Combination of Bacillus licheniformis and Salinomycin: Effect on the Growth Performance and GIT Microbial Populations of Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Birds and Housing
2.3. Diets and Feeding Program
2.4. Bacillus licheniformis and Salinomycin Preparation
2.5. Data and Sample Collection
2.6. Bacterial DNA Extraction and Amplification
2.7. 16 SrDNA Sequencing
2.8. Metagenomic Analysis
2.9. Statistical Analysis
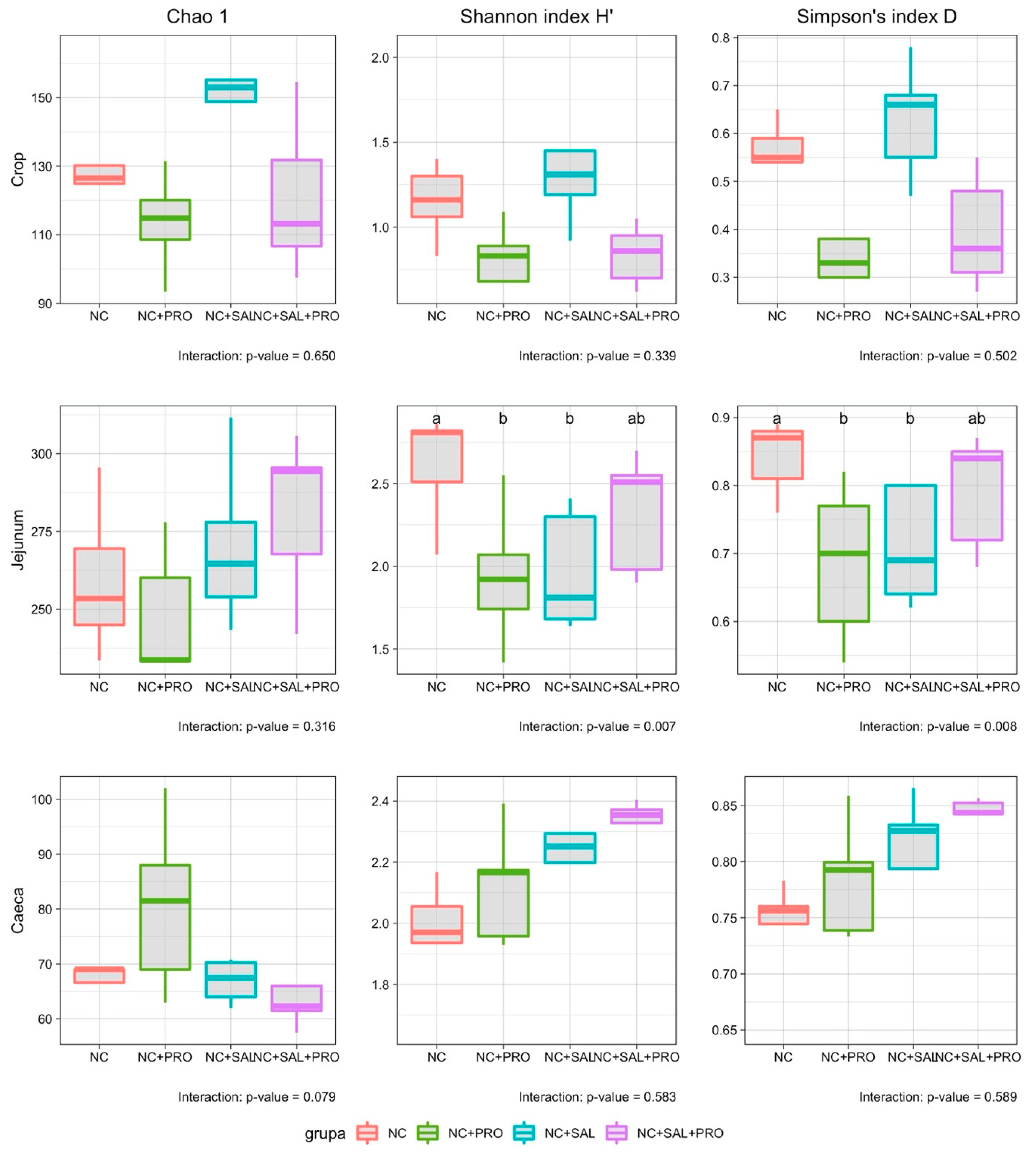
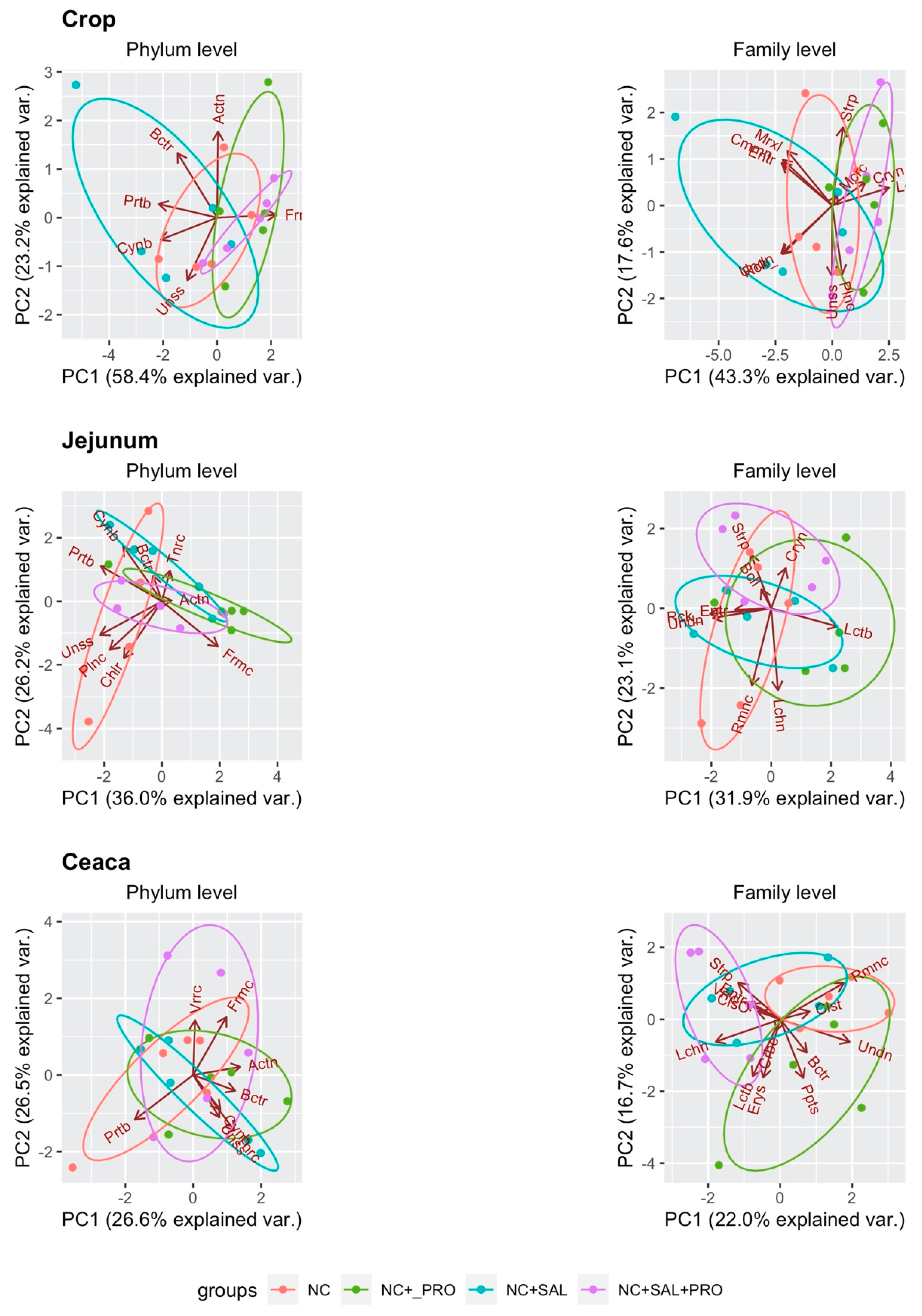
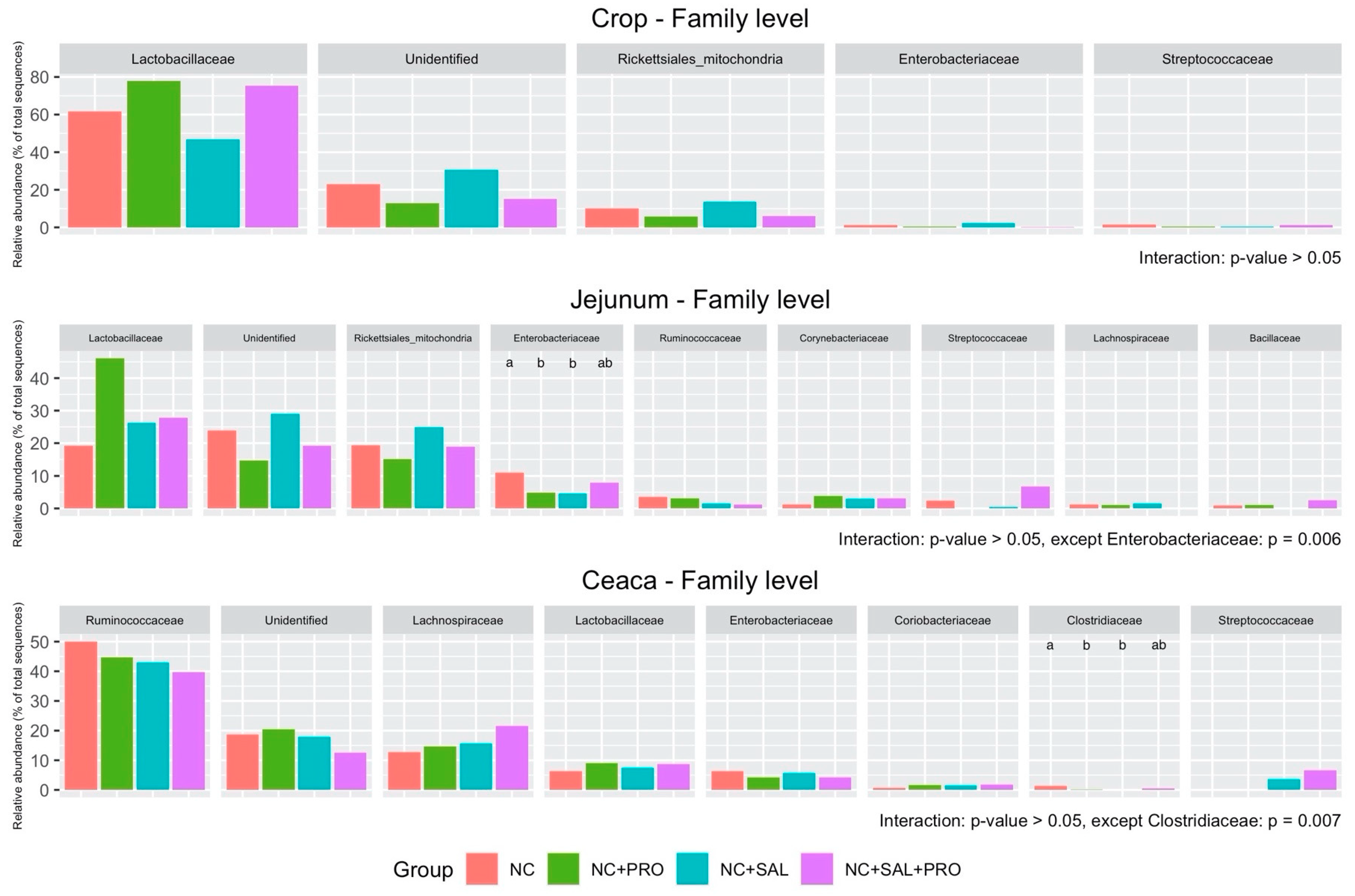
3. Results
3.1. Birds’ Performance
3.2. Morphometric Measurements
3.3. pH Value of Digesta
3.4. Qualitative Determination of the GIT Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Sachan, S.; Karthik, K.; Dhama, K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018, 25, 10611–10618. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.; Ali, S.M.; Mahmoodzadeh Hosseini, H.; Mirhosseini, S.A. A review of dietary probiotics in poultry. J. Appl. Biotechnol. Rep. 2018, 5, 48–54. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.R.; Martínez, M.A. Prebiotics and probiotics in feed and animal health. In Nutraceuticals in Veterinary Medicine; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 261–285. [Google Scholar]
- Kierończyk, B.; Sassek, M.; Pruszyńska-Oszmałek, E.; Kołodziejski, P.; Rawski, M.; Świątkiewicz, S.; Józefiak, D. The physiological response of broiler chickens to the dietary supplementation of the bacteriocin nisin and ionophore coccidiostats. Poult. Sci. 2017, 96, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Kierończyk, B.; Rawski, M.; Mikołajczak, Z.; Świątkiewicz, S.; Józefiak, D. Nisin as a novel feed additive: The effects on gut microbial modulation and activity, histological parameters, and growth performance of broiler chickens. Animals 2020, 10, 101. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Pandey, S.; Pandey, P. Promising future of probiotics for human health: Current scenario. Chron. Young Sci. 2012, 3, 17. [Google Scholar] [CrossRef]
- Hejdysz, M.; Kaczmarek, S.A.; Kubiś, M.; Wiśniewska, Z.; Peris, S.; Budnik, S.; Rutkowski, A. The effect of protease and Bacillus licheniformis on nutritional value of pea, faba bean, yellow lupin and narrow-leaved lupin in broiler chicken diets. Br. Poult. Sci. 2020. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Rudeaux, F.; Kim, I.H. Efficacy of dietary Bacillus subtilis and Bacillus licheniformis supplementation continuously in pullet and lay period on egg production, excreta microflora, and egg quality of Hyline-Brown birds. Poult. Sci. 2019, 98, 4722–4728. [Google Scholar] [CrossRef]
- Luan, S.J.; Sun, Y.B.; Wang, Y.; Sa, R.N.; Zhang, H.F. Bacillus amyloliquefaciens spray improves the growth performance, immune status, and respiratory mucosal barrier in broiler chickens. Poult. Sci. 2019, 98, 1403–1409. [Google Scholar] [CrossRef]
- Wu, Y.; Shao, Y.; Song, B.; Zhen, W.; Wang, Z.; Guo, Y.; Shahid, M.S.; Nie, W. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Sci. Biotechnol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Knap, I.; Lund, B.; Kehlet, A.B.; Hofacre, C.; Mathis, G. Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 2010, 54, 931–935. [Google Scholar] [CrossRef]
- Zhou, M.; Zeng, D.; Ni, X.; Tu, T.; Yin, Z.; Pan, K.; Jing, B. Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids Health Dis. 2016, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, H.; Le Lv, Q.X.; Yin, C.; Zhang, K.; Wang, P.; Hu, J. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas. J. Anim. Sci. 2012, 25, 682. [Google Scholar] [CrossRef] [PubMed]
- Rozs, M.; Manczinger, L.; Vágvölgyi, C.; Kevei, F. Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiol. Lett. 2001, 205, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Becker, S.; Xiao, Y.; Lyu, W.; Yang, Q.; Zhu, H.; Yang, H.; Zhao, J.; Zhang, G. Differential impact of subtherapeutic antibiotics and ionophores on intestinal microbiota of broilers. Microorganisms 2019, 7, 282. [Google Scholar] [CrossRef]
- Broom, L.J. The sub-inhibitory theory for antibiotic growth promoters. Poult. Sci. 2017, 96, 3104–3108. [Google Scholar] [CrossRef]
- Antoszczak, M.; Steverding, D.; Huczyński, A. Anti-parasitic activity of polyether ionophores. Eur. J. Med. Chem. 2019, 166, 32–47. [Google Scholar] [CrossRef]
- Behnamifar, A.R.; Rahimi, S.; Kiaei, M.M.; Fayazi, H. Comparison of the effect of probiotic, prebiotic, salinomycin and vaccine in control of coccidiosis in broiler chickens. Iran. J. Vet. Res. 2019, 20, 51–54. [Google Scholar]
- Taherpour, K.; Moravej, H.; Taheri, H.R.; Shivazad, M. Effect of dietary inclusion of probiotic, prebiotic and butyric acid glycerides on resistance against coccidiosis in broiler chickens. J. Poult. Sci. 2012, 49, 57–61. [Google Scholar] [CrossRef]
- Kierończyk, B.; Pruszyńska-Oszmałek, E.; Świątkiewicz, S.; Rawski, M.; Długosz, J.; Engberg, E.M.; Józefiak, D. The nisin improves broiler chicken growth performance and interacts with salinomycin in terms of gastrointestinal tract microbiota composition. J. Anim. Feed Sci. 2016, 25, 309–316. [Google Scholar] [CrossRef]
- Czerwiński, J.; Højberg, O.; Smulikowska, S.; Engberg, R.M.; Mieczkowska, A. Effects of sodium butyrate and salinomycin upon intestinal microbiota, mucosal morphology and performance of broiler chickens. Arch. Anim. Nutr. 2012, 66, 102–116. [Google Scholar] [CrossRef]
- Diarra, M.S.; Silversides, F.G.; Diarrassouba, F.; Pritchard, J.; Masson, L.; Brousseau, R.; Bonnet, C.; Delaquis, P.; Bach, S.; Skura, B.J. Impact of feed supplementation with antimicrobial agents on growth performance of broiler chickens, Clostridium perfringens and Enterococcus counts, and antibiotic resistance phenotypes and distribution of antimicrobial resistance determinants in Escheric. Appl. Environ. Microbiol. 2007, 73, 6566–6576. [Google Scholar] [CrossRef] [PubMed]
- Aviagen Ross Broiler Management Handbook. 2018. Available online: http://eu.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 15 April 2020).
- Council, N.R. Nutrient Requirements of Poultry: 1994; National Academies Press: Washington, DC, USA, 1994; ISBN 0309048923. [Google Scholar]
- Drew, M.D.; Syed, N.A.; Goldade, B.G.; Laarveld, B.; Van Kessel, A.G. Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens. Poult. Sci. 2004, 83, 414–420. [Google Scholar] [CrossRef]
- Knarreborg, A.; Simon, M.A.; Engberg, R.M.; Jensen, B.B.; Tannock, G.W. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 2002, 68, 5918–5924. [Google Scholar] [CrossRef] [PubMed]
- Kaldhusdal, M.; Hofshagen, M.; Løvland, A.; Langstrand, H.; Redhead, K. Necrotic enteritis challenge models with broiler chickens raised on litter: Evaluation of preconditions, Clostridium perfringens strains and outcome variables. FEMS Immunol. Med. Microbiol. 1999, 24, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Safety and efficacy of B-Act® (Bacillus licheniformis DSM 28710) for chickens for fattening and chickens reared for laying. EFSA J. 2016, 14. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Schumacker, R.; Tomek, S. R Fundamentals BT—Understanding Statistics Using R; Schumacker, R., Tomek, S., Eds.; Springer: New York, NY, USA, 2013; pp. 1–10. ISBN 978-1-4614-6227-9. [Google Scholar]
- De Mendiburu, F.; Simon, R. Agricolae-Ten years of an open source statistical tool for experiments in breeding, agriculture and biology. PeerJ 2015. [Google Scholar] [CrossRef]
- Revelle, W. An introduction to the psych package: Part I: Data entry and data description 2017. Available online: http://www.personality-project.org/r/tutorials/vignettes/intro.pdf (accessed on 15 April 2020).
- Mailund, T. Manipulating Data Frames: Dplyr. In R Data Science Quick Reference; Apress: Berkeley, CA, USA, 2019; pp. 109–160. [Google Scholar]
- Ogle, D.H. Introductory Fisheries Analyses with R; CRC Press: Boca Raton, FL, USA, 2016; Volume 32, ISBN 1482235226. [Google Scholar]
- Rasco, D. An R Companion for Applied Statistics I: Basic Bivariate Techniques; SAGE Publications: Thousand Oaks, CA, USA, 2020; ISBN 1071806300. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Basel, Switzerland, 2016; ISBN 3319242776. [Google Scholar]
- Gong, L.; Wang, B.; Mei, X.; Xu, H.; Qin, Y.; Li, W.; Zhou, Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018, 89, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Musa, B.B.; Duan, Y.; Khawar, H.; Sun, Q.; Ren, Z.; Elsiddig Mohamed, M.A.; Abbasi, I.H.R.; Yang, X. Bacillus subtilis B21 and Bacillus licheniformis B26 improve intestinal health and performance of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Li, Y.L.; Yu, D.Y.; Rajput, I.R.; Li, W.F. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 2013, 92, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Hanuszewska, M.; Blanch, A.; Kozłowski, K.; Rouault, M. Effect of Bacillus subtilis and Bacillus licheniformis inclusion in turkey diets on growth performance. Ann. Wars. Univ. Life Sci. SGGW Anim. Sci. 2018, 57, 95–101. [Google Scholar] [CrossRef]
- Engberg, R.M.; Hedemann, M.S.; Leser, T.D.; Jensen, B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 2000, 79, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Apajalahti, J.; Kettunen, A. Microbes of the chicken gastrointestinal tract. Avian Gut Funct. Heal. Dis. 2006, 28, 124–137. [Google Scholar]
- Pereira, R.; Bortoluzzi, C.; Durrer, A.; Fagundes, N.S.; Pedroso, A.A.; Rafael, J.M.; de Lima Perim, J.E.; Zavarize, K.C.; Napty, G.S.; Andreote, F.D. Performance and intestinal microbiota of chickens receiving probiotic in the feed and submitted to antibiotic therapy. J. Anim. Physiol. Anim. Nutr. 2019, 103, 72–86. [Google Scholar] [CrossRef]
- Feye, K.M.; Baxter, M.F.A.; Tellez-Isaias, G.; Kogut, M.H.; Ricke, S.C. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult. Sci. 2020, 99, 653–659. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Długosz, J.; Świątkiewicz, S.; Józefiak, D. Avian crop function—A review. Ann. Anim. Sci. 2016, 16, 653–678. [Google Scholar] [CrossRef]
- Yeoman, C.J.; Chia, N.; Jeraldo, P.; Sipos, M.; Goldenfeld, N.D.; White, B.A. The microbiome of the chicken gastrointestinal tract. Anim. Heal. Res. Rev. 2012, 13, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.R.; Gong, J. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res. Int. 2011, 44, 3149–3159. [Google Scholar] [CrossRef]
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B.; et al. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Zhang, H.; Wu, S.; Hui, Q.; Yang, C.; Fang, R.; Qi, G. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Front. Physiol. 2019, 9, 1968. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kennes, Y.M.; Lepp, D.; Yin, X.; Wang, Q.; Yu, H.; Yang, C.; Gong, J.; Diarra, M.S. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 2020, 99, 936–948. [Google Scholar] [CrossRef]
- Kumar, S.; Chen, C.; Indugu, N.; Werlang, G.O.; Singh, M.; Kim, W.K.; Thippareddi, H. Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Wang, X.; Farnell, Y.Z.; Kiess, A.S.; Peebles, E.D.; Wamsley, K.G.S.; Zhai, W. Effects of Bacillus subtilis and coccidial vaccination on cecal microbial diversity and composition of Eimeria-challenged male broilers. Poult. Sci. 2019, 98, 3839–3849. [Google Scholar] [CrossRef]
- Ren, H.; Vahjen, W.; Dadi, T.; Saliu, E.-M.; Boroojeni, F.G.; Zentek, J. Synergistic Effects of probiotics and phytobiotics on the intestinal microbiota in young broiler chicken. Microorganisms 2019, 7, 684. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, W.; Zhang, H.; Wang, J.; Zhang, W.; Gao, J.; Wu, S.; Qi, G. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018, 8, 15358. [Google Scholar] [CrossRef]
- Józefiak, A.; Benzertiha, A.; Kierończyk, B.; Łukomska, A.; Wesołowska, I.; Rawski, M. Improvement of cecal commensal microbiome following the insect additive into chicken diet. Animals 2020, 10, 577. [Google Scholar] [CrossRef]
- Singh, P.; Karimi, A.; Devendra, K.; Waldroup, P.W.; Cho, K.K.; Kwon, Y.M. Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens. Poult. Sci. 2013, 92, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.; Perdicaro, D.J.; Brown, A.W.; Hammons, S.; Carden, T.J.; Carr, T.P.; Eskridge, K.M.; Walter, J. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl. Environ. Microbiol. 2013, 79, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Hang, I.; Rinttila, T.; Zentek, J.; Kettunen, A.; Alaja, S.; Apajalahti, J.; Harmoinen, J.; de Vos, W.M.; Spillmann, T. Effect of high contents of dietary animal-derived protein or carbohydrates on canine faecal microbiota. BMC Vet. Res. 2012, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Y. Bacillus licheniformis—Fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2019, 99, 1432–1443. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Manoharan, M.S. Effect of coccidiostat and natustatim on intestinal microflora in caecum of broilers challenged with Eimeria analyzed using bacterial 16S rDNA tag-encoded Flx amplicon pyrosequencing. Master’s Thesis, Stephen F. Austin State University, Nacogdoches, TX, USA, 2010. [Google Scholar]
- Torok, V.A.; Allison, G.E.; Percy, N.J.; Ophel-Keller, K.; Hughes, R.J. Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl. Environ. Microbiol. 2011, 77, 3380–3390. [Google Scholar] [CrossRef]
- Kaczmarek, S.A.; Barri, A.; Hejdysz, M.; Rutkowski, A. Effect of different doses of coated butyric acid on growth performance and energy utilization in broilers. Poult. Sci. 2016, 95, 851–859. [Google Scholar] [CrossRef]
- Ragaa, N.M.; Korany, R.M.S. Studying the effect of formic acid and potassium diformate on performance, immunity and gut health of broiler chickens. Anim. Nutr. 2016, 2, 296–302. [Google Scholar] [CrossRef]
- Apajalahti, J.; Vienola, K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016, 221, 323–330. [Google Scholar] [CrossRef]
- Guo, J.R.; Dong, X.F.; Liu, S.; Tong, J.M. High-throughput sequencing reveals the effect of Bacillus subtilis CGMCC 1.921 on the cecal microbiota and gene expression in ileum mucosa of laying hens. Poult. Sci. 2018, 97, 2543–2556. [Google Scholar] [CrossRef] [PubMed]
- Pourabedin, M.; Zhao, X. Prebiotics and gut microbiota in chickens. FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009, 10, 3531–3546. [Google Scholar] [CrossRef] [PubMed]

| Ingredient (g·kg−1) | Diets | ||
|---|---|---|---|
| 1–10 d | 11–22 d | 23–36 d | |
| Wheat | 360.8 | 362.1 | 356.0 |
| Maize | 250.0 | 250.0 | 250.0 |
| Rapeseed expeller | - | 40.0 | 80.0 |
| Rapeseeds | 40.0 | 80.0 | 60.0 |
| Soy meal, 46.8% | 264.7 | 185.4 | 161.1 |
| Fish meal, 64% | 20.0 | 20.0 | 20.0 |
| Hemoglobin | 5.0 | 5.9 | 5.0 |
| Soy oil | 21.1 | - | - |
| Pig lard | - | 29.4 | 44.4 |
| 1 Vitamin-mineral premix | 3.0 | 3.0 | 3.0 |
| Monocalcium phosphate | 16.8 | 9.3 | 5.5 |
| Limestone | 8.0 | 6.4 | 6.4 |
| NaCl | 1.1 | 1.4 | 1.7 |
| Na2SO4 | 2.2 | 1.5 | 1.2 |
| L-lysine | 2.9 | 2.5 | 2.4 |
| L-methionine | 2.6 | 2.0 | 1.9 |
| L-threonine | 1.3 | 0.9 | 1.4 |
| L-valine | 0.5 | 0.2 | - |
| Calculated nutritive value (g·kg−1) | |||
| 2 AMEN, kcal·kg−1 | 3010 | 3150 | 3230 |
| Crude protein | 216.0 | 200.0 | 196.0 |
| Crude fat | 58.3 | 85.2 | 94.2 |
| Crude fiber | 27.1 | 31.9 | 33.8 |
| Dig. Lys | 12.0 | 10.7 | 10.3 |
| Dig. Met + Cys | 8.9 | 8.1 | 7.9 |
| Calcium-total | 8.5 | 7.0 | 6.5 |
| Treatment | Performance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–10 d | 11–22 d | 23–36 d | 1–36 d | ||||||||||
| Salinomycin | B. licheniformis | BWG 1, g | FI 2, g | FCR 3, g:g | BWG, g | FI, g | FCR, g:g | BWG, g | FI, g | FCR, g:g | BWG, g | FI, g | FCR, g:g |
| - | - | 234 | 314 | 1.34 | 700 | 1006 | 1.44 | 1300 | 2073 | 1.60 | 2234 | 3393 | 1.52 |
| + | - | 230 | 318 | 1.39 | 705 | 1002 | 1.42 | 1330 | 2108 | 1.59 | 2264 | 3428 | 1.52 |
| - | + | 239 | 316 | 1.32 | 725 | 1017 | 1.40 | 1331 | 2125 | 1.60 | 2295 | 3458 | 1.51 |
| + | + | 243 | 319 | 1.32 | 715 | 1008 | 1.41 | 1325 | 2097 | 1.58 | 2283 | 3425 | 1.50 |
| Model RMSE 4 | 11.47 | 8.84 | 0.08 | 22.88 | 32.76 | 0.02 | 56.47 | 11.88 | 0.03 | 76.61 | 102.08 | <0.01 | |
| Model P | 0.070 | 0.553 | 0.170 | 0.081 | 0.786 | 0.003 | 0.575 | 0.476 | 0.525 | 0.302 | 0.572 | 0.018 | |
| Main effects | |||||||||||||
| Salinomycin | |||||||||||||
| None | 237 | 315 | 1.33 | 713 | 1012 | 1.42 | 1315 | 2099 | 1.60 | 2264 | 3425 | 1.51 | |
| 60 mg/kg | 236 | 319 | 1.35 | 710 | 1005 | 1.42 | 1327 | 2103 | 1.59 | 2274 | 3426 | 1.51 | |
| B. licheniformis | |||||||||||||
| None | 232 b | 316 | 1.37 | 702 b | 1004 | 1.43 a | 1315 | 2090 | 1.59 | 2249 | 3410 | 1.52 a | |
| 1.6 × 109 CFU/kg | 241 a | 317 | 1.32 | 720 a | 1013 | 1.41 b | 1328 | 2111 | 1.59 | 2289 | 3441 | 1.50 b | |
| p-value | |||||||||||||
| Salinomycin | 0.962 | 0.177 | 0.455 | 0.723 | 0.550 | 0.565 | 0.510 | 0.880 | 0.146 | 0.705 | 0.970 | 0.205 | |
| B. licheniformis | 0.016 | 0.658 | 0.067 | 0.018 | 0.423 | <0.001 | 0.466 | 0.386 | 0.967 | 0.100 | 0.349 | 0.004 | |
| Interaction terms Salinomycin × B. licheniformis | 0.275 | 0.859 | 0.286 | 0.331 | 0.837 | 0.094 | 0.316 | 0.194 | 0.795 | 0.380 | 0.295 | 0.827 | |
| Treatment | pH | |||
|---|---|---|---|---|
| Salinomycin | B. licheniformis | Crop | Jejunum | Ceca |
| - | - | 4.90 | 5.92 | 5.67 b |
| + | - | 5.61 | 5.93 | 6.03 a |
| - | + | 4.69 | 5.80 | 5.60 b |
| + | + | 4.84 | 5.90 | 5.44 b |
| Model RMSE 1 | 0.43 | 0.16 | 0.40 | |
| Model P | <0.001 | 0.345 | 0.020 | |
| Main effects | ||||
| Salinomycin | ||||
| None | 4.79 b | 5.86 | 5.63 | |
| 60 mg/kg | 5.21 a | 5.92 | 5.72 | |
| B. licheniformis | ||||
| None | 5.23 a | 5.93 | 5.84 a | |
| 1.6 × 109 CFU/kg | 4.77 b | 5.85 | 5.52 b | |
| p-value | ||||
| Salinomycin | 0.005 | 0.350 | 0.494 | |
| B. licheniformis | 0.001 | 0.176 | 0.015 | |
| Interaction terms Salinomycin × B. licheniformis | 0.053 | 0.432 | 0.046 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trela, J.; Kierończyk, B.; Hautekiet, V.; Józefiak, D. Combination of Bacillus licheniformis and Salinomycin: Effect on the Growth Performance and GIT Microbial Populations of Broiler Chickens. Animals 2020, 10, 889. https://doi.org/10.3390/ani10050889
Trela J, Kierończyk B, Hautekiet V, Józefiak D. Combination of Bacillus licheniformis and Salinomycin: Effect on the Growth Performance and GIT Microbial Populations of Broiler Chickens. Animals. 2020; 10(5):889. https://doi.org/10.3390/ani10050889
Chicago/Turabian StyleTrela, Jacek, Bartosz Kierończyk, Veerle Hautekiet, and Damian Józefiak. 2020. "Combination of Bacillus licheniformis and Salinomycin: Effect on the Growth Performance and GIT Microbial Populations of Broiler Chickens" Animals 10, no. 5: 889. https://doi.org/10.3390/ani10050889
APA StyleTrela, J., Kierończyk, B., Hautekiet, V., & Józefiak, D. (2020). Combination of Bacillus licheniformis and Salinomycin: Effect on the Growth Performance and GIT Microbial Populations of Broiler Chickens. Animals, 10(5), 889. https://doi.org/10.3390/ani10050889






