Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Resource Allocation between Maintenance and Productive Life Functions in Early Lactating High-Producing Dairy Cows
2.1. Resource Allocation Theory
2.2. Homeorhetic and Allostatic Control of Nutrient Partitioning
3. Glucose Metabolism to Fuel Milk Synthesis and Immune Functions in Dairy Cows
3.1. Adaptation to Lactation
3.2. Adaptation to Inflammation
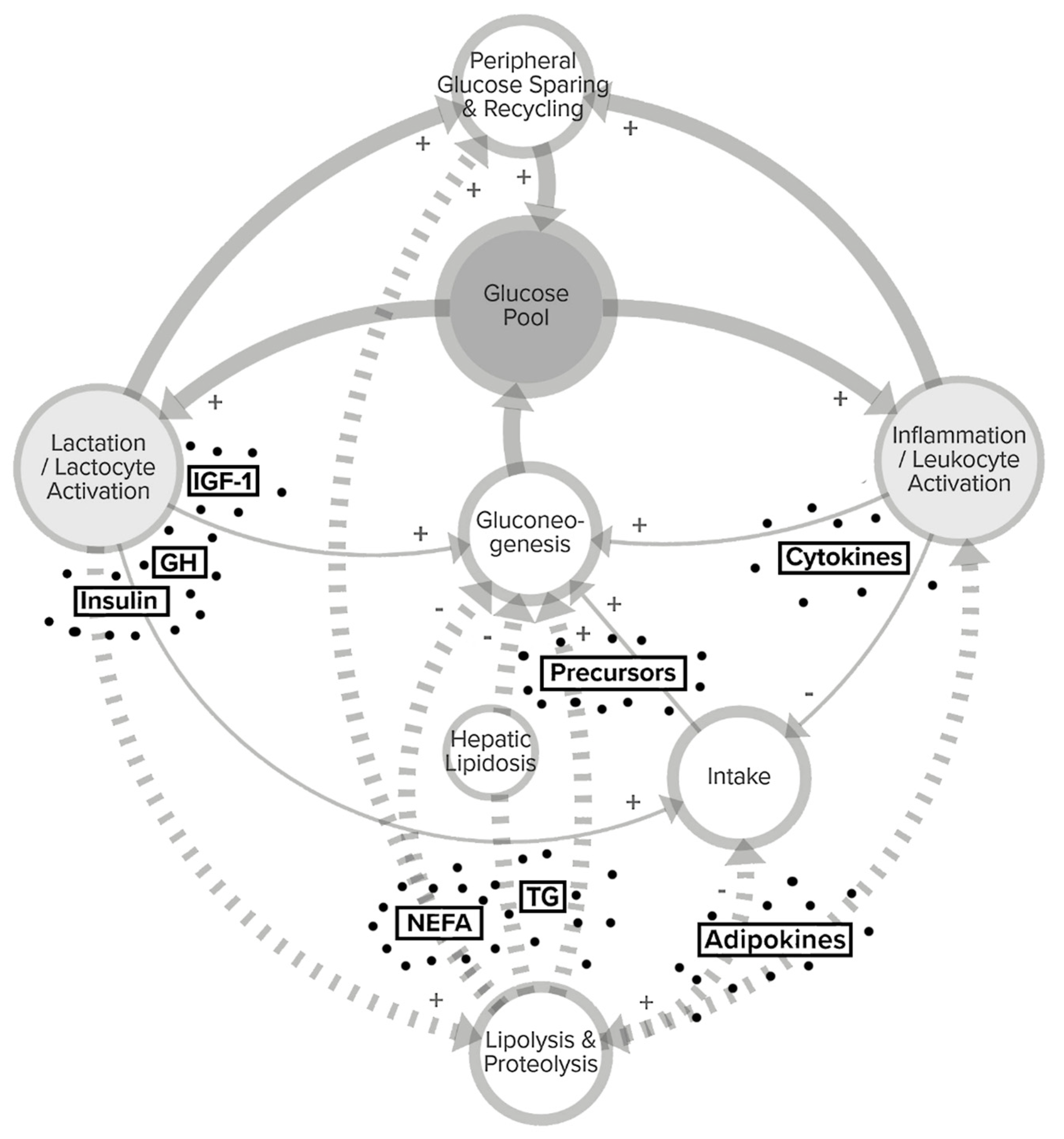
4. Trade-Offs for Glucose between Lactocytes and Leukocytes
4.1. Peripartal Immune Dysfunction
4.2. Metabolic Stress and the Immune System
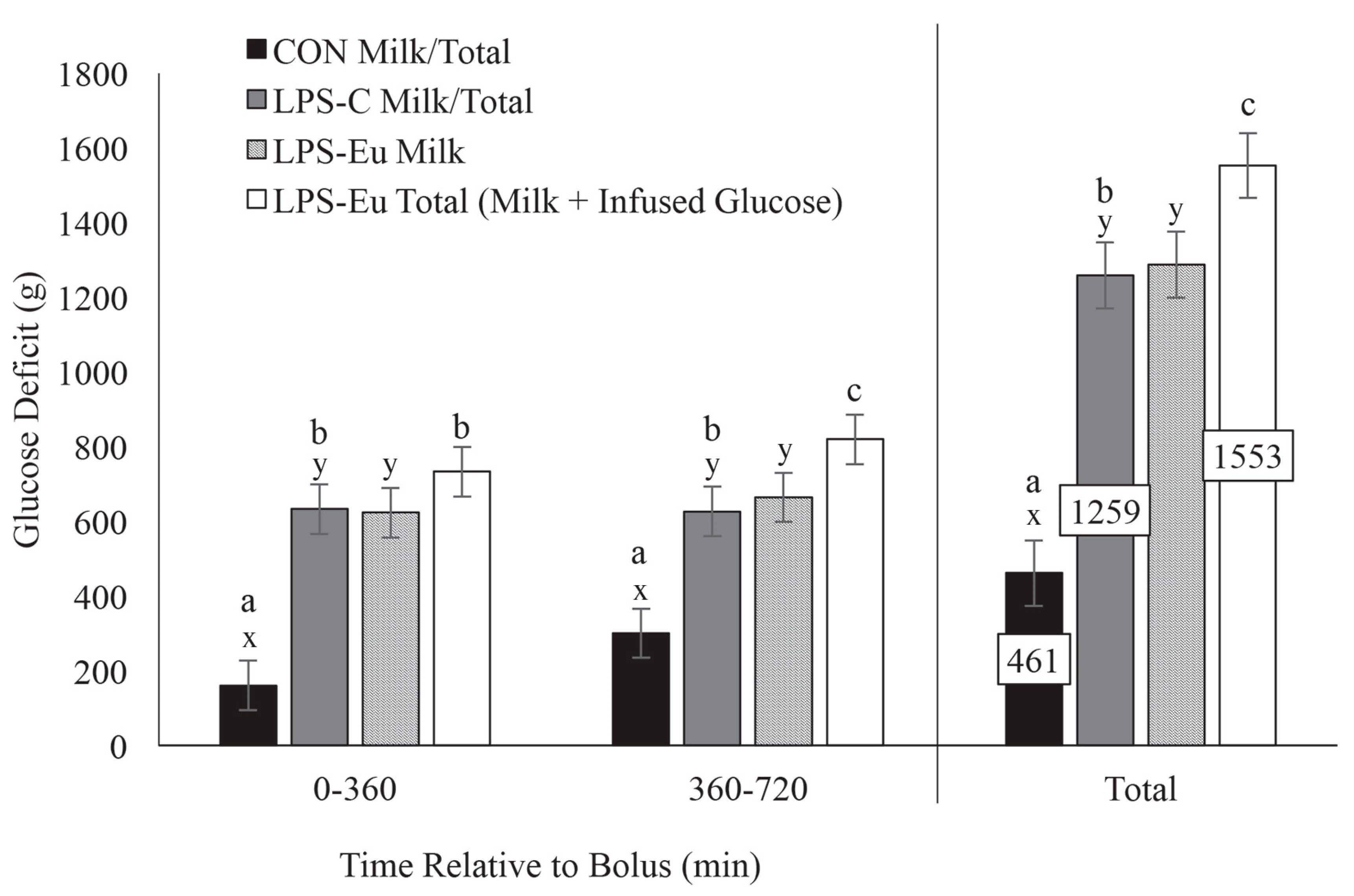
4.3. Competition for Glucose between MEC and Immune Cells
5. Management of High-Producing Dairy Cows that Risk Glucose Shortage
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brunner, N.; Groeger, S.; Canelas Raposo, J.; Bruckmaier, R.M.; Gross, J.J. Prevalence of subclinical ketosis and production diseases in dairy cows in Central and South America, Africa, Asia, Australia, New Zealand, and Eastern Europe1. Transl. Anim. Sci. 2019, 3, 84–92. [Google Scholar] [CrossRef]
- Krieger, M.; Sjöström, K.; Blanco-Penedo, I.; Madouasse, A.; Duval, J.E.; Bareille, N.; Fourichon, C.; Sundrum, A.; Emanuelson, U. Prevalence of production disease related indicators in organic dairy herds in four European countries. Livest. Sci. 2017, 198, 104–108. [Google Scholar] [CrossRef]
- van Soest, F.J.S.; Mourits, M.C.M.; Blanco-Penedo, I.; Duval, J.; Fall, N.; Krieger, M.; Sjöstrom, K.; Hogeveen, H. Farm-specific failure costs of production disorders in European organic dairy herds. Prev. Vet. Med. 2019, 168, 19–29. [Google Scholar] [CrossRef]
- Compton, C.W.R.; Heuer, C.; Thomsen, P.T.; Carpenter, T.E.; Phyn, C.V.C.; McDougall, S. Invited review: A systematic literature review and meta-analysis of mortality and culling in dairy cattle. J. Dairy Sci. 2017, 100, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Friggens, N.C.; Berry, D.P.; Faverdin, P. Isolating the cow-specific part of residual energy intake in lactating dairy cows using random regressions. Animal 2018, 12, 1396–1404. [Google Scholar] [CrossRef]
- De Koster, J.; Salavati, M.; Grelet, C.; Crowe, M.A.; Matthews, E.; O’Flaherty, R.; Opsomer, G.; Foldager, L.; Hostens, M. Prediction of metabolic clusters in early-lactation dairy cows using models based on milk biomarkers. J. Dairy Sci. 2019, 102, 2631–2644. [Google Scholar] [CrossRef]
- Weikard, R.; Goldammer, T.; Brunner, R.M.; Kuehn, C. Tissue-specific mRNA expression patterns reveal a coordinated metabolic response associated with genetic selection for milk production in cows. Physiol. Genom. 2012, 44, 728–739. [Google Scholar] [CrossRef]
- Nayeri, S.; Stothard, P. Tissues, Metabolic Pathways and Genes of Key Importance in Lactating Dairy Cattle. Springer Sci. Rev. 2016, 4, 49–77. [Google Scholar] [CrossRef]
- Bauman, D.E.; Currie, W.B. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Kessel, S.; Stroehl, M.; Meyer, H.H.D.; Hiss, S.; Sauerwein, H.; Schwarz, F.J.; Bruckmaier, R.M. Individual variability in physiological adaptation to metabolic stress during early lactation in dairy cows kept under equal conditions. J. Anim. Sci. 2008, 86, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Graber, M.; Kohler, S.; Müller, A.; Burgermeister, K.; Kaufmann, T.; Bruckmaier, R.M.; van Dorland, H.A. Identification of plasma and hepatic parameters related to metabolic robustness in dairy cows. J. Anim. Physiol. Anim. Nutr. 2012, 96, 75–84. [Google Scholar] [CrossRef] [PubMed]
- van Dorland, H.A.; Richter, S.; Morel, I.; Doherr, M.G.; Castro, N.; Bruckmaier, R.M. Variation in hepatic regulation of metabolism during the dry period and in early lactation in dairy cows. J. Dairy Sci. 2009, 92, 1924–1940. [Google Scholar] [CrossRef] [PubMed]
- Ingvartsen, K.L.; Friggens, N.C. To what extent do variabilities in hormones, metabolites and energy intake explain variability in milk yield? Domest. Anim. Endocrinol. 2005, 29, 294–304. [Google Scholar] [CrossRef]
- Ortigues-Marty, I.; Agabriel, J.; Vernet, J.; Sepchat, B.; Al-Jammas, M.; Nozière, P. Contribution of nutrient fluxes to the evolution of the net energy systems, example of the INRA feeding system for beef cattle. Transl. Anim. Sci. 2019, 3, 1048–1063. [Google Scholar] [CrossRef]
- Bickerstaffe, R.; Annison, E.F.; Linzell, J.L. The metabolism of glucose, acetate, lipids and amino acids in lactating dairy cows. J. Agric. Sci. 1974, 82, 71. [Google Scholar] [CrossRef]
- Linzell, J.L. Mechanism of Secretion of the Aqueous Phase of Milk. J. Dairy Sci. 1972, 55, 1316–1322. [Google Scholar] [CrossRef]
- Rigout, S.; Lemosquet, S.; van Eys, J.E.; Blum, J.W.; Rulquin, H. Duodenal Glucose Increases Glucose Fluxes and Lactose Synthesis in Grass Silage-Fed Dairy Cows. J. Dairy Sci. 2002, 85, 595–606. [Google Scholar] [CrossRef]
- Chaiyabutr, N.; Faulkner, A.; Peaker, M. The utilization of glucose for the synthesis of milk components in the fed and starved lactating goat in vivo. Biochem. J. 1980, 186, 301–308. [Google Scholar] [CrossRef]
- Smith, G.H.; Crabtree, B.; Smith, R. Energy metabolism in the mammary gland. In Biochemistry of Lactation; Mepham, T.B., Ed.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 121–141. ISBN 0444804897. [Google Scholar]
- Ingvartsen, K.L.; Moyes, K.M. Factors contributing to immunosuppression in the dairy cow during the periparturient period. Jpn. J. Vet. Res. 2015, 63 (Suppl. 1), S15–S24. [Google Scholar]
- Kvidera, S.K.; Horst, E.A.; Abuajamieh, M.; Mayorga, E.J.; Fernandez, M.V.S.; Baumgard, L.H. Glucose requirements of an activated immune system in lactating Holstein cows. J. Dairy Sci. 2017, 100, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. IUBMB Life 2010, 62, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Rauw, W.M. Introduction. In Resource Allocation Theory Applied to Farm Animal Production; Rauw, W.M., Ed.; CABI: Wallingford, UK; Cambridge, MA, USA, 2009; pp. 1–21. ISBN 184593394X. [Google Scholar]
- Sundrum, A. Nutrition and Health-Management in Dairy Production. In Livestock Health and Farming; Abubakar, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Knap, P.W. Allocation of Resources to Maintenance. In Resource Allocation Theory Applied to Farm Animal Production; Rauw, W.M., Ed.; CABI: Wallingford, UK; Cambridge, MA, USA, 2009; pp. 110–129. ISBN 184593394X. [Google Scholar]
- Colditz, I.G. Allocation of Resources to Immune Responses. In Resource Allocation Theory Applied to Farm Animal Production; Rauw, W.M., Ed.; CABI: Wallingford, UK; Cambridge, MA, USA, 2009; pp. 192–209. ISBN 184593394X. [Google Scholar]
- Baumgard, L.H.; Collier, R.J.; Bauman, D.E. A 100-Year Review: Regulation of nutrient partitioning to support lactation. J. Dairy Sci. 2017, 100, 10353–10366. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, R.F. Selection for economic efficiency of dairy cattle using information on live weight and feed intake: A review. J. Dairy Sci. 1998, 81, 1109–1119. [Google Scholar] [CrossRef]
- Huber, K. Invited review: Resource allocation mismatch as pathway to disproportionate growth in farm animals-prerequisite for a disturbed health. Animal 2018, 12, 528–536. [Google Scholar] [CrossRef]
- Ortigues-Marty, I.; Cantalapiedra-Hijar, G.; Vernet, J.; Nozière, P. De l’énergie de la ration à l’utilisation des nutriments chez les ruminants: Quel rôle pour les tissus splanchniques? Cah. Nutr. Diététique 2017, 52, 45–53. [Google Scholar] [CrossRef]
- Tyrrell, H.F.; Moe, P.W. Effect of Intake on Digestive Efficiency. J. Dairy Sci. 1975, 58, 1151–1163. [Google Scholar] [CrossRef]
- Ledinek, M.; Gruber, L.; Steininger, F.; Fuerst-Waltl, B.; Zottl, K.; Royer, M.; Krimberger, K.; Mayerhofer, M.; Egger-Danner, C. Analysis of lactating cows on commercial Austrian dairy farms: The influence of genotype and body weight on efficiency parameters. Arch. Anim. Breed. 2019, 62, 491–500. [Google Scholar] [CrossRef]
- Loncke, C.; Nozière, P.; Vernet, J.; Lapierre, H.; Bahloul, L.; Al-Jammas, M.; Sauvant, D.; Ortigues-Marty, I. Net hepatic release of glucose from precursor supply in ruminants: A meta-analysis. Animal 2020, 14, 1–16. [Google Scholar] [CrossRef]
- Oltenacu, P.A.; Broom, D.M. The impact of genetic selection for increased milk yield on the welfare of dairy cows. Anim. Welf. 2010, 19, 39–49. [Google Scholar]
- Domblides, C.; Lartigue, L.; Faustin, B. Metabolic Stress in the Immune Function of T Cells, Macrophages and Dendritic Cells. Cells 2018, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Ingvartsen, K.L.; Dewhurst, R.J.; Friggens, N.C. On the relationship between lactational performance and health: Is it yield or metabolic imbalance that cause production diseases in dairy cattle? A position paper. Livest. Prod. Sci. 2003, 83, 277–308. [Google Scholar] [CrossRef]
- Sterling, P. Principles of Allostasis: Optimal Design, Predictive Regulation, Pathophysiology, and Rational Therapeutics. In Allostasis, Homeostasis and the Costs of Physiological Adaptation; Schulkin, J., Ed.; Cambridge University Press: Cambridge, UK, 2004; pp. 17–64. ISBN 9781316257081. [Google Scholar]
- Friggens, N.C.; Newbold, J.R. Towards a biological basis for predicting nutrient partitioning: The dairy cow as an example. Animal 2007, 1, 87–97. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Wingfield, J.C. What’s in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2009, 57, 105. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P. Biological response to stress: Implications for animal welfare. In The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare, Reprinted; Moberg, G.P., Mench, J.A., Eds.; CABI Publ.: Wallingford, UK, 2001; ISBN 0851993591. [Google Scholar]
- Tucker, H.A. Physiological Control of Mammary Growth, Lactogenesis, and Lactation. J. Dairy Sci. 1981, 64, 1403–1421. [Google Scholar] [CrossRef]
- Capuco, A.V.; Wood, D.L.; Baldwin, R.; Mcleod, K.; Paape, M.J. Mammary Cell Number, Proliferation, and Apoptosis During a Bovine Lactation: Relation to Milk Production and Effect of bST. J. Dairy Sci. 2001, 84, 2177–2187. [Google Scholar] [CrossRef]
- Akers, R.M.; Bauman, D.E.; Capuco, A.V.; Goodman, G.T.; Tucker, H.A. Prolactin regulation of milk secretion and biochemical differentiation of mammary epithelial cells in periparturient cows. Endocrinology 1981, 109, 23–30. [Google Scholar] [CrossRef]
- Akers, R.M. Lactation and the Mammary Gland, 1st ed.; Blackwell Publishing: Ames, Iowa, 2002; pp. 129–198. ISBN 9781119264880. [Google Scholar]
- Vernon, R.G. Endocrine control of metabolic adaptation during lactation. Proc. Nutr. Soc. 1989, 48, 23–32. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Boyd, C.K.; Bracken, C.J.; Lamberson, W.R.; Keisler, D.H.; Lucy, M.C. Reduced growth hormone receptor (GHR) messenger ribonucleic acid in liver of periparturient cattle is caused by a specific down-regulation of GHR 1A that is associated with decreased insulin-like growth factor I. Endocrinology 1999, 140, 3947–3954. [Google Scholar] [CrossRef]
- Lucy, M.C.; Jiang, H.; Kobayashi, Y. Changes in the Somatotrophic Axis Associated with the Initiation of Lactation. J. Dairy Sci. 2001, 84, E113–E119. [Google Scholar] [CrossRef]
- Rhoads, R.P.; Kim, J.W.; Leury, B.J.; Baumgard, L.H.; Segoale, N.; Frank, S.J.; Bauman, D.E.; Boisclair, Y.R. Insulin increases the abundance of the growth hormone receptor in liver and adipose tissue of periparturient dairy cows. J. Nutr. 2004, 134, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Flores-Morales, A.; Greenhalgh, C.J.; Norstedt, G.; Rico-Bautista, E. Negative regulation of growth hormone receptor signaling. Mol. Endocrinol. 2006, 20, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Radcliff, R.P.; McCormack, B.L.; Crooker, B.A.; Lucy, M.C. Plasma Hormones and Expression of Growth Hormone Receptor and Insulin-Like Growth Factor-I mRNA in Hepatic Tissue of Periparturient Dairy Cows. J. Dairy Sci. 2003, 86, 3920–3926. [Google Scholar] [CrossRef]
- Hue, L.; Taegtmeyer, H. The Randle cycle revisited: A new head for an old hat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Hametner, C.; Tuchscherer, A.; Losand, B.; Kanitz, E.; Otten, W.; Sauerwein, H.; Bruckmaier, R.M.; Becker, F.; Kanitz, W.; et al. Hepatic gene expression involved in glucose and lipid metabolism in transition cows: Effects of fat mobilization during early lactation in relation to milk performance and metabolic changesade. J. Dairy Sci. 2013, 96, 5670–5681. [Google Scholar] [CrossRef]
- De Koster, J.D.; Opsomer, G. Insulin resistance in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef]
- Greenfield, R.B.; Cecava, M.J.; Donkin, S.S. Changes in mRNA Expression for Gluconeogenic Enzymes in Liver of Dairy Cattle During the Transition to Lactation1. J. Dairy Sci. 2000, 83, 1228–1236. [Google Scholar] [CrossRef]
- Grummer, R.R. Etiology of Lipid-Related Metabolic Disorders in Periparturient Dairy Cows. J. Dairy Sci. 1993, 76, 3882–3896. [Google Scholar] [CrossRef]
- Zarrin, M.; Grossen-Rösti, L.; Bruckmaier, R.M.; Gross, J.J. Elevation of blood β-hydroxybutyrate concentration affects glucose metabolism in dairy cows before and after parturition. J. Dairy Sci. 2017, 100, 2323–2333. [Google Scholar] [CrossRef]
- Zarrin, M.; De Matteis, L.; Vernay, M.C.M.B.; Wellnitz, O.; van Dorland, H.A.; Bruckmaier, R.M. Long-term elevation of β-hydroxybutyrate in dairy cows through infusion: Effects on feed intake, milk production, and metabolism. J. Dairy Sci. 2013, 96, 2960–2972. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Bai, G.; Chen, H.; Deng, Q.; Liu, Z.; Zhang, L.; Liu, G.; Wang, Z. Effects of non-esterified fatty acids on the gluconeogenesis in bovine hepatocytes. Mol. Cell. Biochem. 2012, 359, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Herdt, T.H. Variability Characteristics and Test Selection In Herdlevel Nutritional And Metabolic Profile Testing. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 387–403. [Google Scholar] [CrossRef]
- Ruoff, J.; Borchardt, S.; Heuwieser, W. Short communication: Associations between blood glucose concentration, onset of hyperketonemia, and milk production in early lactation dairy cows. J. Dairy Sci. 2017, 100, 5462–5467. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Bauman, D.E. Adaptations of Glucose Metabolism During Pregnancy and Lactation. J. Mammary Gland Biol. Neoplasia 1997, 2, 265–278. [Google Scholar] [CrossRef]
- Davey, A.W.F.; Grainger, C.; MacKenzie, D.D.S.; Flux, D.S.; Wilson, G.F.; Brookes, I.M.; Holmes, C.W. Nutritional and physiological studies of differences between Friesian cows of high and low genetic merit. Proc. N. Z. Soc. Anim. Prod. 1983, 67–70. [Google Scholar]
- Kerestes, M.; Faigl, V.; Kulcsár, M.; Balogh, O.; Földi, J.; Fébel, H.; Chilliard, Y.; Huszenicza, G. Periparturient insulin secretion and whole-body insulin responsiveness in dairy cows showing various forms of ketone pattern with or without puerperal metritis. Domest. Anim. Endocrinol. 2009, 37, 250–261. [Google Scholar] [CrossRef]
- Zachut, M.; Honig, H.; Striem, S.; Zick, Y.; Boura-Halfon, S.; Moallem, U. Periparturient dairy cows do not exhibit hepatic insulin resistance, yet adipose-specific insulin resistance occurs in cows prone to high weight loss. J. Dairy Sci. 2013, 96, 5656–5669. [Google Scholar] [CrossRef]
- Ostrowska, M.; Jarczak, J. Glucose transporters in cattle—A review. Anim. Sci. Pap. Rep. 2015, 33, 191–212. [Google Scholar]
- Komatsu, T.; Itoh, F.; Kushibiki, S.; Hodate, K. Changes in gene expression of glucose transporters in lactating and nonlactating cows. J. Anim. Sci. 2005, 83, 557–564. [Google Scholar] [CrossRef]
- Mattmiller, S.A.; Corl, C.M.; Gandy, J.C.; Loor, J.J.; Sordillo, L.M. Glucose transporter and hypoxia-associated gene expression in the mammary gland of transition dairy cattle. J. Dairy Sci. 2011, 94, 2912–2922. [Google Scholar] [CrossRef]
- Prosser, C.G.; Davis, S.R.; Farr, V.C.; Lacasse, P. Regulation of Blood Flow in the Mammary Microvasculature1. J. Dairy Sci. 1996, 79, 1184–1197. [Google Scholar] [CrossRef]
- Davis, S.R.; Collier, R.J. Mammary Blood Flow and Regulation of Substrate Supply for Milk Synthesis. J. Dairy Sci. 1985, 68, 1041–1058. [Google Scholar] [CrossRef]
- Davis, S.R.; Collier, R.J.; McNamara, J.P.; Head, H.H.; Croom, W.J.; Wilcox, C.J. Effects of thyroxine and growth hormone treatment of dairy cows on mammary uptake of glucose, oxygen and other milk fat precursors. J. Anim. Sci. 1988, 66, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Kronfeld, D.S.; Raggi, F.; Ramberg, C.F. Mammary blood flow and ketone body metabolism in normal, fasted, and ketotic cows. Am. J. Physiol. 1968, 215, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Samanc, H.; Kirovski, D.; Lakić, N.; Celeska, I.; Bojković-Kovačević, S.; Sladojević, Z.; Ivanov, I. A comparison of the concentrations of energy-balance-related variables in jugular and mammary vein blood of dairy cows with different milk yield. Acta Vet. Hung. 2014, 62, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Sadri, H.; Bruckmaier, R.M.; Rahmani, H.R.; Ghorbani, G.R.; Morel, I.; van Dorland, H.A. Gene expression of tumour necrosis factor and insulin signalling-related factors in subcutaneous adipose tissue during the dry period and in early lactation in dairy cows. J. Anim. Physiol. Anim. Nutr. 2010, 94, e194–e202. [Google Scholar] [CrossRef]
- Kuhla, B.; Nürnberg, G.; Albrecht, D.; Görs, S.; Hammon, H.M.; Metges, C.C. Involvement of skeletal muscle protein, glycogen, and fat metabolism in the adaptation on early lactation of dairy cows. J. Proteome Res. 2011, 10, 4252–4262. [Google Scholar] [CrossRef]
- Larsen, M.; Kristensen, N.B. Precursors for liver gluconeogenesis in periparturient dairy cows. Animal 2013, 7, 1640–1650. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef]
- Minuti, A.; Palladino, A.; Khan, M.J.; Alqarni, S.; Agrawal, A.; Piccioli-Capelli, F.; Hidalgo, F.; Cardoso, F.C.; Trevisi, E.; Loor, J.J. Abundance of ruminal bacteria, epithelial gene expression, and systemic biomarkers of metabolism and inflammation are altered during the peripartal period in dairy cows. J. Dairy Sci. 2015, 98, 8940–8951. [Google Scholar] [CrossRef]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Fadden, A.N.; Traber, M.G.; Bobe, G. Potential risk indicators of retained placenta and other diseases in multiparous cows. J. Dairy Sci. 2014, 97, 4151–4165. [Google Scholar] [CrossRef] [PubMed]
- Huzzey, J.M.; Mann, S.; Nydam, D.V.; Grant, R.J.; Overton, T.R. Associations of peripartum markers of stress and inflammation with milk yield and reproductive performance in Holstein dairy cows. Prev. Vet. Med. 2015, 120, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, G.D. The placenta, prostaglandins and parturition: A review. Reprod. Fertil. Dev. 1991, 3, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Newby, N.C.; Leslie, K.E.; Dingwell, H.D.P.; Kelton, D.F.; Weary, D.M.; Neuder, L.; Millman, S.T.; Duffield, T.F. The effects of periparturient administration of flunixin meglumine on the health and production of dairy cattle. J. Dairy Sci. 2017, 100, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Pond, C.M.; Newsholme, E.A. Coping with metabolic stress in wild and domesticated animals. Bsap Occas. Publ. 1999, 24, 9–20. [Google Scholar] [CrossRef]
- Liu, S.M.; Smith, T.L.; Karlsson, L.J.E.; Palmer, D.G.; Besier, R.B. The costs for protein and energy requirements by nematode infection and resistance in Merino sheep. Livest. Prod. Sci. 2005, 97, 131–139. [Google Scholar] [CrossRef]
- Rathmell, J.C.; Fox, C.J.; Plas, D.R.; Hammerman, P.S.; Cinalli, R.M.; Thompson, C.B. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol. Cell. Biol. 2003, 23, 7315–7328. [Google Scholar] [CrossRef]
- Galvão, K.N.; Flaminio, M.J.B.F.; Brittin, S.B.; Sper, R.; Fraga, M.; Caixeta, L.; Ricci, A.; Guard, C.L.; Butler, W.R.; Gilbert, R.O. Association between uterine disease and indicators of neutrophil and systemic energy status in lactating Holstein cows. J. Dairy Sci. 2010, 93, 2926–2937. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids, dietary lipids and lymphocyte functions. Biochem. Soc. Trans. 1995, 23, 302–309. [Google Scholar] [CrossRef]
- Castell, L.M.; Newsholme, E.A. Glutamine and the effects of exhaustive exercise upon the immune response. Can. J. Physiol. Pharmacol. 1998, 76, 524–532. [Google Scholar] [CrossRef]
- Gross, J.J.; Grossen-Rösti, L.; Héritier, R.; Tröscher, A.; Bruckmaier, R.M. Inflammatory and metabolic responses to an intramammary lipopolysaccharide challenge in early lactating cows supplemented with conjugated linoleic acid. J. Anim. Physiol. Anim. Nutr. 2018, 102, e838–e848. [Google Scholar] [CrossRef] [PubMed]
- Noleto, P.G.; Saut, J.P.E.; Sheldon, I.M. Short communication: Glutamine modulates inflammatory responses to lipopolysaccharide in ex vivo bovine endometrium. J. Dairy Sci. 2017, 100, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.T. On the Treatment of Glycosuria and Diabetes Mellitus with Sodium Salicylate. Br. Med. J. 1901, 1, 760–762. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed]
- Horst, E.A.; Kvidera, S.K.; Dickson, M.J.; McCarthy, C.S.; Mayorga, E.J.; Al-Qaisi, M.; Ramirez, H.A.; Keating, A.F.; Baumgard, L.H. Effects of continuous and increasing lipopolysaccharide infusion on basal and stimulated metabolism in lactating Holstein cows. J. Dairy Sci. 2019, 102, 3584–3597. [Google Scholar] [CrossRef]
- Zhao, F.-Q.; Keating, A.F. Expression and regulation of glucose transporters in the bovine mammary gland. J. Dairy Sci. 2007, 90 (Suppl. 1), E76–E86. [Google Scholar] [CrossRef]
- Eger, M.; Hussen, J.; Koy, M.; Danicke, S.; Schuberth, H.-J.; Breves, G. Glucose transporter expression differs between bovine monocyte and macrophage subsets and is influenced by milk production. J. Dairy Sci. 2016, 99, 2276–2287. [Google Scholar] [CrossRef]
- Maratou, E.; Dimitriadis, G.; Kollias, A.; Boutati, E.; Lambadiari, V.; Mitrou, P.; Raptis, S.A. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur. J. Clin. Investig. 2007, 37, 282–290. [Google Scholar] [CrossRef]
- Mamedova, L.K.; Yuan, K.; Laudick, A.N.; Fleming, S.D.; Mashek, D.G.; Bradford, B.J. Toll-like receptor 4 signaling is required for induction of gluconeogenic gene expression by palmitate in human hepatic carcinoma cells. J. Nutr. Biochem. 2013, 24, 1499–1507. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic shifts in immunity and inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Wannemacher, R.W.; Beall, F.A.; Canonico, P.G.; Dinterman, R.E.; Hadick, C.L.; Neufeld, H.A. Glucose and alanine metabolism during bacterial infections in rats and rhesus monkeys. Metabolism 1980, 29, 201–212. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.J.; Bruckmaier, R.M. Invited review: Metabolic challenges and adaptation during different functional stages of the mammary gland in dairy cows: Perspectives for sustainable milk production. J. Dairy Sci. 2019, 102, 2828–2843. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, M.; Minuti, A.; Piccioli-Cappelli, F.; Amadori, M.; Bionaz, M.; Trevisi, E. The role of altered immune function during the dry period in promoting the development of subclinical ketosis in early lactation. J. Dairy Sci. 2019. [Google Scholar] [CrossRef]
- Gao, Y.; Lin, X.; Shi, K.; Yan, Z.; Wang, Z. Bovine mammary gene expression profiling during the onset of lactation. PLoS ONE 2013, 8, e70393. [Google Scholar] [CrossRef]
- Goff, J.P.; Horst, R.L. Physiological Changes at Parturition and Their Relationship to Metabolic Disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Kehrli, M.E.; Nonnecke, B.J.; Roth, J.A. Alterations in bovine lymphocyte function during the periparturient period. Am. J. Vet. Res. 1989, 50, 215–220. [Google Scholar]
- van Kampen, C.; Mallard, B.A. Effects of peripartum stress and health on circulating bovine lymphocyte subsets. Vet. Immunol. Immunopathol. 1997, 59, 79–91. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Koiwa, M.; Fukuda, S.; Satoh, Y.; Hayashi, T.; Hoshi, F.; Yoshino, T.-O.; Kawamura, S.-I. Changes in peripheral leukocyte subsets in dairy cows with inflammatory diseases after calving. J. Vet. Med. Sci. 2004, 66, 905–909. [Google Scholar] [CrossRef]
- Lamote, I.; Meyer, E.; Duchateau, L.; Burvenich, C. Influence of 17β-Estradiol, Progesterone, and Dexamethasone on Diapedesis and Viability of Bovine Blood Polymorphonuclear Leukocytes. J. Dairy Sci. 2004, 87, 3340–3349. [Google Scholar] [CrossRef]
- Saama, P.M.; Jacob, J.B.; Kehrli, M.E.; Freeman, A.E.; Kelm, S.C.; Kuck, A.L.; Tempelman, R.J.; Burton, J.L. Genetic Variation in Bovine Mononuclear Leukocyte Responses to Dexamethasone. J. Dairy Sci. 2004, 87, 3928–3937. [Google Scholar] [CrossRef]
- Burton, J.L.; Madsen, S.A.; Chang, L.-C.; Weber, P.S.D.; Buckham, K.R.; van Dorp, R.; Hickey, M.-C.; Earley, B. Gene expression signatures in neutrophils exposed to glucocorticoids: A new paradigm to help explain “neutrophil dysfunction” in parturient dairy cows. Vet. Immunol. Immunopathol. 2005, 105, 197–219. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Minuti, A.; Trevisi, E. Assessment of immune response in periparturient dairy cows using ex vivo whole blood stimulation assay with lipopolysaccharides and carrageenan skin test. Vet. Immunol. Immunopathol. 2015, 165, 119–126. [Google Scholar] [CrossRef]
- Lee, E.K.; Kehrli, M.E. Expression of adhesion molecules on neutrophils of periparturient cows and neonatal calves. Am. J. Vet. Res. 1998, 59, 37–43. [Google Scholar]
- Kimura, K.; Goff, J.P.; Kehrli, M.E. Effects of the presence of the mammary gland on expression of neutrophil adhesion molecules and myeloperoxidase activity in periparturient dairy cows. J. Dairy Sci. 1999, 82, 2385–2392. [Google Scholar] [CrossRef]
- Kimura, K.; Goff, J.P.; Kehrli, M.E.; Harp, J.A.; Nonnecke, B.J. Effects of Mastectomy on Composition of Peripheral Blood Mononuclear Cell Populations in Periparturient Dairy Cows1. J. Dairy Sci. 2002, 85, 1437–1444. [Google Scholar] [CrossRef]
- Nonnecke, B.J.; Kimura, K.; Goff, J.P.; Kehrli, M.E. Effects of the Mammary Gland on Functional Capacities of Blood Mononuclear Leukocyte Populations from Periparturient Cows1. J. Dairy Sci. 2003, 86, 2359–2368. [Google Scholar] [CrossRef]
- Minuti, A.; Bionaz, M.; Lopreiato, V.; Janovick, N.A.; Rodriguez-Zas, S.L.; Drackley, J.K.; Loor, J.J. Prepartum dietary energy intake alters adipose tissue transcriptome profiles during the periparturient period in Holstein dairy cows. J. Anim. Sci. Biotechnol. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Minuti, A.; Jahan, N.; Lopreiato, V.; Piccioli-Cappelli, F.; Bomba, L.; Capomaccio, S.; Loor, J.J.; Ajmone-Marsan, P.; Trevisi, E. Evaluation of circulating leukocyte transcriptome and its relationship with immune function and blood markers in dairy cows during the transition period. Funct. Integr. Genom. 2020, 20, 293–305. [Google Scholar] [CrossRef]
- Contreras, G.A.; Strieder-Barboza, C.; De Koster, J. Symposium review: Modulating adipose tissue lipolysis and remodeling to improve immune function during the transition period and early lactation of dairy cows. J. Dairy Sci. 2018, 101, 2737–2752. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Association of oxidative status and insulin sensitivity in periparturient dairy cattle: An observational study. J. Anim. Physiol. Anim. Nutr. 2016, 100, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, X.; Li, Y.; Li, N.; Shi, X.; Ding, H.; Zhang, Y.; Li, X.; Liu, G.; Wang, Z. Non-esterified fatty acids activate the ROS-p38-p53/Nrf2 signaling pathway to induce bovine hepatocyte apoptosis in vitro. Apoptosis 2014, 19, 984–997. [Google Scholar] [CrossRef]
- Contreras, G.A.; O’Boyle, N.J.; Herdt, T.H.; Sordillo, L.M. Lipomobilization in periparturient dairy cows influences the composition of plasma nonesterified fatty acids and leukocyte phospholipid fatty acids. J. Dairy Sci. 2010, 93, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Olsen, J.R.; Jeffress, E.J.; Moore, D.A.; Kastelic, J.P. Associations among serum pro-and anti-inflammatory cytokines, metabolic mediators, body condition, and uterine disease in postpartum dairy cows. Reprod. Biol. Endocrinol. 2013, 11, 103. [Google Scholar] [CrossRef]
- Mecitoglu, Z.; Senturk, S.; Akgul, G.; Udum, D.; Uzabacı, E.; Kasap, S.; Catik, S. Changes in circulating adiponectin and tumour necrosis factor-α and their relationship with insulin resistance in periparturient dairy cows. J. Vet. Res. 2016, 60, 163–167. [Google Scholar] [CrossRef]
- Loor, J.J.; Dann, H.M.; Everts, R.E.; Oliveira, R.; Green, C.A.; Guretzky, N.A.J.; Rodriguez-Zas, S.L.; Lewin, H.A.; Drackley, J.K. Temporal gene expression profiling of liver from periparturient dairy cows reveals complex adaptive mechanisms in hepatic function. Physiol. Genom. 2005, 23, 217–226. [Google Scholar] [CrossRef]
- Wathes, D.C.; Cheng, Z.; Chowdhury, W.; Fenwick, M.A.; Fitzpatrick, R.; Morris, D.G.; Patton, J.; Murphy, J.J. Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Physiol. Genom. 2009, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moyes, K.M.; Drackley, J.K.; Morin, D.E.; Rodriguez-Zas, S.L.; Everts, R.E.; Lewin, H.A.; Loor, J.J. Mammary gene expression profiles during an intramammary challenge reveal potential mechanisms linking negative energy balance with impaired immune response. Physiol. Genom. 2010, 41, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Moyes, K.M.; Drackley, J.K.; Salak-Johnson, J.L.; Morin, D.E.; Hope, J.C.; Loor, J.J. Dietary-induced negative energy balance has minimal effects on innate immunity during a Streptococcus uberis mastitis challenge in dairy cows during midlactation. J. Dairy Sci. 2009, 92, 4301–4316. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.H.; VandeHaar, M.J.; Burton, J.L.; Liesman, J.S.; Erskine, R.J.; Elsasser, T.H. Clinical responses to intramammary endotoxin infusion in dairy cows subjected to feed restriction. J. Dairy Sci. 2002, 85, 1724–1731. [Google Scholar] [CrossRef]
- Perkins, K.H.; VandeHaar, M.J.; Tempelman, R.J.; Burton, J.L. Negative energy balance does not decrease expression of leukocyte adhesion or antigen-presenting molecules in cattle. J. Dairy Sci. 2001, 84, 421–428. [Google Scholar] [CrossRef]
- Bjerre-Harpøth, V.; Friggens, N.C.; Thorup, V.M.; Larsen, T.; Damgaard, B.M.; Ingvartsen, K.L.; Moyes, K.M. Metabolic and production profiles of dairy cows in response to decreased nutrient density to increase physiological imbalance at different stages of lactation. J. Dairy Sci. 2012, 95, 2362–2380. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; van Dorland, H.; Bruckmaier, R.M.; Schwarz, F. Performance and metabolic profile of dairy cows during a lactational and deliberately induced negative energy balance with subsequent realimentation. J. Dairy Sci. 2011, 94, 1820–1830. [Google Scholar] [CrossRef]
- Eger, M.; Hussen, J.; Drong, C.; Meyer, U.; von Soosten, D.; Frahm, J.; Daenicke, S.; Breves, G.; Schuberth, H.-J. Impacts of parturition and body condition score on glucose uptake capacity of bovine monocyte subsets. Vet. Immunol. Immunopathol. 2015, 166, 33–42. [Google Scholar] [CrossRef]
- Bouvier-Muller, J.; Allain, C.; Tabouret, G.; Enjalbert, F.; Portes, D.; Noirot, C.; Rupp, R.; Foucras, G. Whole blood transcriptome analysis reveals potential competition in metabolic pathways between negative energy balance and response to inflammatory challenge. Sci. Rep. 2017, 7, 2379. [Google Scholar] [CrossRef]
- Shin, D.H.; Jeong, J.K.; Choi, I.S.; Moon, S.H.; Lee, S.C.; Kang, H.G.; Park, S.B.; Kim, I.H. Associations between serum haptoglobin concentration and peri-and postpartum disorders, milk yield, and reproductive performance in dairy cows. Livest. Sci. 2018, 213, 14–18. [Google Scholar] [CrossRef]
- Winkler, A.; Gessner, D.K.; Koch, C.; Romberg, F.-J.; Dusel, G.; Herzog, E.; Most, E.; Eder, K. Effects of a plant product consisting of green tea and curcuma extract on milk production and the expression of hepatic genes involved in endoplasmic stress response and inflammation in dairy cows. Arch. Anim. Nutr. 2015, 69, 425–441. [Google Scholar] [CrossRef]
- Carpenter, A.J.; Ylioja, C.M.; Vargas, C.F.; Mamedova, L.K.; Mendonça, L.G.; Coetzee, J.F.; Hollis, L.C.; Gehring, R.; Bradford, B.J. Hot topic: Early postpartum treatment of commercial dairy cows with nonsteroidal antiinflammatory drugs increases whole-lactation milk yield. J. Dairy Sci. 2016, 99, 672–679. [Google Scholar] [CrossRef]
- Gross, J.; van Dorland, H.; Wellnitz, O.; Bruckmaier, R.M. Glucose transport and milk secretion during manipulated plasma insulin and glucose concentrations and during LPS-induced mastitis in dairy cows. J. Anim. Physiol. Anim. Nutr. 2014, 99. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.S.; Lohakare, J.; Bionaz, M. Biosynthesis of milk fat, protein, and lactose: Roles of transcriptional and posttranscriptional regulation. Physiol. Genom. 2016, 48, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Kreipe, L.; Vernay, M.C.M.B.; Oppliger, A.; Wellnitz, O.; Bruckmaier, R.M.; van Dorland, H.A. Induced hypoglycemia for 48 hours indicates differential glucose and insulin effects on liver metabolism in dairy cows. J. Dairy Sci. 2011, 94, 5435–5448. [Google Scholar] [CrossRef] [PubMed]
- Lindmark-Månsson, H.; Bränning, C.; Aldén, G.; Paulsson, M. Relationship between somatic cell count, individual leukocyte populations and milk components in bovine udder quarter milk. Int. Dairy J. 2006, 16, 717–727. [Google Scholar] [CrossRef]
- Moyes, K.M.; Drackley, J.K.; Morin, D.E.; Bionaz, M.; Rodriguez-Zas, S.L.; Everts, R.E.; Lewin, H.A.; Loor, J.J. Gene network and pathway analysis of bovine mammary tissue challenged with Streptococcus uberis reveals induction of cell proliferation and inhibition of PPARgamma signaling as potential mechanism for the negative relationships between immune response and lipid metabolism. BMC Genom. 2009, 10, 542. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, K.; Liu, J. Effects of glucose availability on expression of the key genes involved in synthesis of milk fat, lactose and glucose metabolism in bovine mammary epithelial cells. PLoS ONE 2013, 8, e66092. [Google Scholar] [CrossRef]
- Lehtolainen, T.; Suominen, S.; Kutila, T.; Pyörälä, S. Effect of Intramammary Escherichia coli Endotoxin in Early- vs. Late-Lactating Dairy Cows. J. Dairy Sci. 2003, 86, 2327–2333. [Google Scholar] [CrossRef]
- Shuster, D.E.; Lee, E.K.; Kehrli, M.E. Bacterial growth, inflammatory cytokine production, and neutrophil recruitment during coliform mastitis in cows within ten days after calving, compared with cows at midlactation. Am. J. Vet. Res. 1996, 57, 1569–1575. [Google Scholar]
- Yuan, K.; Farney, J.K.; Mamedova, L.K.; Sordillo, L.M.; Bradford, B.J. TNFα altered inflammatory responses, impaired health and productivity, but did not affect glucose or lipid metabolism in early-lactation dairy cows. PLoS ONE 2013, 8, e80316. [Google Scholar] [CrossRef]
- Hoeben, D.; Burvenich, C.; Trevisi, E.; Bertoni, G.; Hamann, J.; Bruckmaier, R.M.; Blum, J.W. Role of endotoxin and TNF-alpha in the pathogenesis of experimentally induced coliform mastitis in periparturient cows. J. Dairy Res. 2000, 67, 503–514. [Google Scholar] [CrossRef]
- Dickson, M.J.; Kvidera, S.K.; Horst, E.A.; Wiley, C.E.; Mayorga, E.J.; Ydstie, J.; Perry, G.A.; Baumgard, L.H.; Keating, A.F. Impacts of chronic and increasing lipopolysaccharide exposure on production and reproductive parameters in lactating Holstein dairy cows. J. Dairy Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.A.; Mamedova, L.K.; Minton, J.E.; Jones, M.L.; Carroll, J.A.; Bradford, B.J. Continuous low-dose infusion of tumor necrosis factor alpha in adipose tissue elevates adipose tissue interleukin 10 abundance and fails to alter metabolism in lactating dairy cows. J. Dairy Sci. 2014, 97, 4897–4906. [Google Scholar] [CrossRef] [PubMed]
- Snijders, S.E.M.; Dillon, P.G.; O’Farrell, K.J.; Diskin, M.; Wylie, A.R.G.; O’Callaghan, D.; Rath, M.; Boland, M.P. Genetic merit for milk production and reproductive success in dairy cows. Anim. Reprod. Sci. 2001, 65, 17–31. [Google Scholar] [CrossRef]
- Veerkamp, R.F.; Beerda, B.; van der Lende, T. Effects of genetic selection for milk yield on energy balance, levels of hormones, and metabolites in lactating cattle, and possible links to reduced fertility. Livest. Prod. Sci. 2003, 83, 257–275. [Google Scholar] [CrossRef]
- Chagas, L.M.; Lucy, M.C.; Back, P.J.; Blache, D.; Lee, J.M.; Gore, P.J.S.; Sheahan, A.J.; Roche, J.R. Insulin resistance in divergent strains of Holstein-Friesian dairy cows offered fresh pasture and increasing amounts of concentrate in early lactation. J. Dairy Sci. 2009, 92, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Bossaert, P.; Leroy, J.L.M.R.; De Campeneere, S.; de Vliegher, S.; Opsomer, G. Differences in the glucose-induced insulin response and the peripheral insulin responsiveness between neonatal calves of the Belgian Blue, Holstein-Friesian, and East Flemish breeds. J. Dairy Sci. 2009, 92, 4404–4411. [Google Scholar] [CrossRef] [PubMed]
- Hart, I.; Bines, J.; Morant, S.; Ridley, J. Endocrine control of energy metabolism in the cow: Comparison of the level of hormones (prolactin, growth hormone, insulin and thyroxine) and metabolites in the plasma of high- and low-yielding cattle at various stages of lactation. J. Endocrinol. 1978, 77, 333–345. [Google Scholar] [CrossRef]
- Newsholme, P.; Costa Rosa, L.F.; Newsholme, E.A.; Curi, R. The importance of fuel metabolism to macrophage function. Cell Biochem. Funct. Cell. Biochem. Its Modul. Act. Agents Dis. 1996, 14, 1–10. [Google Scholar] [CrossRef]
- Alba-Loureiro, T.C.; Munhoz, C.D.; Martins, J.O.; Cerchiaro, G.A.; Scavone, C.; Curi, R.; Sannomiya, P. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz. J. Med. Biol. Res. 2007, 40, 1037–1044. [Google Scholar] [CrossRef]
- Forsyth, I.A. The Insulin-Like Growth Factor and Epidermal Growth Factor Families in Mammary Cell Growth in Ruminants: Action and Interaction with Hormones. J. Dairy Sci. 1996, 79, 1085–1096. [Google Scholar] [CrossRef]
- Flint, D.J.; Gardner, M. Evidence that growth hormone stimulates milk synthesis by direct action on the mammary gland and that prolactin exerts effects on milk secretion by maintenance of mammary deoxyribonucleic acid content and tight junction status. Endocrinology 1994, 135, 1119–1124. [Google Scholar] [CrossRef]
- Welniak, L.A.; Sun, R.; Murphy, W.J. The role of growth hormone in T-cell development and reconstitution. J. Leukoc. Biol. 2002, 71, 381–387. [Google Scholar] [PubMed]
- Okamura, C.S.; Bader, J.F.; Keisler, D.H.; Lucy, M.C. Growth hormone receptor expression in two dairy breeds during the periparturient period. J. Dairy Sci. 2009, 92, 2706–2710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lucy, M.C.; Crooker, B.A.; Beal, W.E. Expression of growth hormone receptor 1A mRNA is decreased in dairy cows but not in beef cows at parturition. J. Dairy Sci. 2005, 88, 1370–1377. [Google Scholar] [CrossRef]
- Trevisi, E.; Minuti, A. Assessment of the innate immune response in the periparturient cow. Res. Vet. Sci. 2018, 116, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Beever, D.E. The impact of controlled nutrition during the dry period on dairy cow health, fertility and performance. Anim. Reprod. Sci. 2006, 96, 212–226. [Google Scholar] [CrossRef]
- Mann, S.; Leal Yepes, F.A.; Duplessis, M.; Wakshlag, J.J.; Overton, T.R.; Cummings, B.P.; Nydam, D.V. Dry period plane of energy: Effects on glucose tolerance in transition dairy cows. J. Dairy Sci. 2016, 99, 701–717. [Google Scholar] [CrossRef]
- Lucy, M.C.; Escalante, R.C.; Keisler, D.H.; Lamberson, W.R.; Mathew, D.J. Short communication: Glucose infusion into early postpartum cows defines an upper physiological set point for blood glucose and causes rapid and reversible changes in blood hormones and metabolites. J. Dairy Sci. 2013, 96, 5762–5768. [Google Scholar] [CrossRef]
- Mesgaran, M.D.; Koolabadi, G.; Banikamalil, A.; Mesgaran, S.D. Effect on animal performance of the source of energy (glucogenic vs. lipogenic) of early lactating dairy cow diets with similar content of metabolizable energy. In Energy and Protein Metabolism and Nutrition. 3rd EAAP International Symposium on Energy and Protein Metabolism and Nutrition, Parma, Italy, 6–10 September 2010; Wageningen Academic Publishers: Wageningen, The Netherlands, 2010; pp. 335–336. ISBN 9789086861538. [Google Scholar]
- Kokkonen, T.; Taponen, J.; Anttila, T.; Syrjala-Qvist, L.; Delavaud, C.; Chilliard, Y.; Tuori, M.; Tesfa, A.T. Effect of body fatness and glucogenic supplement on lipid and protein mobilization and plasma leptin in dairy cows. J. Dairy Sci. 2005, 88, 1127–1141. [Google Scholar] [CrossRef]
- van Knegsel, A.T.M.; van den Brand, H.; Dijkstra, J.; van Straalen, W.M.; Heetkamp, M.J.W.; Tamminga, S.; Kemp, B. Dietary Energy Source in Dairy Cows in Early Lactation: Energy Partitioning and Milk Composition. J. Dairy Sci. 2007, 90, 1467–1476. [Google Scholar] [CrossRef]
- Bar-Pelled, U.; Maltz, E.; Bruckental, I.; Folman, Y.; Kali, Y.; Gacitua, H.; Lehrer, A.R.; Knight, C.H.; Robinson, B.; Voet, H.; et al. Relationship Between Frequent Milking or Suckling in Early Lactation and Milk Production of High Producing Dairy Cows1. J. Dairy Sci. 1995, 78, 2726–2736. [Google Scholar] [CrossRef]
- Stelwagen, K.; Phyn, C.V.C.; Davis, S.R.; Guinard-Flament, J.; Pomiès, D.; Roche, J.R.; Kay, J.K. Invited review: Reduced milking frequency: Milk production and management implications. J. Dairy Sci. 2013, 96, 3401–3413. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, P.; Vanacker, N.; Ollier, S.; Ster, C. Innovative dairy cow management to improve resistance to metabolic and infectious diseases during the transition period. Res. Vet. Sci. 2018, 116, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.; Garcia, M.; Ylioja, C.; Mamedova, L.K.; Bradford, B.J. Effects of anti-inflammatory treatment and milking frequency on mRNA abundance of adipose tissue from early lactation cows. In Abstracts of the 2018 American Dairy Science Association Annual Meeting, Proceedings of the American Dairy Science Association Annual Meeting 2018, Knoxville, TN, USA, 24–27 June 2018; Lucy, M.C., Ed.; Journal of Dairy Science: Champaign, IL, USA, 2018; p. 61820. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habel, J.; Sundrum, A. Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows. Animals 2020, 10, 1028. https://doi.org/10.3390/ani10061028
Habel J, Sundrum A. Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows. Animals. 2020; 10(6):1028. https://doi.org/10.3390/ani10061028
Chicago/Turabian StyleHabel, Jonas, and Albert Sundrum. 2020. "Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows" Animals 10, no. 6: 1028. https://doi.org/10.3390/ani10061028
APA StyleHabel, J., & Sundrum, A. (2020). Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows. Animals, 10(6), 1028. https://doi.org/10.3390/ani10061028



