The Effect of Iron Dextran Injection on Daily Weight Gain and Haemoglobin Values in Whole Milk Fed Calves
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Analysis
2.3. Ethical Considerations
3. Results
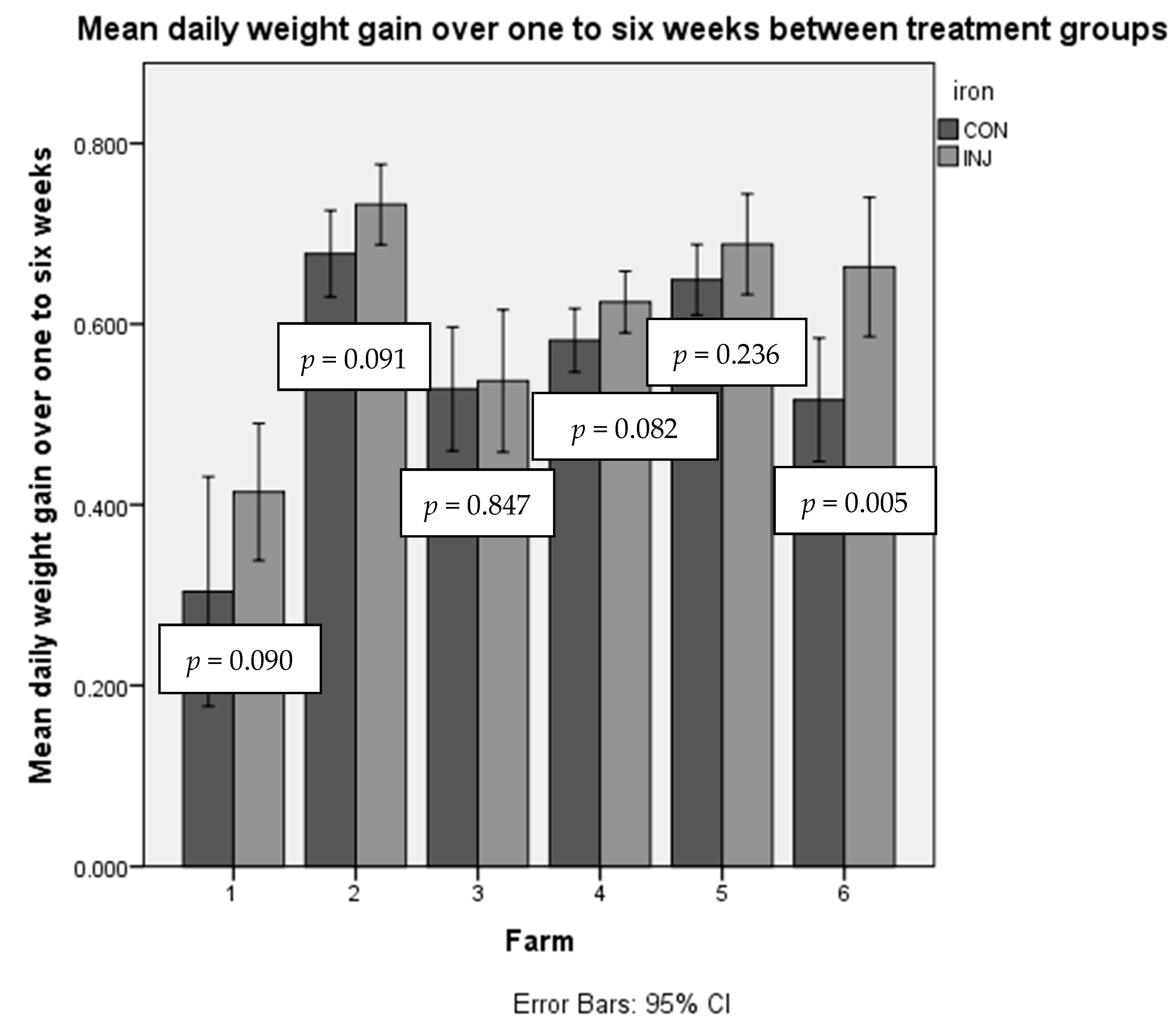
3.1. Daily Live Weight Gain
3.1.1. One to Six Weeks DG
3.1.2. Six- to Twelve-Week DG
3.1.3. One- to Twelve-Week DG
3.2. Haemoglobin Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Farm | Number of Calves Enrolled | Number of Calves in CON Group | Number of Calves in INJ Group | Dead One to Six Weeks | Dead One to 12 Weeks | Missed Bloods or Weights | Excluded for no Date of Birth | Excluded Hb under 72.5 g/L at Week One |
|---|---|---|---|---|---|---|---|---|
| 1 | 28 | 13 | 15 | 6 | 1 | 0 | 1 | 0 |
| 2 | 38 | 19 | 19 | 1 | 1 | 1 | 6, one is also under 72.5 g/L | 3 |
| 3 | 18 | 8 | 10 | 0 | 0 | 5 | 2, one is also under 72.5 g/L | 3 |
| 4 | 65 | 33 | 32 | 1 | 1 | 3 | 0 | 0 |
| 5 | 40 | 19 | 21 | 2 | 0 | 4 | 0 | 1 |
| 6 | 48 | 25 | 23 | 0 | 0 | 1 | 0 | 1 |
| Total | 237 | 117 | 120 | 10 | 3 | 14 | 9 | 8 |
| Farm | Treatment Group (g/d) (SD) | Control Group (g/d) (SD) | Difference (g/d) | p-Value from Independent T-Test for Each Farm | Overall Daily Live Weight Gain (g/d) (SD) |
|---|---|---|---|---|---|
| 1 | 414 (126) | 304 (165) | 110 | 0.090 | 369 (150) |
| 2 | 732 (74) | 678 (90) | 54 | 0.091 | 702 (86) |
| 3 | 537 (94) | 528 (65) | 9 | 0.847 | 533 (80) |
| 4 | 624 (93) | 582 (98) | 42 | 0.082 | 603 (97) |
| 5 | 688 (116) | 649 (79) | 39 | 0.236 | 669 (100) |
| 6 | 663 (178) | 516 (162) | 147 | 0.005 | 588 (184) |
| Average | 625 (151) | 566 (170) | 59 |
| Farm | Treatment Group (g/d) (SD) | Control Group (g/d) (SD) | Difference (g/d) | Overall Daily Live Weight Gain (g/d) (SD) |
|---|---|---|---|---|
| 1 | 861 (178) | 846 (75) | 15 | 855 (145) |
| 2 | 980 (142) | 1014 (174) | –34 | 998 (158) |
| 3 | 1017 (139) | 906 (147) | 111 | 970 (148) |
| 4 | 1017 (134) | 980 (108) | 37 | 998 (122) |
| 5 | 872 (107) | 937 (137) | –65 | 904 (126) |
| 6 | 725 (125) | 708 (167) | 17 | 717 (147) |
| Average | 903 (174) | 899 (178) | 4 |
| Farm | Treatment Group (g/d) (SD) | Control Group (g/d) (SD) | Difference (g/d) | Overall Daily Live Weight Gain (g/d) (SD) |
|---|---|---|---|---|
| 1 | 632 (124) | 591 (60) | 41 | 617 (105) |
| 2 | 865 (73) | 852 (93) | 13 | 858 (83) |
| 3 | 776 (84) | 715 (85) | 61 | 750 (87) |
| 4 | 768 (80) | 730 (81) | 38 | 749 (82) |
| 5 | 780 (99) | 792 (86) | –12 | 786 (91) |
| 6 | 696 (72) | 621 (72) | 75 | 658 (81) |
| Average | 750 (108) | 722 (115) | 28 |
References
- Knoop, C.; Krauss, W.; Washburn, R. The Development of Nutritional Anemia in Dairy Calves. J. Dairy Sci. 1935, 18, 337–347. [Google Scholar] [CrossRef]
- Hibbs, J.; Conrad, H.; Vandersall, J.; Gale, C. Occurrence of Iron Deficiency Anemia in Dairy Calves at Birth and Its Alleviation by Iron Dextran Injection. J. Dairy Sci. 1963, 46, 1118–1124. [Google Scholar] [CrossRef]
- Cherayil, B.J. The role of iron in the immune response to bacterial infection. Immunol. Res. 2011, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Perri, A.M.; Friendship, R.M.; Harding, J.C.S.; O’Sullivan, T.L. An investigation of iron deficiency and anemia in piglets and the effect of iron status at weaning on post-weaning performance. J. Swine Health Prod. 2016, 24, 10–20. [Google Scholar]
- Hunt, C.; Nielsen, F. Nutritional Aspects of Minerals in Bovine and Human Milks. In Advanced Dairy Chemistry; McSweeney, P., Fox, P.F., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 391–456. [Google Scholar]
- National Research Council. Minerals. In Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Ramin, A.G.; Asri-Rezaei, S.; Paya, K.; Eftekhari, Z.; Jelodary, M.; Akbari, H.; Ramin, S. Evaluation of Anemia in Calves up to 4 Months of Age in Holstein Dairy Herds. İstanbul Üniversitesi Veteriner Fakültesi Dergisi 2014, 40, 1. [Google Scholar] [CrossRef]
- Bhattarai, S.; Nielsen, J. Early indicators of iron deficiency in large piglets at weaning. J. Swine Health Prod. 2015, 23, 10–17. [Google Scholar]
- Pu, Y.; Li, S.; Xiong, H.; Zhang, X.; Wang, Y.; Du, H.-H. Iron Promotes Intestinal Development in Neonatal Piglets. Nutrients 2018, 10, 726. [Google Scholar] [CrossRef]
- Svoboda, M.; Drabek, J. Iron deficiency in suckling piglets: Ethiology, clinical aspects and diagnosis. Folia Vet. 2005, 49, 104–111. [Google Scholar]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, S568–S580. [Google Scholar] [CrossRef]
- Rajabian, F.; Mohri, M.; Heidarpour, M. Relationships between oxidative stress, haematology and iron profile in anaemic and non-anaemic calves. Veter. Rec. 2017, 181, 265. [Google Scholar] [CrossRef]
- Bünger, U.; Schmoldt, P.; Ponge, J. Oral and parenteral control of iron deficit in relation to the course of diseases in milk fed calves originating from different farms. Monatsh. Veterinarmed. 1986, 41, 302–306. (In German) [Google Scholar]
- Volker, H.; Rotermund, L. The possibilities of oral iron supply in calves to maintain their health status. Ger. Vet. Wkly. 2000, 107, 16–22. (In German) [Google Scholar]
- Mohri, M.; Poorsina, S.; Sedaghat, R. Effects of Parenteral Supply of Iron on RBC Parameters, Performance, and Health in Neonatal Dairy Calves. Boil. Trace Element Res. 2009, 136, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Allison, R.W. Evaluation of the Ruminant Complete Blood Cell Count. Veter. Clin. North Am. Food Anim. Pr. 2007, 23, 377–402. [Google Scholar] [CrossRef]
- RSPCA. RSPCA Welfare Standards for Dairy Cattle. 2018. Available online: https://www.ciwf.org.uk/media/5235182/Statistics-Dairy-cows.pdf (accessed on 8 July 2018).
- Dillane, P.; Krump, L.; Kennedy, A.; Sayers, R.G.; Sayers, G.P. Establishing blood gas ranges in healthy bovine neonates differentiated by age, sex, and breed type. J. Dairy Sci. 2018, 101, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- UK legislation. The Welfare of Farmed Animals (England) Regulations 2007, Animal Welfare Act 2006. Available online: http://www.legislation.gov.uk/uksi/2007/2078/schedule/6/made (accessed on 9 September 2017).
- Macfarlane, J.A.; Grove-White, D.H.; Royal, M.D.; Smith, R. Use of plasma samples to assess passive transfer in calves using refractometry: Comparison with serum and clinical cut-off point. Veter. Rec. 2014, 174, 303. [Google Scholar] [CrossRef] [PubMed]
- Elsohaby, I.; McClure, J.; Waite, L.; Cameron, M.; Heider, L.; Keefe, G. Using serum and plasma samples to assess failure of transfer of passive immunity in dairy calves. J. Dairy Sci. 2019, 102, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Brickell, J.; Bourne, N.; McGowan, M.; Wathes, D.C. Effect of growth and development during the rearing period on the subsequent fertility of nulliparous Holstein-Friesian heifers. Theriogenology 2009, 72, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Soberón, F.; Raffrenato, E.; Everett, R.; Van Amburgh, M. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 2012, 95, 783–793. [Google Scholar] [CrossRef]
- Wathes, D.C.; Pollott, G.E.; Johnson, K.F.; Richardson, H.; Cooke, J.S. Heifer fertility and carry over consequences for life time production in dairy and beef cattle. Anim. 2014, 8, 91–104. [Google Scholar] [CrossRef]
- Cherayil, B.J. Iron and Immunity: Immunological Consequences of Iron Deficiency and Overload. Arch. Immunol. Ther. Exp. 2010, 58, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Cassat, J.E.; Skaar, E.P. Iron in infection and immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Bostedt, H.; Hospes, R.; Wehrend, A.; Schramel, P. Effects of parenteral administration of iron preparations in the early development of calves. Vet. Rev. 2000, 55, 305–315. (In German) [Google Scholar]
- Kume, S.-I.; Tanabe, S. Effect of Supplemental Lactoferrin with Ferrous Iron on Iron Status of Newborn Calves. J. Dairy Sci. 1996, 79, 459–464. [Google Scholar] [CrossRef]
- Mohri, M.; Sarrafzadeh, F.; Seifi, H. Effect of oral iron supplementation on hematocrit, live weight gain and health of neonatal dairy calves. Iranian J. Vet. Med. Res. 2006, 14, 34–37. [Google Scholar]
- BNF. Iron Dextran, Institute for Health and Care Excellence, National. 2019. Available online: https://bnf.nice.org.uk/drug/iron-dextran.html (accessed on 29 December 2019).
- Matrone, G.; Conley, C.; Wise, G.; Waugh, R. A Study of Iron and Copper Requirements of Dairy Calves. J. Dairy Sci. 1957, 40, 1437–1447. [Google Scholar] [CrossRef]
- Thomas, J.; Okamoto, M.; Jacobson, W.; Moore, L. A Study of Hemoglobin Levels in the Blood of Young Dairy Calves and the Alleviation of Anemia by Iron. J. Dairy Sci. 1954, 37, 805–812. [Google Scholar] [CrossRef]
- Blaxter, K.L.; Sharman, G.A.M.; Macdonald, A.M. Iron-deficiency anaemia in calves. Br. J. Nutr. 1957, 11, 234–246. [Google Scholar] [CrossRef]
- Potthoff, B. Influence of Oral Iron Supplements (Iron Amino Acid Chelate) in the Dry Period on Parameters of the Iron Metabolism of Cows and Their Newborn Calves. Dissertation Thesis, University of Veterinary Medicine Hannover, Hannover, Germany, 2011. Available online: https://elib.tiho-hannover.de/receive/etd_mods_00001087 (accessed on 20 September 2019). (In German).
| Farm Factors | Farm 1 | Farm 2 | Farm 3 | Farm 4 | Farm 5 | Farm 6 |
|---|---|---|---|---|---|---|
| Volume of whole milk fed per day (fed via bucket with teat) | 6 L | 6 L | 5 L | 5 L | 6 L | 6 L |
| Starter ration | Yes, from birth | Yes, from birth | Yes, from week 1 | Yes, from 2–3 weeks | Yes, from birth | Yes, from birth |
| Calving pattern | All year round | Spring | All year round | Spring | Spring | Spring |
| Number of calves enrolled | 28 | 38 | 18 | 65 | 40 | 48 |
| Average bodyweight at first weighing (SD) * | 39.3 (4.4) | 43.7 (7.9) | 42.7 (3.6) | 34.7 (4.3) | 38.7 (4.5) | 37.1 (4.7) |
| Average haemoglobin (g/L) levels at first measurement (SD) | 114.0 (20.0) | 105.0 (16.3) | 105.6 (17.1) | 110.3 (13.4) | 108.6 (12.9) | 102.9 (13.7) |
| Farm | Average Hb Six Weeks (g/L) (SD) | Average Change in Hb Level One to Six Weeks (g/L) (SD) | Number of Calves with HB Levels under 90 g/L at Six Weeks | |||||
|---|---|---|---|---|---|---|---|---|
| INJ | CON | Difference between Groups | INJ | CON | Difference between Groups | INJ | CON | |
| 1 | 120.9 (14.6) | 106.9 (10.0) | 14 | 9.8 (18.0) | −6.2 (20.9) | 16 | 0 | 0 |
| 2 | 108.1 (6.4) | 87.9 (12.2) | 18.9 | 5.2 (11.3) | −16.8 (10.7) | 19.3 | 0 | 8 (2) * |
| 3 | 105.0 (10.4) | 83.5 (5.2) | 19.8 | 0.4 (5.7) | −12.3 (6.8) | 11.8 | 0 | 4 |
| 4 | 109.1 (11.5) | 95.5 (10.8) | 13.6 | −0.5 (11.5) | −15.4 (12.6) | 14.9 | 2 | 13 |
| 5 | 113.6 (13.9) | 95.9 (13.1) | 17.7 | 3.9 (15.5) | −11.2 (12.9) | 15.1 | 2 | 4 (1) * |
| 6 | 108.0 (11.9) | 95.6 (15.6) | 12.4 | 4.4 (12.6) | −7.3 (19.0) | 11.7 | 2 | 7 (1) * |
| Overall average | 110.7 (12.4) | 94.9 (13.2) | 15.3 (1.7) | 3.4 (13.3) | −12.1 (15.1) | 15.2 (1.9) | 6 total | 40 total |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allan, J.; Plate, P.; Van Winden, S. The Effect of Iron Dextran Injection on Daily Weight Gain and Haemoglobin Values in Whole Milk Fed Calves. Animals 2020, 10, 853. https://doi.org/10.3390/ani10050853
Allan J, Plate P, Van Winden S. The Effect of Iron Dextran Injection on Daily Weight Gain and Haemoglobin Values in Whole Milk Fed Calves. Animals. 2020; 10(5):853. https://doi.org/10.3390/ani10050853
Chicago/Turabian StyleAllan, Jennifer, Peter Plate, and Steven Van Winden. 2020. "The Effect of Iron Dextran Injection on Daily Weight Gain and Haemoglobin Values in Whole Milk Fed Calves" Animals 10, no. 5: 853. https://doi.org/10.3390/ani10050853
APA StyleAllan, J., Plate, P., & Van Winden, S. (2020). The Effect of Iron Dextran Injection on Daily Weight Gain and Haemoglobin Values in Whole Milk Fed Calves. Animals, 10(5), 853. https://doi.org/10.3390/ani10050853





