Use of Propylene-Glycol as a Cosolvent for GnRH in Synchronization of Estrus and Ovulation in Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Issues
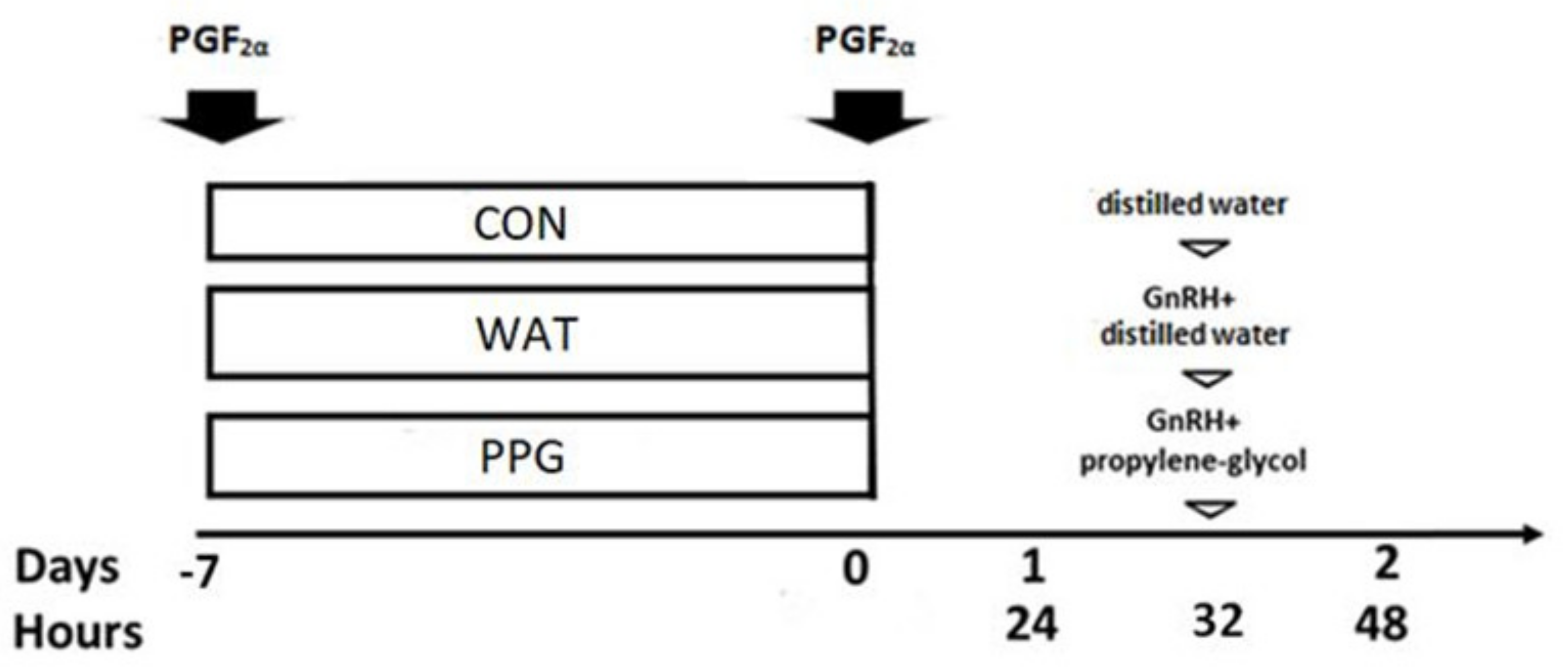
2.2. Experimental Design
2.3. Occurrence and Timing of Estrus Behavior
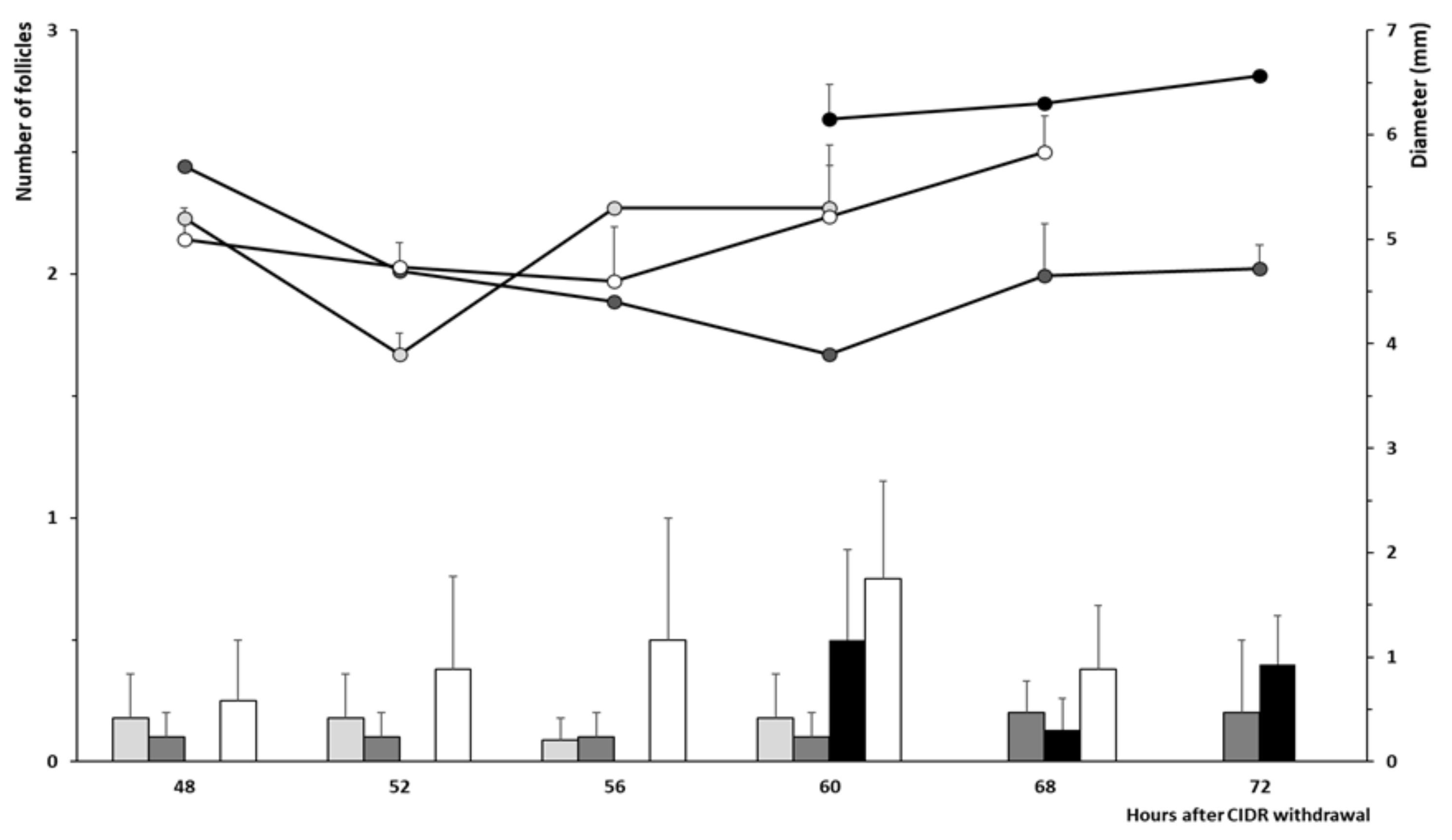
2.4. Development of Preovulatory Follicles during the Follicular Phase and Timing of Ovulation
2.5. Plasma Estradiol Concentrations During the Follicular Phase
2.6. Number and Functionality of Corpora Lutea
2.7. Statistical Analysis
3. Results
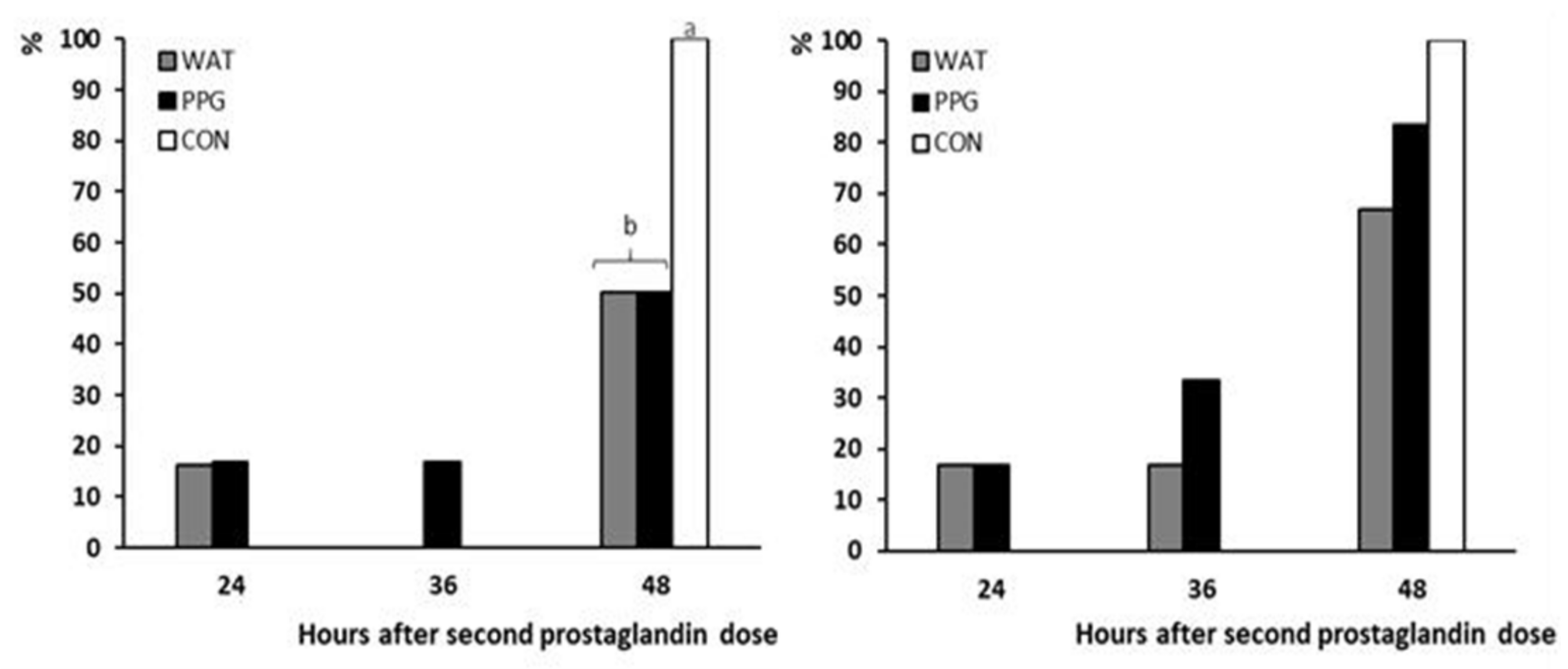
3.1. Trial 1: Effect of GnRH Administration in Prostaglandin-Based Protocols
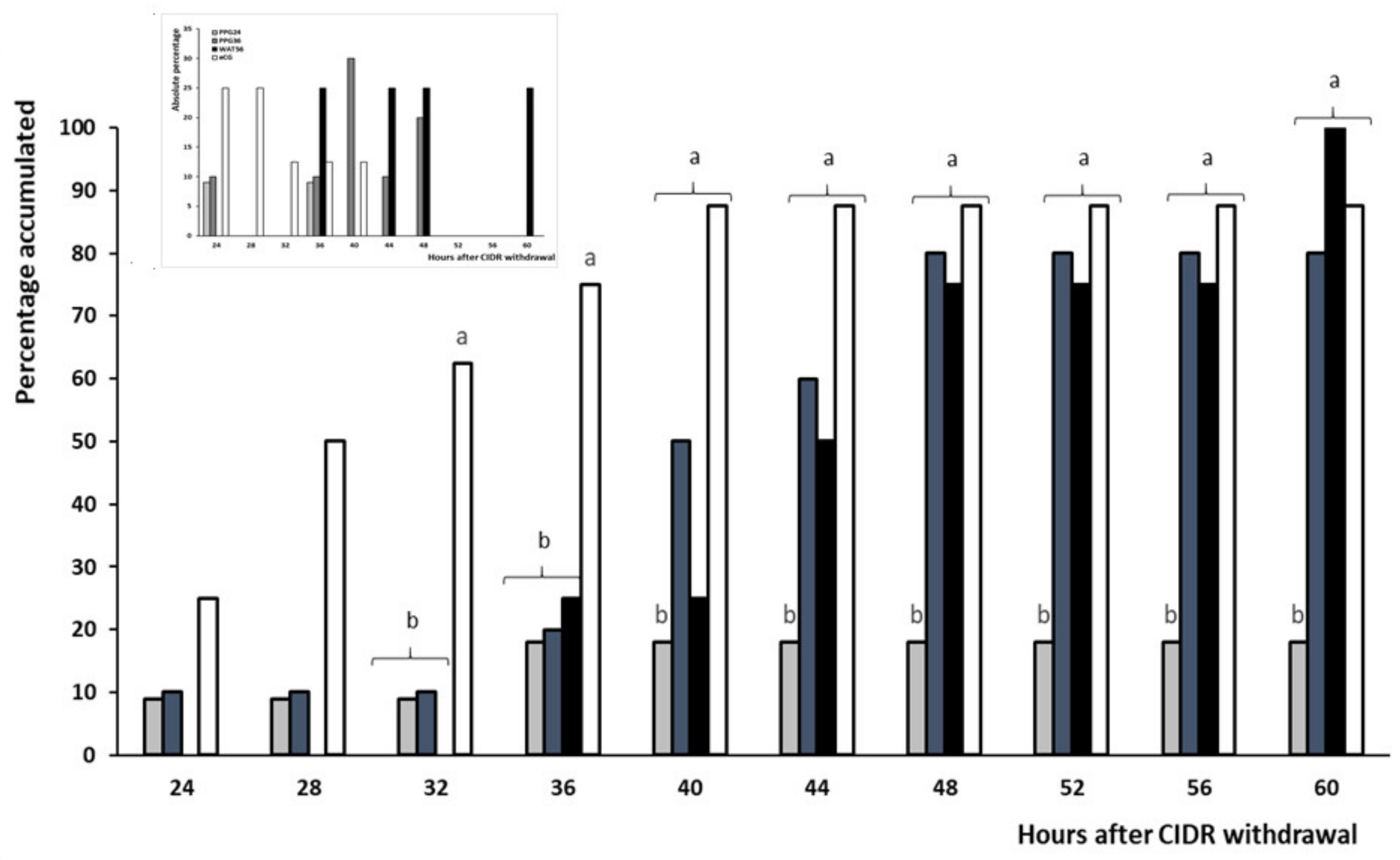
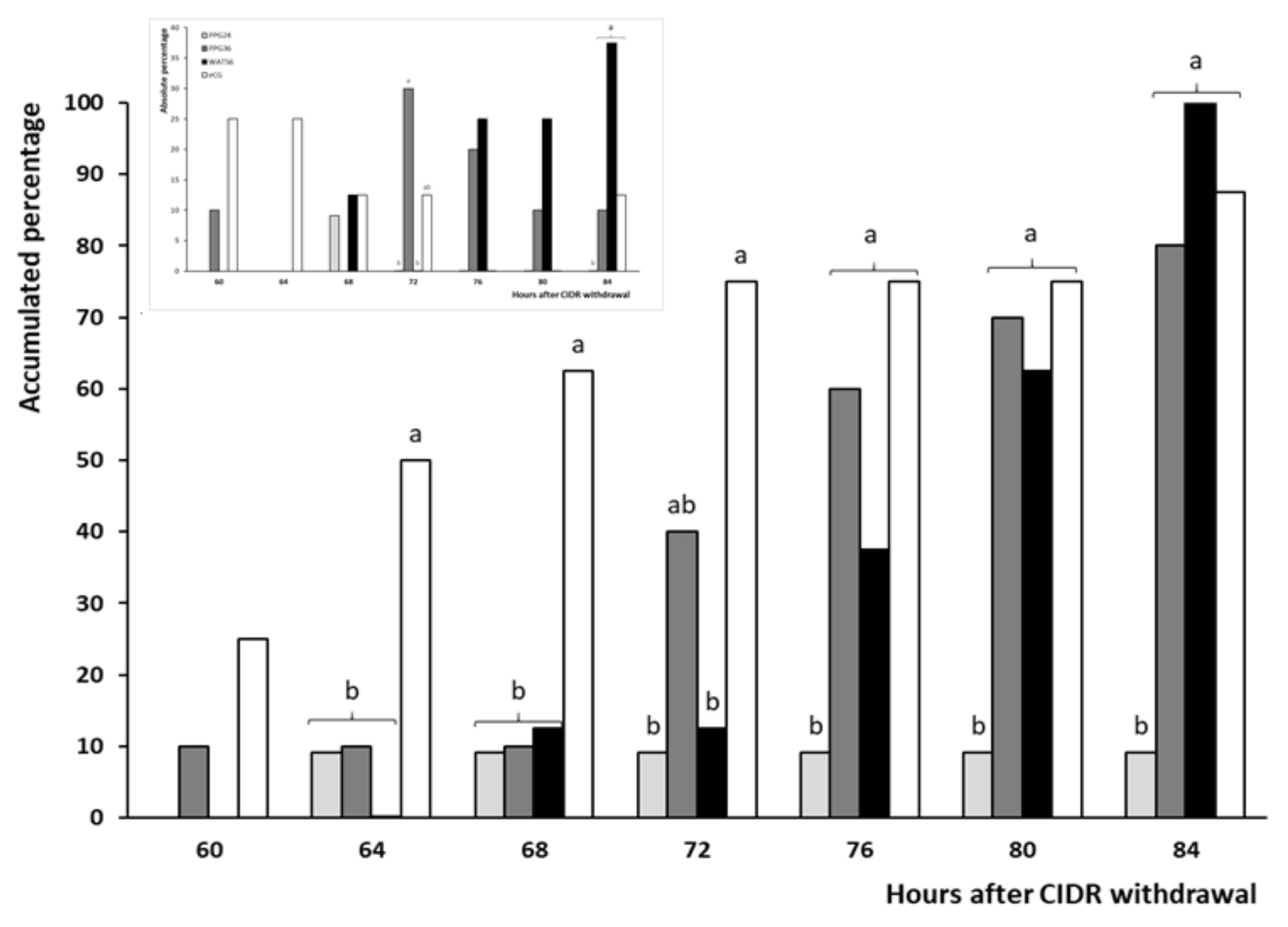
3.2. Trial 2: Efficiency and Timing of GnRH Administration in Progesterone-Based Protocols
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abecia, J.A.; Forcada, F.; Gonzalez-Bulnes, A. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 2012, 130, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Dutt, R.H. Induction of oestrus and ovulation in anestrual ewes by the use of progesterone and pregnant mares serum. J. Anim. Sci. 1952, 11, 792. [Google Scholar]
- Robinson, T.J. Role of progesterone in the mating behaviour of the ewe. Nature 1952, 170, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.J. Use of progestagen-impregnated sponges inserted intravaginally or subcutaneously for the control of the oestrous cycle in the sheep. Nature 1965, 206, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.N. Identification of progestogens of high activity for the control of the oestrous cycle in the sheep. Nature 1965, 206, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Manteca-Vilanova, X.; De Briyne, N.; Beaver, B.; Turner, P.V. Horse welfare during equine Chorionic Gonadotropin (eCG) production. Animals 2019, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Bulnes, A.; Menchaca, A.; Martin, G.B.; Martinez-Ros, P. Seventy years of progestagen treatments for management of the sheep oestrous cycle: Where we are and where we should go. Reprod. Fertil. Dev. 2020, 32, 441–452. [Google Scholar] [CrossRef]
- Martinez-Ros, P.; Gonzalez-Bulnes, A. Efficiency of CIDR-based protocols including GnRH instead of eCG for estrus synchronization in sheep. Animals 2019, 9, 146. [Google Scholar] [CrossRef] [Green Version]
- Eppleston, J.; Evans, G.; Roberts, E.M. Effect of time of PMSG and GnRH on the time of ovulation, LH secretion and reproductive performance after intrauterine insemination with frozen ram semen. Anim. Reprod. Sci. 1991, 26, 227–237. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, A. Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. J. Hum. Reprod. Sci. 2014, 7, 170–174. [Google Scholar] [CrossRef]
- Baird, D.T. Factors regulating the growth of the preovulatory follicle in sheep and human. J. Reprod. Fertil. 1983, 69, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukanich, B.; Gehring, R.; Webb, A.I.; Craigmill, A.L.; Riviere, J.E. Effect of formulation and route of administration on tissue residues and withdrawal times. J. Am. Vet. Med. Assoc. 2005, 227, 1574–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klink, P.R.; Ferguson, T.H.; Magruder, J.A. Formulation of veterinary dosage forms. In Development and Formulation of Veterinary Dosage Forms, 2nd ed.; Hardee, G.E., Baggot, J.D., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 145–229. [Google Scholar]
- Baggot, J.D.; Brown, S.A. Basis for selection of the dosage form. In Development and Formulation of Veterinary Dosage Forms; Marcel Dekker: New York, NY, USA, 1998; pp. 7–43. [Google Scholar]
- Gao, P.; Guyton, M.E.; Huang, T.; Bauer, J.M.; Stefanski, K.J.; Lu, Q. Enhanced oral bioavailability of a poorly water soluble drug PNU-91325 by supersaturatable formulations. Drug Dev. Ind. Pharm. 2004, 30, 221–229. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S.; McKenna, E.A.; Hubner, R.P.; Tardiff, R.G. A review of the comparative mammalian toxicity of ethylene glycol and propylene glycol. Crit. Rev. Toxicol. 1999, 29, 331–365. [Google Scholar] [CrossRef]
- Lopez-Sebastian, A.; Gomez-Brunet, A.; Lishman, A.W.; Johnson, S.K.; Inskeep, E.K. Modification by propylene glycol of ovulation rate in ewes in response to a single injection of FSH. J. Reprod. Fertil. 1993, 99, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Solis, I.; Gomez-Brunet, A.; Encinas, T.; Gonzalez-Bulnes, A.; Santiago-Moreno, J.; Lopez-Sebastian, A. Influence of vehicle on kinetics of exogenous progesterone administered either by subcutaneous and intramuscular routes to sheep. Res. Vet. Sci. 2008, 85, 162–165. [Google Scholar] [CrossRef]
- Brazeau, A.G.; Cooper, B.; Svetic, K.A.; Smith, C.L.; Gupta, P. Current perspectives on pain upon injection of drugs. J. Pharm. Sci. 1998, 87, 667–677. [Google Scholar] [CrossRef]
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Encinas, T.; McNeilly, A.S.; Gonzalez-Bulnes, A. Timing of preovulatory LH surge and ovulation in superovulated sheep are affected by follicular status at start of the FSH treatment. Reprod. Domest. Anim. 2008, 43, 92–98. [Google Scholar] [CrossRef]
- Wildeus, S. Current concepts y synchronization of estrus: Sheep and goats. J. Anim. Sci. 2000, 77, 1–14. [Google Scholar] [CrossRef]
- Hay, M.F.; Moor, R.M. Functional and structural relationships in the Graafian follicle population of the sheep ovary. J. Reprod. Fertil. 1975, 45, 583–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martemucci, G.; D’Alessandro, A.G. Synchronization of oestrus and ovulation by short time combined FGA, PGF2α, GnRH, eCG treatments for natural service or AI fixed-time. Anim. Reprod. Sci. 2011, 123, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Muzante, J.; Gil, J.; Viñoles, C.; Fierro, S. Reproductive outcome with GnRH inclusion at 24 or 36 h following a prostaglandin F2α-based protocol for timed AI in ewes. Anim. Reprod. Sci. 2013, 138, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Veiga-Lopez, A.; Garcia, P.; Garcia-Garcia, R.M.; Ariznavarreta, C.; Sanchez, M.A.; Tresguerres, J.A.F.; Cocero, M.J.; Flores, J.M. Effects of progestagens and prostaglandin analogues on ovarian function and embryo viability in sheep. Theriogenology 2005, 63, 2523–2534. [Google Scholar] [CrossRef]
- Garcia-Palencia, P.; Sanchez, M.A.; Nieto, A.; Vilar, M.P.; Gonzalez, M.; Veiga-Lopez, A.; Gonzalez-Bulnes, A.; Flores, J.M. Sex steroid receptor expression in the oviduct and uterus of sheep with estrus synchronized with progestagen or prostaglandin analogues. Anim. Reprod. Sci. 2007, 97, 25–35. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, I.; Sanchez, M.A.; Garcia-Fernandez, R.A.; Garcia-Palencia, P.; Sanchez, B.; Letelier, C.A.; Gonzalez-Bulnes, A.; Flores, J.M. Endometrial expression of IFNAR-1 and oxytocin receptor (OTR) is not improved by prostaglandin analogues when compared to progestagens in ewes. Reprod. Domest. Anim. 2012, 47, 274–280. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, I.; Sanchez, M.A.; Garcia-Fernandez, R.A.; Garcia-Palencia, P.; Sanchez, B.; Gonzalez-Bulnes, A.; Flores, J.M. Different influence of ovine estrus synchronization treatments on caruncular early angiogenesis. Histol. Histopathol. 2013, 28, 373–383. [Google Scholar]
- Titi, H.H.; Kridli, R.T.; Alnimer, M.A. Estrus synchronization in sheep and goats using combinations of GnRH, progestagen and prostaglandin F2alpha. Reprod. Domest. Anim. 2010, 45, 594–599. [Google Scholar]
- Joseph, I.B.J.K.; Currie, W.D.; Rawlings, N.C. Effects of time after ovariectomy, season and oestradiol on luteinizing hormone and follicle-stimulating hormone secretion in ovariectomized ewes. J. Reprod. Fertil. 1992, 94, 511–523. [Google Scholar] [CrossRef] [Green Version]


| Group | PPG | WAT | CON |
|---|---|---|---|
| Occurrence of estrus (%) | 83.3 (5/6) a | 66.7 (4/6) b | 100 (6/6) a |
| Onset of estrus (h) | 40.8 ± 4.3 | 42.0 ± 4.8 | 48.0 ± 0.1 |
| Length of estrus (h) | 32.4 ± 8.0 | 27.0 ± 4.6 | 38.0 ± 4.8 |
| Plasma estradiol at estrus (pg/mL) | 3.0 ± 1.6 | 2.9 ± 0.2 | 5.5 ± 1.2 |
| Occurrence of ovulation (%) | 100 (6/6) a | 50 (3/6) b | 100 (6/6) a |
| Ovulation rate (n) | 1.6 ± 0.3 | 1.3 ± 0.2 | 1.5 ± 0.2 |
| Group | PPG24 | PPG36 | WAT56 | eCG |
|---|---|---|---|---|
| Occurrence of estrus (%) | 18.2 (2/11) b | 80.0 (8/10) a | 100 (8/8) a | 87.5 (7/8) a |
| Timing of estrus (h) | 30 ± 2.5 a | 40 ± 2.4 b | 47 ± 3.2 b | 30.1 ± 1.5 a |
| Occurrence of ovulation (%) | 100 (11/11) | 100 (10/10) | 100 (8/8) | 100 (8/8) |
| Timing of ovulation (h) | 68 a | 74.5 ± 2.3 a,b | 79.5 ± 2.2 b | 66.3 ± 3.8 a |
| Interval estrus-ovulation (h) | 40 | 34 ± 1.3 | 32.5 ± 1.9 | 36.1 ± 2.5 |
| Ovulation rate (n) | 1.7 ± 0.2 | 1.8 ± 0.2 | 1.6 ± 0.1 | 1.5 ± 0.2 |
| Plasma progesterone (ng/mL) | 4.5 ± 0.4 a | 6.1 ± 0.6 b | 5.1 ± 0.6 b | 5.3 ± 1.3 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Jimenez, Z.; Guillen-Gargallo, S.; Encinas, T.; Berlinguer, F.; Veliz-Deras, F.G.; Martinez-Ros, P.; Gonzalez-Bulnes, A. Use of Propylene-Glycol as a Cosolvent for GnRH in Synchronization of Estrus and Ovulation in Sheep. Animals 2020, 10, 897. https://doi.org/10.3390/ani10050897
Santos-Jimenez Z, Guillen-Gargallo S, Encinas T, Berlinguer F, Veliz-Deras FG, Martinez-Ros P, Gonzalez-Bulnes A. Use of Propylene-Glycol as a Cosolvent for GnRH in Synchronization of Estrus and Ovulation in Sheep. Animals. 2020; 10(5):897. https://doi.org/10.3390/ani10050897
Chicago/Turabian StyleSantos-Jimenez, Zurisaday, Sara Guillen-Gargallo, Teresa Encinas, Fiammetta Berlinguer, Francisco G. Veliz-Deras, Paula Martinez-Ros, and Antonio Gonzalez-Bulnes. 2020. "Use of Propylene-Glycol as a Cosolvent for GnRH in Synchronization of Estrus and Ovulation in Sheep" Animals 10, no. 5: 897. https://doi.org/10.3390/ani10050897
APA StyleSantos-Jimenez, Z., Guillen-Gargallo, S., Encinas, T., Berlinguer, F., Veliz-Deras, F. G., Martinez-Ros, P., & Gonzalez-Bulnes, A. (2020). Use of Propylene-Glycol as a Cosolvent for GnRH in Synchronization of Estrus and Ovulation in Sheep. Animals, 10(5), 897. https://doi.org/10.3390/ani10050897







