22.2 | 27.3 | 2.9 | 0.02 | <0.01 | 0.09 |
| Variable | Days Open | SEM | Transition Treatments | SEM | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Short (<80 d) | Mid (80–130 d) | Long (>130 d) | Not Pregnant 3 | 0-d DP (LOW) | 0-d DP (STD) | 30-d DP (STD) | Days Open | P | TT | TT × Days Open | |||
| Cows, n | 37 | 37 | 37 | 17 | 42 | 43 | 43 | ||||||
| Calving to first AI 4 (days) | 60.42 a | 68.85 a | 80.16 b | 68.07 a | 5.55 | 71.02 | 67.44 | 69.66 | 6.03 | <0.01 | 0.48 | 0.97 | 0.83 |
| Services per pregnancy | 1.44 a | 2.71 b | 4.09 c | 0.36 | 2.36 | 3.31 | 2.57 | 0.16 | <0.01 | 0.35 | 0.79 | 0.79 | |
| Days to first OLA 5 | 24.73 b | 22.20 a | 23.76 c | 24.68 c | 1.36 | 19.26 | 25.53 | 26.73 | 2.84 | <0.01 | <0.01 | <0.01 | <0.01 |
| Cycle number (per cow within 100 DIM) | 1.62 a | 2.49 c | 2.00 b | 1.74 a | 0.06 | 2.32 | 1.77 | 1.80 | 0.20 | <0.01 | <0.01 | <0.01 | 0.22 |
| Normal cycles (per cow within 100 DIM) (%) | 47.96 a | 43.98 a | 30.47 b | 38.83 a b | 4.66 | 42.28 | 40.02 | 38.62 | 6.41 | <0.01 | 0.41 | 0.82 | <0.01 |
| Short cycles (per cow within 100 DIM) (%) | 18.01 a | 14.93 a | 13.74 a | 6.76 b | 3.06 | 14.40 | 10.65 | 15.04 | 6.45 | <0.01 | <0.01 | 0.19 | <0.01 |
| Prolonged cycles (per cow within 100 DIM) (%) | 35.03 a | 40.92 a | 55.31 b | 52.90 b | 4.88 | 42.17 | 49.26 | 46.68 | 9.75 | <0.01 | 0.10 | 0.66 | <0.01 |
| 1st ovarian cycle postpartum (days) | |||||||||||||
| Luteal phase length | 17.94 a | 18.22 a | 22.03 b | 18.29 b | 0.74 | 20.18 | 21.01 | 16.16 | 2.92 | <0.01 | 0.82 | <0.01 | 0.05 |
| Follicular phase length | 7.02 | 7.33 | 9.72 | 7.73 | 0.77 | 8.83 | 6.59 | 8.43 | 1.38 | 0.07 | 0.01 | <0.01 | 0.31 |
| Cycle length | 25.00 a | 25.28 a | 31.89 b | 25.56 a | 1.59 | 28.58 | 27.56 | 24.66 | 3.19 | <0.01 | 0.30 | <0.01 | <0.01 |
| Days to OLA | SEM | Transition Treatments | SEM | p-Value 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <21 d | ≥21 d | 0-d DP (LOW) | 0-d DP (STD) | 30-d DP (STD) | OLA | TT | P | W | OLA × TT | OLA × P | OLA × W | |||
| Cows, n | 66 | 53 | ||||||||||||
| Glucose (mmol/L) 4 | 3.92 a | 3.71 b | 0.05 | 3.89 | 3.90 | 3.66 | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | 0.21 | 0.15 | 0.65 |
| NEFA (mmol/L) 4 | 0.11 a | 0.20 b | 0.12 | 0.12 | 0.23 | <0.01 | <0.01 | <0.01 | <0.01 | 0.47 | 0.01 | 0.24 | ||
| (0.10–0.13) | (0.17–0.22) | (0.11–0.14) | (0.10–0.14) | (0.20–0.26) | ||||||||||
| BHB (mmol/L )4 | 0.63 a | 0.73 b | 0.66 | 0.64 | 0.74 | <0.01 | 0.02 | <0.01 | 0.01 | 0.01 | 0.07 | 0.36 | ||
| (0.59–0.67) | (0.68–0.79) | (0.60–0.72) | (0.59–0.69) | (0.69–0.80) | ||||||||||
| IGF-1 (ng/mL) 4 | 129.11 a | 95.31 b | 6.48 | 122.63 | 121.82 | 92.17 | 5.39 | <0.01 | <0.01 | <0.01 | <0.01 | 0.29 | 0.43 | 0.01 |
| Insulin (µIU/mL) 4 | 15.86 a | 11.41 b | 0.82 | 14.58 | 15.51 | 10.81 | 0.68 | <0.01 | <0.01 | 0.86 | <0.01 | 0.10 | 0.71 | 0.08 |
| EB (kJ/kg0.75 ·day) (week 1 till 7) 5 | −15.52 a | −153.05 b | 26.61 | −55.59 | −18.33 | −178.94 | 22.11 | <0.01 | <0.01 | 0.06 | <0.01 | 0.22 | <0.01 | 0.19 |
| Days Open | SEM | Transition Treatments | SEM | p-Value 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <80 d | 80-130 d | >130 d | Not Pregnant 5 | 0-d DP (LOW) | 0-d DP (STD) | 30-d DP (STD) | Days Open | TT | P | W | Days Open × P | Days Open × TT | Days Open × W | |||
| 34 | 34 | 37 | 13 | |||||||||||||
| Glucose (mmol/L) 3 | 3.86 | 3.81 | 3.81 | 3.71 | 0.62 | 3.87 | 3.91 | 3.64 | 0.05 | 0.89 | <0.01 | 0.04 | <0.01 | 0.73 | 0.88 | 0.28 |
| NEFA (mmol/L) 3 | 0.12 | 0.15 | 0.15 | 0.16 | 0.13 | 0.10 | 0.22 | 0.12 | <0.01 | <0.01 | <0.01 | <0.01 | 0.44 | 0.53 | ||
| (0.09–0.14) | (0.13–0.18) | (0.12–0.18) | (0.12–0.20) | (0.11–0.16) | (0.08–0.12) | (0.19–0.26) | ||||||||||
| BHB (mmol/L) 3 | 0.66 | 0.67 | 0.73 | 0.66 | 0.69 | 0.62 | 0.73 | 0.53 | 0.03 | 0.12 | <0.01 | 0.43 | 0.85 | 0.53 | ||
| (0.59–0.73) | (0.61–0.74) | (0.66–0.80) | (0.58–0.76) | (0.62–0.76) | (0.56–0.68) | (0.67–0.80) | ||||||||||
| IGF-1 (ng/mL) 3 | 122.26 | 116.49 | 111.48 | 105.99 | 7.24 | 122.04 | 128.34 | 91.79 | 6.61 | 0.72 | 0.73 | 0.37 | <0.01 | 0.42 | 0.87 | 0.10 |
| Insulin (µIU/mL) 3 | 14.88 a | 14.07 a | 13.75 a b | 12.12 b | 0.98 | 14.04 | 16.42 | 10.65 | 0.78 | <0.01 | <0.01 | 0.42 | <0.01 | <0.01 | 0.92 | 0.57 |
| EB (kJ/kg0.75 ·day) (week 1 till 7) 4 | −48.34 a | 93.09b | 92.98b | −118.71 b | 31.64 | −87.88 | 2.54 | −179.51 | 38.84 | <0.01 | 0.09 | 0.90 | <0.01 | <0.01 | 0.58 | 0.21 |
| EB (kJ/kg0.75·day) (week 1 till 14) | −15.86 | −26.47 | −10.98 | −33.50 | 21.53 | −23.32 | 49.36 | −91.15 | 20.60 | 0.05 | 0.02 | 0.02 | 0.36 | 0.05 | 0.72 | 0.37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; van Hoeij, R.J.; Bruckmaier, R.M.; Kok, A.; Lam, T.J.G.M.; Kemp, B.; van Knegsel, A.T.M. Consequences of Transition Treatments on Fertility and Associated Metabolic Status for Dairy Cows in Early Lactation. Animals 2020, 10, 1100. https://doi.org/10.3390/ani10061100
Ma J, van Hoeij RJ, Bruckmaier RM, Kok A, Lam TJGM, Kemp B, van Knegsel ATM. Consequences of Transition Treatments on Fertility and Associated Metabolic Status for Dairy Cows in Early Lactation. Animals. 2020; 10(6):1100. https://doi.org/10.3390/ani10061100
Chicago/Turabian StyleMa, Junnan, Renny J. van Hoeij, Rupert M. Bruckmaier, Akke Kok, Theo J. G. M. Lam, Bas Kemp, and Ariette T. M. van Knegsel. 2020. "Consequences of Transition Treatments on Fertility and Associated Metabolic Status for Dairy Cows in Early Lactation" Animals 10, no. 6: 1100. https://doi.org/10.3390/ani10061100
APA StyleMa, J., van Hoeij, R. J., Bruckmaier, R. M., Kok, A., Lam, T. J. G. M., Kemp, B., & van Knegsel, A. T. M. (2020). Consequences of Transition Treatments on Fertility and Associated Metabolic Status for Dairy Cows in Early Lactation. Animals, 10(6), 1100. https://doi.org/10.3390/ani10061100





